Abstract
T cells simultaneously producing multiple cytokines and possessing cytotoxic capacity termed polyfunctional cells (PFCs) are increasingly recognized as the immune correlate of protection against pathogenic viruses. We investigated co-expression of four cytokines (interferon-γ, macrophage inflammatory protein 1-α, tumour necrosis factor-α and interleukin-2) and degranulation capacity (CD107a surface expression) of Epstein–Barr virus (EBV) -specific CD4+ and CD8+ T cells upon stimulation by overlapping peptides of EBV lytic (BZLF1) and latent (EBNA1, EBNA3 and LMP2) proteins, in 20 healthy Chinese long-term carriers. Two patients with post-transplant lymphoproliferative disorder (PTLD), who had impaired T-cell immunity, were studied for comparison. Both EBV-specific CD4+ and CD8+ PFCs were readily generated in long-term carriers and showed immunodominance hierarchies of latent proteins (EBNA1 > EBNA3/LMP2 and EBNA3 > LMP2 > EBNA1 for CD4+ and CD8+ T cells, respectively), as evidenced by a higher proportion of PFCs generated by immunodominant EBV proteins than by subdominant viral proteins. In contrast, the proportion of EBV-specific PFCs was markedly decreased in patients with PTLD. The EBV-specific PFCs produced more cytokine per cell than single-functional T cells and comprised different subsets. Five-functional CD4+ and CD8+ T cells were detected and four-functional CD4+ T cells were mainly CD107a negative and expressed all four cytokines whereas four-functional CD8+ T cells were mainly CD107a positive and expressed three of the four cytokines (interleukin-2-negative). We conclude that EBV-specific PFCs are generated in much higher proportions in the long-term carriers than in the patients with PTLD and maintain the immunodominant characteristics of the virus.
Keywords: Epstein–Barr virus, immunodominance hierarchies, lytic and latent proteins, polyfunctional T cells
Introduction
Epstein–Barr Virus (EBV), a ubiquitous human γ-herpesvirus, is capable of establishing persistent infection in the majority of world populations.1 Primary EBV infection usually occurs in an asymptomatic manner, but some individuals may present with a self-limiting lymphoproliferative condition, namely infectious mononucleosis, which is clinically characterized by fever, lymphadenopathy, tonsillopharyngitis and hepatosplenomegaly.2 Transmission of EBV is primarily through saliva contact.1 After oral transmission, the virus replicates in the oropharyngeal epithelial cells before infecting naive B cells and subsequently entering the long-lived memory B-cell pool where it establishes lifelong latency expressing very few or no latent proteins.3 Persistent EBV infection is closely associated with a number of malignancies such as Burkitt's lymphoma, Hodgkin's lymphoma, nasopharyngeal carcinoma and gastric carcinoma.1,4 Recent studies suggested that EBV might also contribute to certain autoimmune diseases including multiple sclerosis, systemic lupus erythematosus, rheumatoid arthritis and primary Sjögren syndrome.5–7
The immune responses to EBV encompass both innate and adaptive immunity. The innate immunity mainly consists of production of interferons (IFNs) by the phagocytes and natural killer cells to reduce immediate B-cell infection and virus replication.8 The adaptive immunity (particularly T cells) is crucial to control EBV-infected B-cell proliferation, especially during the persistent infection stage.1 A remarkable expansion of EBV-specific CD8+ T cells producing IFN-γ and exerting cytotoxic effects was observed in acute EBV infection.9 The CD4+ T cells, despite having a weaker response, are also activated by EBV to produce functional cytokines and to recognize EBV-transformed lymphoblastoid cell lines and inhibit EBV-induced B-cell transformation.10–12 Therefore, immunodeficient individuals such as HIV-infected patients or patients with post-transplant lymphoproliferative disorder (PTLD) could develop uncontrolled EBV infections.13,14 PTLD is predominantly an EBV-driven lymphoproliferative disorder occurring in solid organ and stem cell transplant recipients and arising from suppression of T-cell immunity by immunosuppressive drugs in the post-transplant period.15
Previously, most of the research on virus-specific T cells either solely examined single cytokine production (e.g. IFN-γ secretion by ELISPOT) or cytotoxicity, which might underestimate the total virus-specific immune responses and ignore some important functions of the responsive T cells.16–18 Although majority of T cells react to antigens with only one function, studies showed that T cells with multiple functions, namely polyfunctional T cells (PFCs), might exert more effective control of human viral infection.17,18 Betts et al.18 showed that HIV non-progressors maintained more highly polyfunctional HIV-specific CD8+ T cells than HIV progressors who failed to control HIV and that CD8+ PFCs could be used to evaluate HIV vaccine efficacy.
To investigate whether virus-specific PFCs are important in the control of EBV infection, we set out to study EBV-specific PFCs in healthy long-term carriers who have established long-term control of EBV and to compare them with those in patients with PTLD who have defective anti-EBV immunity. We also study the characteristics of PFCs in response to both lytic and latent EBV peptides. A nine-colour flow cytometric assay was used to simultaneously analyse five functions of CD4+ and CD8+ T cells: production of IFN-γ, macrophage inflammatory protein 1-α (MIP1-α), tumour necrosis factor-α (TNF-α) and interleukin-2 (IL-2), and surface mobilization of CD107a (degranulation marker indicating cytotoxic potential), upon stimulation by overlapping peptides of EBV lytic (BZLF1) and latent (EBNA1, EBNA3A-C and LMP2) proteins.
Materials and methods
Donors
The study protocol was approved by the Institutional Review Board of The University of Hong Kong. Informed consent was obtained from each participant before entry into the study. Venous blood samples of 20 healthy Hong Kong Chinese (12 men and eight women aged 22–50 years) were collected in the Department of Paediatrics and Adolescent Medicine, the University of Hong Kong and the Hong Kong Red Cross. All participants were confirmed serologically to have persistent EBV infection (VCAIgG+ EBNA+). The plasma virus loads of each volunteer, as measured by quantitative PCR, were negligible. Venous blood samples from two children (girls aged 3·25 years and 14 months, respectively) who underwent orthotopic liver transplantation for congenital biliary atresia and were diagnosed with PTLD at 22 and 7 months after the date of transplantation, respectively, were obtained. Both patients had serological confirmation of EBV infection and had high viral loads in plasma (3·53 × 106 copies/ml and 4·57 × 104 copies/ml, respectively) and in peripheral blood mononuclear cells (2·59 × 106 copies/million PBMCs and 3·49 × 104 copies/million PBMCs, respectively) at the time of diagnosis of PTLD.
Synthetic EBV peptides
Fifteen-mer peptides overlapped by 11 amino acids spanning each of the EBV latent proteins EBNA1, EBNA3A, EBNA3B, EBNA3C and LMP2 and EBV lytic protein BZLF1 were purchased from JPT Peptide Technologies, Berlin, Germany. Totals of 158, 234, 279, 265, 122 and 59 peptides spanning the entire EBNA1, EBNA3A, EBNA3B and EBNA3C, LMP2 and BZLF1 proteins, respectively, were used in the experiments. The peptide pools of each EBV protein were prepared according to the manufacturer's instructions. Briefly, lyophilized peptides (25 μg/vial) were reconstituted in 40 μl pure DMSO and diluted with 1210 μl PBS. Final concentration of overlapping peptides was 2 μg/ml per million cells in all experiments. Final concentration of DMSO was approximately 0·3% which did not have detectable toxicity to the cells.
Preparation of PBMCs
Peripheral blood was collected in a falcon tube containing sodium heparin (15 U/ml) followed by centrifugation at 805 g for 10 min. The plasma was collected and the remaining blood was diluted 1 : 1 in Hanks’ buffered salt solution (Invitrogen, Carlsbad, CA). The PBMCs were isolated by the standard density centrifugation through the Ficoll-Paque Plus (Amersham Bioscience, Uppsala, Sweden) gradient and washed twice in Hanks’ buffered salt solution. Cells were resuspended in 10% DMSO in 100% fetal bovine serum (FBS) (Invitrogen) for storage in liquid nitrogen until use.
Overlapping peptide stimulation
Cryopreserved PBMCs were thawed with 10% FBS/RPMI (Invitrogen) and rested overnight in six-well plates at a concentration of 2 × 106/ml of 10% FBS/RPMI at 37° in 5% CO2. The PBMCs were washed once the next day with 10% FBS/RPMI and concentrated to 1 million cells per 100 μl 10% FBS/RPMI before the experiment. Then, 100 μl PBMC suspension was transferred to 5-ml polyethylene tube and phycoerythrin (PE)-Cy5-conjugated monoclonal antibody (mAb) to CD107a (BD Pharmingen, Heidelberg, Germany), co-stimulatory reagents containing anti-CD28 mAb (1 μg/ml; BD Pharmingen), anti-CD49d mAb (1 μg/ml; BD Pharmingen) and brefeldin A (10 μg/ml; BD Pharmingen) were added to each tube. The final volume was made up to 200 μl. Overlapping peptide pools of BZLF1, EBNA1, EBNA3A, EBNA3B, EBNA3C and LMP2 were added respectively to the tubes at a final concentration of 2 μg/ml. The PBMCs stimulated with 1 μg/ml staphylococcal enterotoxin B were used as a positive control and unstimulated PBMCs with culture medium only were a negative control. The tubes were incubated for 6 hr at 37°, 5% CO2.
Cell surface and intracellular cytokine staining and nine-colour flow cytometric assay
After incubation for 6 hr, the cells were washed once in PBS according to the manufacturer's instruction and stained with Aqua Blue Dye (Dead cell exclusion; Invitrogen Molecular Probes, Eugene, OR) for 20 min. The cells were washed once in PBS and stained for 20 min with allophycocyanin-Cy7-conjugated anti-CD3 (BD Pharmingen), PE-Texas Red-conjugated anti-CD4 (Invitrogen) and Pacific Blue-conjugated anti-CD8 (BD Pharmingen). The cells were washed, fixed and permeabilized using a BD FACS fixation/permeabilization kit (BD Bioscience). The cells were washed followed by staining with FITC-conjugated anti-IFN-γ, PE-conjugated anti-MIP1-α, PE-Cy7-conjugated anti-TNF-α and allophycocyanin-conjugated anti-IL-2 (BD Pharmingen). After 30 min of intracellular staining, the cells were washed with PBS and resuspended in 1% paraformaldehyde before the flow cytometric analysis. Approximately 300 000 cells were acquired on the FACS LSR-II flow cytometer (BD Bioscience, San Jose, CA). The FACS data were analysed using flowjo software (Tree Star, San Carlos, CA) and the distribution of polyfunctional T cells was analysed using spice 5 (Mario Roederer, ImmunoTechnology Section, Vaccine Research Center, NIAID, NIH, Bethesda, MD).19
Statistical analysis
The cell frequencies were compared between CD4+ and CD8+ T cells responding to the same overlapping peptide pools and between T cells stimulated by two different peptide pools. The cytokine production measured by median fluorescent intensity (MFI) was also compared between single-functional and polyfunctional T cells. Comparisons were performed using two-tailed Wilcoxon's signed rank test, one-way analysis of variance test, and Student's t-test. A P-value < 0·05 was regarded as statistically significant. Prism 5 (Graphpad Software, La Jolla, CA) was used for calculations and illustrations.
Results
Overlapping peptide pools of EBV lytic and latent proteins activated EBV-specific CD4+ and CD8+ T cells that possessed multiple functions
The PBMCs were stimulated by overlapping peptide pools for 6 hr and subjected to the nine-colour flow cytometric assay, as described in the Materials and methods section. The negative population in the biological control (unstimulated PBMCs incubated with appropriate concentrations of DMSO and co-stimulatory reagent, and stained with the whole panel of mAbs) was used to determine the location of positive gates. Low backgrounds (< 0·05%) were achieved in every staining and were subtracted from positive results in each individual sample. Staphylococcus enterotoxin B stimulation served as the positive control. The gating scheme and T-cell responses to the overlapping peptide pool of EBNA3B in a representative healthy long-term carrier (Fig. 1a) and in a patient with PTLD (Fig. 1b) are illustrated in Fig. 1. For long-term carriers, EBNA3B could induce CD4+ and CD8+ T-cell responses characterized by any of the five functions (production of cytokines or surface mobilization of CD107a). The functional hierarchy of CD4+ T cells consisted of expression of IFN-γ and IL-2 followed by TNF-α, with minor expression of MIP1-α and CD107a whereas that of CD8+ T cells consisted of production of IFN-γ, CD107a, MIP1-α, TNF-α and IL-2 in descending order. The proportion of IFN-γ-producing CD8+ T cells was higher than that of CD4+ T cells whereas the reverse was observed for IL-2 production. Similar patterns were observed among other overlapping peptide pools. Surface expression of CD107a was detectable in some CD4+ T cells, indicating that a subset of EBV-specific CD4+ T cells could rapidly degranulate upon stimulation and might exert direct cytotoxic function. For patients with PTLD, both CD4+ and CD8+ T-cell responses showed distinct patterns of cytokine and CD107a expression compared with those in healthy long-term carriers, as demonstrated by the high expression of CD107a and TNF-α and the markedly suppressed production of IFN-γ, and to a lesser extent, IL-2. This functional profile was evident in both CD4+ and CD8+ T cells but was accentuated in the CD8+ T cells.
Figure 1.
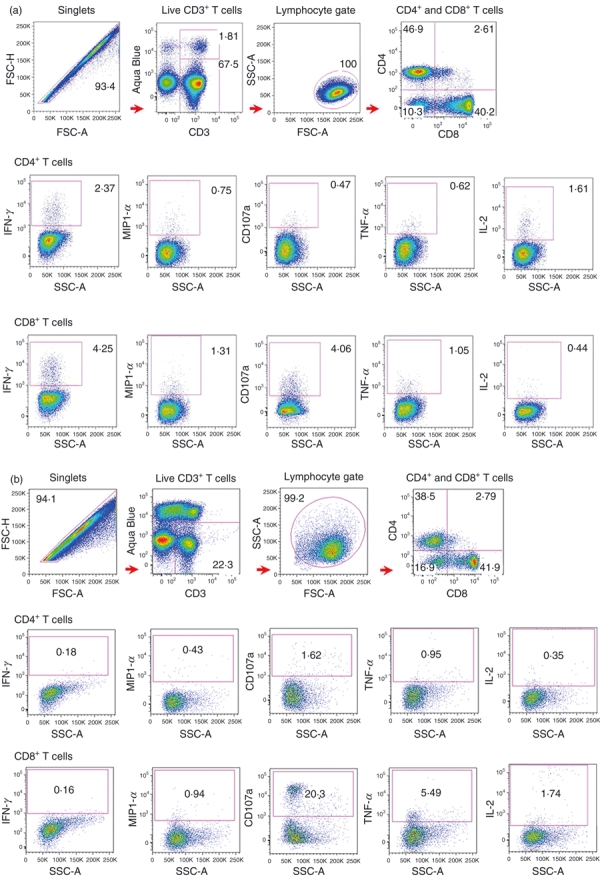
Nine-colour flow cytometry for evaluation of Epstein–Barr virus (EBV)-specific T-cell responses. (a) Analysis of five functions of CD4+ and CD8+ T cells reactive to overlapping peptide pool of EBNA3B in a representative healthy long-term carrier. Gating scheme used to identify EBV-specific T cells was shown. Doublets were excluded from the single cells based on the Forward Scatter-Area (FSC-A) versus Forward Scatter-Height (FSC-H) profile. Live CD3-positive cells were selected by gating the CD3-positive and Aqua Blue-negative population. As CD3+ population form a ‘tail’ into the Aqua Blue channel, a back-gating procedure was performed, confirming that these cells were living CD4+ or CD8+ T cells. Lymphocytes were gated based on the FSC versus Side Scatter (SSC) profile. CD4+ and CD8+ T cells were analysed separately. (b) Analysis of five functions of EBNA3B-specific CD4+ and CD8+ T in a patient with post-transplant lymphoproliferative disorder (PTLD). The same gating strategies as those of long-term carriers were applied. The numbers in the flow diagrams represent the frequencies of cytokine-producing or degranulating T cells. IFN-γ, interferon-γ; MIP1-α, macrophage inflammatory protein 1-α; TNF-α, tumour necrosis factor-α; IL-2, interleukin-2.
We used the Boolean Gating function of flowjo software to analyse the functional profiles of T cells with single and multiple functions (details not shown). We measured the co-expression of IFN-γ, MIP1-α, TNF-α, IL-2 and CD107a, and categorized the responsive T cells into 31 different subsets constituting quintuple, quadruple, triple, double and single functional cells. Both EBV-specific CD4+ and CD8+ T cells could have more than one function in response to EBV proteins. In all cases, the magnitude of total responses was measured by the proportion of T cells possessing at least one function. For healthy long-term carriers, CD4+ T cells showed relatively stronger responses to EBNA1 than other proteins, whereas CD8+ T cells showed relatively weaker responses to EBNA1 but stronger responses to EBNA3 family proteins (Fig. 2), but these differences were not statistically significant (one-way anova test, P-value > 0·05). For each individual, CD4+ and CD8+ T-cell response magnitudes to six overlapping peptide pools were investigated respectively (data not shown). There was a clear trend that for most individuals EBNA1 protein induced relatively stronger CD4+ T-cell responses, whereas three EBNA3 proteins activated more CD8+ T cells. The proportion of CD4+ and CD8+ T-cell responses to the same overlapping peptide pools, on the other hand, differed significantly (Wilcoxon signed-rank test, P < 0·05). The magnitude of CD8+ T-cell responses was approximately threefold that of CD4+ T cells in terms of proportion of responsive cells. EBNA3A-C activated, on average, 3–4% of total CD8+ T cells in 20 individuals whereas EBNA1, LMP2 and BZLF1 induced weaker responses (2% of total CD8+ T cells), and all six peptide pools elicited approximately 1% of total CD4+ T cells.
Figure 2.
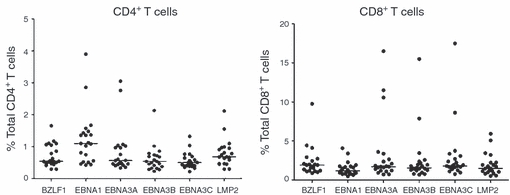
Comparison of response magnitudes of CD4+ and CD8+ T cells to Epstein–Barr virus (EBV) proteins. Responses to EBV lytic (BZLF1) and latent (EBNA1, EBNA3A-C, LMP2) overlapping peptide pools were analysed for both CD4+ and CD8+ T cells, respectively. Each dot represents the percentages of responsive CD4+ or CD8+ T cells for an individual. The median percentages of responsive T cells in 20 long-term carriers for each peptide pool were shown as a horizontal line. There was no significant difference among the response magnitudes towards six EBV overlapping peptide pools for either CD4+ or CD8+ T cells (one-way anova test, P-value > 0.05). There was significant difference between CD4+ and CD8+ T-cell response magnitudes towards the same overlapping peptide pools (two-sided Wilcoxon's signed-rank test, P-value < 0.05).
T cells responsive to immunodominant EBV proteins had higher proportions of PFCs than those responsive to subdominant proteins
Different peptide pools had distinct abilities to induce PFCs for both CD4+ and CD8+ T cells. Long-term carriers generated substantially higher proportion of PFCs than patients with PTLD (Fig. 3). In long-term carriers, a relatively higher proportion of CD4+ PFCs was elicited by EBNA1 and BZLF1 than EBNA3A–C and LMP2, although the differences among the EBV proteins were not as prominent as those in CD8+ T cells. BZLF1 and EBNA3A–C activated larger proportions of CD8+ PFCs than did EBNA1 and LMP2 (Fig. 3a). When stimulated by the same peptide pool, the proportion of CD8+ T cells with two or more functions (PFCs) was higher than that of CD4+ T cells. EBNA3A–C could activate CD8+ PFCs contributing up to approximately half of the total responsive CD8+ T cells whereas CD4+ PFCs accounted for 25–30% of the total responsive CD4+ T cells. These results suggested that immunodominant EBV proteins could stimulate higher proportions of PFCs in both CD4+ and CD8+ T-cell responses, and the PFCs were quantitatively different between CD4+ and CD8+ T cells with respect to the same overlapping peptide pool. In patients with PTLD, however, over 90% of peptide-responsive CD4+ and CD8+ T cells possessed only one function. Whereas long-term carriers could generate substantial proportions of PFCs with three, four and five functions, patients with PTLD had greatly impaired ability to generate EBV-specific PFCs in response to the EBV peptides with no five-functional PFCs, markedly diminished three-functional and four-functional PFCs and small proportion of two-functional PFCs (Fig. 3b). In addition, no immunodominance hierarchies were observed in the T-cell responses of patients with PTLD.
Figure 3.
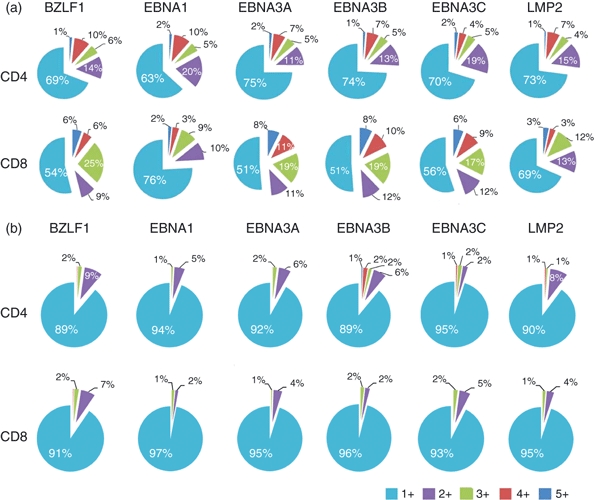
Proportions of polyfunctional CD4+ and CD8+ T cells to Epstein–Barr virus (EBV) proteins. Proportions of T cells with single, double, triple, quadruple and quintuple functions in response to EBV lytic (BZLF1) and latent (EBNA1, EBNA3A, 3B, 3C, LMP2) overlapping peptide pools were shown for 20 healthy long-term carriers (a) and for two patients with post-transplant lymphoproliferative disorder (PTLD) (b). The numbers in the pie charts represent the mean percentages (rounded up to integer) of T-cell subpopulations with respect to total responding CD4+ or CD8+ T cells. 5+ denotes T cells that have five functions; 4+ denotes T cells that have four functions and so on. In long-term carriers, EBNA3 proteins clearly stimulated larger proportions of polyfunctional CD8+ T cells among total responsive CD8+ T cells than other latent EBV proteins. BZLF1 protein stimulated similar proportions of polyfunctional CD8+ T cells to EBNA3 proteins. EBNA1 protein appeared to stimulate a higher proportion of polyfunctional CD4+ T cells among all the latent EBV proteins. In patients with PTLD, most T cells possessed only one function with none exhibiting five functions and rare cells showing three and four functions. No immunodominance hierarchies of EBV proteins were observed.
EBV-specific PFCs produced more cytokines per cell
We calculated the MFI for each of the five functional parameters for quintuple, quadruple, triple, double and single functional T-cell populations stimulated by each overlapping peptide pool in every subject. As a result of the great variation of MFI among individuals, data were logarithmically transformed before comparison. In long-term carriers, EBV-specific CD4+ and CD8+ PFCs (two or more functions) could produce larger amounts of IFN-γ (Fig. 4a) as well as MIP1-α, TNF-α and IL-2 (data not shown) than the corresponding single functional T cells (paired Student's t-test, P-value < 0·05). However, CD107a expression level did not differ significantly between PFCs and single functional T cells. We also compared the MFI of cytokines for all functional permutations, i.e. two functional, three functional, four functional and five functional CD4+ and CD8+ T cells (data not shown). T cells with more functions generally produced larger amounts of cytokines than the T cells with fewer functions.
Figure 4.
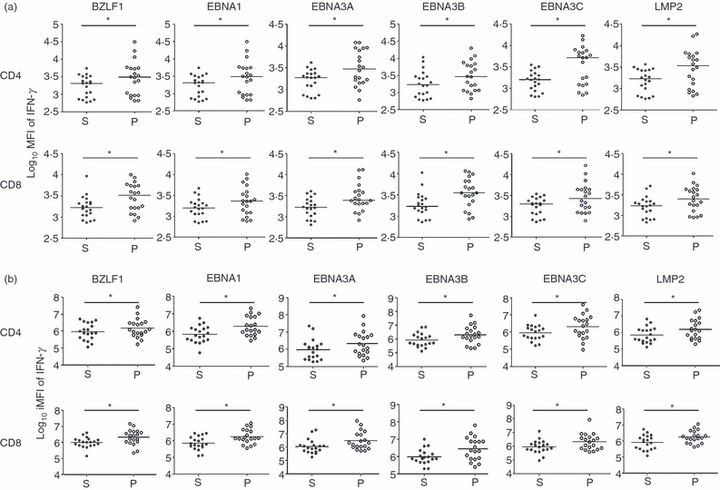
Comparison of cytokine production by single and polyfunctional T cells (PFCs). (a) Median fluorescent intensity (MFI) of interferon-γ (IFN-γ) of virus-specific CD4+ and CD8+ T cells of each long-term carrier for each peptide pool was calculated. The log10 MFI of IFN-γ of T cells with single function (denoted as S) and with two or more functions (denoted as P) were compared. Each dot represents one subject. For all peptide pools, PFCs consistently produced more IFN-γ per cell than the single functional T cells (paired Student's t-test, *P < 0.05). (b) Integrated MFI (iMFI) of IFN-γ of virus-specific CD4+ and CD8+ T cells of each long-term carrier for each peptide pool was obtained. The log10 iMFI of IFN-γ of T cells with single function (denoted as S) and with two or more functions (denoted as P) were compared. Each dot represented one subject. For all peptide pools, PFCs consistently had a larger value of iMFI of IFN-γ than that of single functional T cells (paired Student's t-test, *P < 0.05).
The MFI reflects the amount of cytokine produced per cell and so assesses the quality of the individual cellular response. To more comprehensively compare the functional difference between single functional T cells and PFCs, we incorporated both magnitude and quality of T-cell responses by introducing a new parameter termed the integrated MFI (iMFI) defined as the product of MFI of cytokines and corresponding cell frequencies.20 We compared the iMFI between single functional and polyfunctional T cells for each peptide pool (Fig. 4b). Because of the great variation among individuals, we applied logarithmic transformation to iMFI before comparison. The PFCs had significantly higher values of iMFI of IFN-γ than the single functional T cells (paired Student's t test, P < 0·05). These findings applied to both CD4+ and CD8+ T cells.
EBV-specific CD4+ and CD8+ PFCs have distinct functional subsets
The complexity of measuring five functional parameters simultaneously was challenging, and the results must be interpreted with caution because of the limited number of events. In long-term carriers, we analysed 31 (25−1) unique subsets for both CD4+ and CD8+ T cells, according to every combination of the five functional parameters (IFN-γ, MIP1-α, TNF-α, IL-2 and CD107a), by using the software spice 5 (Mario Roederer).19 Each T-cell subset was illustrated in terms of frequency ± SEM (Fig. 5).
Figure 5.
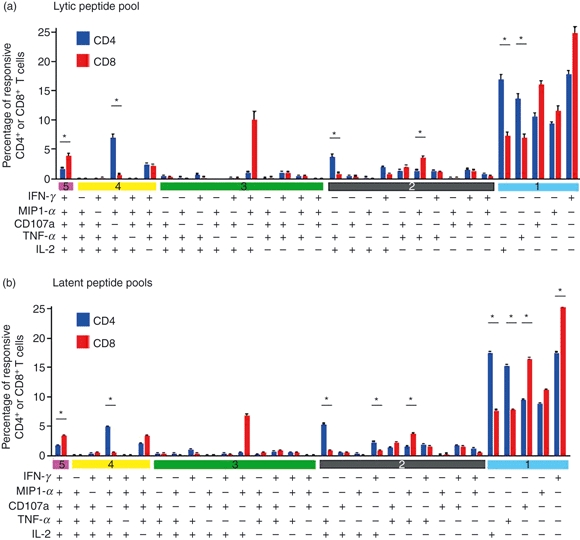
Subset analysis of Epstein–Barr virus (EBV) -specific T cells. (a) Subset analysis of CD4+ and CD8+ T-cell responses to overlapping peptide pool of BZLF1 protein. (b) Subset analysis of CD4+ and CD8+ T-cell responses to overlapping peptide pools of latent EBV proteins. The graph displayed the mean frequencies ± SEM of different T-cell subsets with respect to total responsive T cells in all 20 long-term carriers. Comparison of frequencies between responsive CD4+ and CD8+ T cells was performed by Wilcoxon's signed-rank test at a significant level of 0.05. Asterisk indicates statistically significant difference between frequencies of responsive CD4+ and CD8+ T cells.
It was clear that most of the responding CD4+ or CD8+ T cells present only one of the tested functions but cells with more than one function were also pronounced. A small proportion of CD4+ T cells possessed five functions upon stimulation by lytic-cycle and latent-cycle overlapping peptide pools. Most of the four-functional CD4+ T cells secreted IFN-γ, MIP1-α, TNF-α and IL-2 without CD107a surface mobilization. Four-functional CD4+ T cells without IL-2 production were also detected. The frequencies of other combinations of four functions appeared minor. Three-functional and two-functional EBV-specific CD4+ T-cell responses were heterogeneous without a specific pattern. Among the single functional T cells, IFN-γ-secreting and IL-2-secreting CD4+ T cells were the most dominant subsets, but a substantial proportion of CD4+ T cells expressed CD107a.
Likewise, five-functional CD8+ T cells were readily detected. For four-functional subsets, unlike CD4+ T cells, CD8+ T cells produced IFN-γ, MIP1-α, TNF-α and expressed CD107a at the cell surface without production of IL-2. Similarly, three-functional and two-functional EBV-specific CD8+ T-cell responses were heterogeneous without a specific pattern. Strikingly, a subset of three-functional CD8+ T cells expressing triple IFN-γ, MIP1-α and IL-2 with neither TNF-α nor CD107a was observed but the frequency of this population varied substantially among the 20 long-term carriers. As for the single functional subsets, IFN-γ-secreting, TNF-α-secreting and CD107a-expressing CD8+ T cells were the dominant single functional populations.
Comparing the characteristics of CD4+ and CD8+ PFCs, we observed a higher proportion of five-functional CD8+ T cells than CD4+ T cells and most four-functional CD8+ PFCs were CD107a+ IL2− in contrast to CD4+ T cells being CD107a− IL2+. However, these observations should not be over-interpreted because the variation among the 20 long-term carriers was substantial. Among the latent peptide pools, we also examined the T-cell responses towards EBNA1, EBNA3 family and LMP2 proteins separately (data not shown). Although the distribution of functional T-cell subsets appeared similar, EBNA3 proteins appeared to induce higher proportion of CD4+ and CD8+ PFCs than did LMP2 and EBNA1, which was consistent with the notion that higher proportions of PFCs can be stimulated by immunodominant EBV proteins.
Discussion
The EBV-specific T-cell responses had been mainly measured by IFN-γ production using ELISPOT or tetramer staining assays.21,22 However, measurement of T-cell responses by IFN-γ secretion alone might correlate poorly with immune protection. In a study of HIV vaccine-induced responses, the frequency of T cells producing IFN-γ was strikingly low and a substantial proportion of the HIV-specific T-cell responses would have been missed by measuring IFN-γ alone.23 In long-term EBV carriers, we found that IFN-γ producing EBV-specific T cells accounted for only about 40% and 50% of the total responsive CD4+ and CD8+ T cells, respectively, indicating that more than half of the EBV-specific T cells were actually IFN-γ negative. We believed that PFCs play an active role in long-term effective immunity against EBV. We simultaneously measured different aspects of T-cell functions including degranulation (surface mobilization of CD107a) and production of three cytokines (IFN-γ, TNF-α and IL-2) and one chemokine (MIP1-α). Indeed, the simultaneous measurement of these five functions had been employed to evaluate T-cell responses in other studies.18,24,25 Betts et al.18 pointed out that although the frequency of HIV-specific CD8+ T-cell responses in HIV-infected non-progressors was not dramatically different from that in progressor subjects, functional profiling of HIV-specific CD8+ T-cell responses demonstrated that non-progressors had more PFCs that exhibited higher degrees of functionality (possessing four or five functions) than progressors. These studies supported the notion that anti-microbial T-cell responses should be assessed both quantitatively (frequency of responding T cells) and qualitatively (number of functions of T cells).
Using our nine-colour flow cytometric assay, we demonstrated that every long-term carrier could readily generate EBV-specific CD4+ and CD8+ PFCs with high degrees of functionality (three to five functions). To further investigate whether the PFCs have a possible role in the control of EBV infection, we performed the same evaluation on blood samples from two children who underwent liver transplantation and subsequently developed EBV-driven PTLD. These patients had impaired T-cell immunity because of continual immunosuppressive drug therapy such as prednisolone and tacrolimus. Notably, EBV-specific T cells of the patients with PTLD produced almost no IFN-γ and less IL-2 but retained the ability to degranulate and produce TNF-α vigorously. There seemed to be a shift of the functional profile from one that produced anti-viral cytokines to one that was mainly pro-inflammatory. In addition, the PFCs seen in PTLD had a markedly reduced degree of functionality with predominantly single-functional and a diminished proportion of two-functional T cells and did not exhibit the immunodominant characteristics of EBV peptides (Fig. 3). The T-cell response data in patients with PTLD, who are seropositive for EBV but do not control their EBV infection, supported that EBV-specific PFCs seen in long-term carriers might indeed have a functional role in the control of EBV infection. However, the distinct T-cell responses between long-term carriers and patients with PTLD (elevated CD107a and TNF-α expression in patients with PTLD) might also be influenced by the duration of EBV infection (chronic in long-term carriers versus sub-acute in patients with PTLD) and age (patients with PTLD being younger). Hence, further work in investigating the protective immune function of EBV-specific PFCs is needed. Other supporting evidence of robust immune protective role of PFCs comes from Smith et al.26 who showed that galectin-1, a known immunosuppressive molecule expressed on Hodgkin lymphoma cells, could inhibit the proliferation and cytokine production of LMP1-, LMP2- and EBNA1-specific CD8+ T-cell responses in healthy EBV carriers. However, EBV-specific PFCs generated by EBV-polyepitope stimulation could overcome the inhibitory effects of galectin-1, implying that the PFCs might confer stronger immune protection.
Epstein–Barr virus is a large DNA virus exhibiting distinct hierarchies of immunodominance among its lytic and latent cycle proteins.1 CD4+ T-cell responses were largely focused on epitopes derived from EBNA1 followed by EBNA2 and EBNA3C.11,27,28 The responses to EBNA3A, EBNA3B, LMP2 and BZLF1 remained lower in the immunodominance hierarchy.1 CD8+ T-cell responses were markedly focused on epitopes drawn from EBNA3A–C proteins followed by BZLF1, LMP2 and EBNA1 proteins, respectively.1,29 Using six overlapping peptide pools of one lytic and five latent EBV proteins, we investigated whether EBV-specific PFCs could retain similar immunodominance patterns to those of EBV-specific T cells demonstrated in single cytokine (IFN-γ) studies. In long-term carriers, immunodominance of EBNA3 proteins over other latent proteins in total responsive CD8+ T-cell responses was observed whereas the magnitude of EBNA1-, EBNA3- and LMP2-specific responses among total CD4+ T-cell responses appeared similar with slight dominance of EBNA1-specific responses (Fig. 2). Interestingly, when we examined the relationship between EBV-specific PFCs and immunodominance hierarchies further, we found that viral proteins situated higher in the immunodominance hierarchy could stimulate higher proportions of responding PFCs in both CD4+ and CD8+ T-cell responses (Fig. 3a). The difference in proportion of PFCs across the tested peptide pools was less pronounced in CD4+ than CD8+ T cells, which corresponded to the difference in immunodominance patterns of EBV-specific CD4+ and CD8+ T cells.1
The EBV-specific PFCs produced more cytokine per cell than the single functional T cells (Fig. 4). This characteristic was true for IFN-γ, MIP1-α, TNF-α and IL-2 in both responding CD4+ and CD8+ T cells among all six peptide pools. We also observed a trend of increased surface CD107a mobilization in EBV-specific PFCs. Similarly, the ability to produce more cytokines by CD4+ and CD8+ PFCs was also observed in cytomegalovirus (CMV) -specific, vaccinia virus-specific, HIV-2-specific, modified vaccinia virus Ankara (MVA)-nef-induced HIV-1 specific, and bacillus Calmette–Guérin-specific T cells.20,23,30,31 Our results inferred that EBV-specific PFCs were functionally superior to their single functional counterparts because they have greater production of cytokines and degranulation capacity. We also examined the iMFI of cytokines, which is defined as the product of MFI and corresponding cell frequency. The iMFI can be viewed as a parameter that measures total functional responses. Our results showed that in response to all peptide pools, PFCs had a higher value of iMFI of cytokines than single functional T cells, which was true for both CD4+ and CD8+ T cells, suggesting that PFCs had a relatively stronger total functional response. Nevertheless, more definitive proof of the enhanced functional capability of virus-specific PFCs would require enrichment of sufficient numbers of CD4+ and CD8+ PFCs for direct testing in conventional cytotoxic and cell proliferation assays.
Distribution of subsets of EBV-specific CD4+ and CD8+ PFCs showed difference (Fig. 5). A relatively higher proportion of five-functional CD8+ T cells than of CD4+ T cells was observed. The majority of four-functional CD8+ T cells were CD107a+ IL-2− whereas four-functional CD4+ T cells were CD107a− IL-2+. The four-functional CD8+ T-cell subset was also found in HIV-2-infected patients and HIV-1-infected non-progressors,18,24 supporting the theory that this specific CD8+ PFC subset might confer protective immune function in different viral infections. In contrast to the prevalence of IL-2+ CD4+ PFCs in long-term EBV carriers, CD4+ T cells responding to CMV pp65 were predominantly IL-2 negative,32 indicating that distinct functional subsets of virus-specific CD4+ T cells might occur in different viral infections. Interleukin-2 was produced in both CD4+ and CD8+ EBV-specific PFCs (Fig. 5) and probably functioned as an autocrine proliferative factor. It would be interesting to incorporate a CFSE assay to test whether there is enhanced proliferative capacity of EBV-specific PFCs. Guerreiro et al.33 tested for EBV-specific CD4+ and CD8+ T-cell responses towards EBNA1 and BZLF1 proteins and found a predominance of TNF-α+ and IFN-γ+ ΤΝF-α+ T cells, particularly after expansion and re-stimulation of such reactive T cells. Analysis of our data showed that TNF-α+ IFN-γ+ CD4+ T cells accounted for approximately 14·5% and 15·1% of the total reactive CD4+ T cells to BZLF1 and EBNA1 proteins, respectively and TNF-α+ IFN-γ+ CD8+ T cells accounted for approximately 10·7% and 11·9% of the total reactive CD8+ T cells to BZLF1 and EBNA1 proteins, respectively (data derived from Fig. 5). Further examination showed that the majority of such TNF-α+ IFN-γ+ T cells were indeed five-functional and four-functional CD4+ and CD8+ T cells (Fig. 5). We interpreted these data as indicating that these highly functional T cells increased in proportion and became more evident upon in vitro expansion by recombinant IL-2 and re-stimulation with peptides as in Guerreiro et al.'s study, supporting our observations that highly functional EBV-specific T cells can be readily generated in long-term EBV carriers.
The EBV-specific CD4+ T cells expressing surface CD107a were consistently detected in long-term EBV carriers (refer to Figs 1 and 5), suggesting the presence of potential cytotoxic function of CD4+ T cells. CD107a expression itself is not equivalent to cytotoxicity because it is a marker for degranulation, although the ability to degranulate provides an indication of cytotoxic potential. In this study, we have not directly measured cytotoxicity in terms of perforin production or cellular cytolytic assay. Betts et al.34 had studied the relationship between degranulation (CD107a expression) and cytotoxicity and found that CMV-specific CD8+ T cells could degranulate (expressing CD107a) and mediate cytotoxic activity simultaneously. In some other studies, CMV-specific CD4+ T cells with surface CD107a expression were shown to have direct cytolytic activity against antigen-loaded autologous B-lymphoblastoid cell lines and CD4+ T-cell clones to some EBNA1 epitopes could recognize and kill appropriately HLA-matched B-lymphoblastoid cell lines and EBNA1-positive Burkitt's lymphoma lines.32,35,36 But the importance of such a direct effector function of CD4+ T cells in vivo compared with that of CD8+ cytotoxic T cells still awaits clarification.1 Taken together, it seemed reasonable to use CD107a expression as a marker of potential cytolytic function of virus-specific PFCs.
In conclusion, our experimental results revealed that healthy long-term EBV carriers mounted significant polyfunctional T-cell responses to EBV lytic and latent cycle proteins. Immunodominant EBV proteins induced greater proportions of PFCs than subdominant ones. The EBV-specific PFCs were functionally superior to single-functional cells, as evidenced by enhanced production of multiple cytokines and rapid degranulation. On the contrary, patients with PTLD, who have impaired T-cell immunity, could only generate a low proportion of PFCs with markedly reduced degrees of functionality compared with that of long-term carriers. Evaluation of EBV-specific PFCs can be extended to studies of T-cell immunity in other EBV-associated diseases.
Acknowledgments
This work was supported by RGC-GRF grant (#HKU763407M) and CRCG grant #10400665 of A.K.S.C. Part of this work was presented at the 14th Biennial Conference of the International Association for Research on Epstein–Barr Virus & Associated Diseases.
Disclosures
All authors of this paper have no conflicts of interest.
References
- 1.Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein–Barr virus. Annu Rev Immunol. 2007;25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- 2.Vetsika EK, Callan M. Infectious mononucleosis and Epstein–Barr virus. Expert Rev Mol Med. 2004;6:1–16. doi: 10.1017/S1462399404008440. [DOI] [PubMed] [Google Scholar]
- 3.Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. EBV persistence in memory B cells in vivo. Immunity. 1998;9:395–404. doi: 10.1016/s1074-7613(00)80622-6. [DOI] [PubMed] [Google Scholar]
- 4.Cohen JI. Clinical aspects of Epstein–Barr virus infection. In: Robertson ES, editor. Epstein–Barr Virus. Norfolk, UK: Caister Academic Press; 2005. pp. 35–55. [Google Scholar]
- 5.Salvetti M, Giovannoni G, Aloisi F. Epstein–Barr virus and multiple sclerosis. Curr Opin Neurol. 2009;22:201–6. doi: 10.1097/WCO.0b013e32832b4c8d. [DOI] [PubMed] [Google Scholar]
- 6.James JA, Harley JB, Scofield RH. Epstein–Barr virus and systemic lupus erythematosus. Curr Opi Rheumatol. 2006;18:462–7. doi: 10.1097/01.bor.0000240355.37927.94. [DOI] [PubMed] [Google Scholar]
- 7.Toussirot É, Roudier J. Epstein–Barr virus in autoimmune diseases. Best Pract Res Clin Rheumatol. 2008;22:883–96. doi: 10.1016/j.berh.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Lotz M, Tsoukas CD, Fong S, Carson DA, Vaughan JH. Regulation of Epstein–Barr virus infection by recombinant interferons: selected sensitivity to interferon-γ. Eur J Immunol. 1985;15:520–5. doi: 10.1002/eji.1830150518. [DOI] [PubMed] [Google Scholar]
- 9.Callan MF, Steven N, Krausa P, et al. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat Med. 1996;2:906–11. doi: 10.1038/nm0896-906. [DOI] [PubMed] [Google Scholar]
- 10.Woodberry T, Suscovich TJ, Henry LM, et al. Differential targeting and shifts in the immunodominance of Epstein–Barr virus-specific CD8 and CD4 T cell responses during acute and persistent infection. J Infect Dis. 2005;192:1513–24. doi: 10.1086/491741. [DOI] [PubMed] [Google Scholar]
- 11.Long HM, Haigh TA, Gudgeon NH, et al. CD4+ T-cell responses to Epstein–Barr virus (EBV) latent-cycle antigens and the recognition of EBV-transformed lymphoblastoid cell lines. J Virol. 2005;79:4896–907. doi: 10.1128/JVI.79.8.4896-4907.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikiforow S, Bottomly K, Miller G. CD4+ T-cell effectors inhibit Epstein–Barr virus-induced B-cell proliferation. J Virol. 2001;75:3740–52. doi: 10.1128/JVI.75.8.3740-3752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilmour KC, Gaspar HB. Pathogenesis and diagnosis of X-linked lymphoproliferative disease. Expert Rev Mol Diagn. 2003;3:549–61. doi: 10.1586/14737159.3.5.549. [DOI] [PubMed] [Google Scholar]
- 14.Piriou E, van Dort K, Nanlohy NM, van Oers MHJ, Miedema F, van Baarle D. Loss of EBNA1-specific memory CD4+ and CD8+ T cells in HIV-infected patients progressing to AIDS-related non-Hodgkin lymphoma. Blood. 2005;106:3166–74. doi: 10.1182/blood-2005-01-0432. [DOI] [PubMed] [Google Scholar]
- 15.Lim WH, Russ GR, Coates PTH. Review of Epstein–Barr virus and post-transplant lymphoproliferative disorder post-solid organ transplantation. Nephrology. 2006;11:355–66. doi: 10.1111/j.1440-1797.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- 16.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–58. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 17.Makedonas G, Betts M. Polyfunctional analysis of human T cell responses: importance in vaccine immunogenicity and natural infection. Springer Semin Immunopathol. 2006;28:209–19. doi: 10.1007/s00281-006-0025-4. [DOI] [PubMed] [Google Scholar]
- 18.Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79A:167–74. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 21.Scherrenburg J, Piriou ER, Nanlohy NM, van Baarle D. Detailed analysis of Epstein–Barr virus-specific CD4+ and CD8+ T cell responses during infectious mononucleosis. Clin Exp Immunol. 2008;153:231–9. doi: 10.1111/j.1365-2249.2008.03699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuzushima K, Hayashi N, Kudoh A, Akatsuka Y, Tsujimura K, Morishima Y, Tsurumi T. Tetramer-assisted identification and characterization of epitopes recognized by HLA A*2402-restricted Epstein–Barr virus-specific CD8+ T cells. Blood. 2003;101:1460–8. doi: 10.1182/blood-2002-04-1240. [DOI] [PubMed] [Google Scholar]
- 23.Betts MR, Exley B, Price DA, et al. Characterization of functional and phenotypic changes in anti-Gag vaccine-induced T cell responses and their role in protection after HIV-1 infection. Proc Natl Acad Sci U S A. 2005;102:4512–7. doi: 10.1073/pnas.0408773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duvall MG, Precopio ML, Ambrozak DA, et al. Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur J Immunol. 2008;38:350–63. doi: 10.1002/eji.200737768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilkinson KA, Wilkinson RJ. Polyfunctional T cells in human tuberculosis. Eur J Immunol. 2010;40:2139–42. doi: 10.1002/eji.201040731. [DOI] [PubMed] [Google Scholar]
- 26.Smith C, Beagley L, Khanna R. Acquisition of polyfunctionality by Epstein–Barr virus specific CD8+ T cells correlates with increased resistance to galectin-1-mediated suppression. J Virol. 2009;83:6192–8. doi: 10.1128/JVI.00239-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munz C, Bickham KL, Subklewe M, et al. Human CD4+ T lymphocytes consistently respond to the latent Epstein–Barr virus nuclear antigen EBNA1. J Exp Med. 2000;191:1649–60. doi: 10.1084/jem.191.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leen A, Meij P, Redchenko I, Middeldorp J, Bloemena E, Rickinson A, Blake N. Differential immunogenicity of Epstein–Barr virus latent-cycle proteins for human CD4+ T-helper 1 responses. J Virol. 2001;75:8649–59. doi: 10.1128/JVI.75.18.8649-8659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray RJ, Kurilla MG, Brooks JM, Thomas WA, Rowe M, et al. Identification of target antigens for the human cytotoxic T cell response to Epstein–Barr virus (EBV): implications for the immune control of EBV-positive malignancies. J Exp Med. 1992;176:157–68. doi: 10.1084/jem.176.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol. 2007;81:8468–76. doi: 10.1128/JVI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kutscher S, Allgayer S, Dembek CJ, Bogner JR, Protzer U, Goebel FD, Erfle V, Cosma A. MVA-nef induces HIV-1-specific polyfunctional and proliferative T-cell responses revealed by the combination of short- and long-term immune assays. Gene Ther. 2010;17:1372–83. doi: 10.1038/gt.2010.90. [DOI] [PubMed] [Google Scholar]
- 32.Casazza JP, Betts MR, Price DA, et al. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med. 2006;23:2865–77. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guerreiro M, Na I-K, Letsch A, et al. Human peripheral blood and bone marrow Epstein–Barr virus-specific T-cell repertoire in latent infection reveals distinct memory T-cell subsets. Eur J Immunol. 2010;40:1566–76. doi: 10.1002/eji.200940000. [DOI] [PubMed] [Google Scholar]
- 34.Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 35.Voo KS, Fu T, Heslop HE, Brenner MK, Rooney CM, Wang RF. Identification of HLA-DP3-restricted peptides from EBNA1 recognized by CD4+ T cells. Cancer Res. 2002;62:7195–9. [PubMed] [Google Scholar]
- 36.Paludan C, Bickham K, Nikiforow S, et al. Epstein–Barr nuclear antigen 1-specific CD4+ Th1 cells kill Burkitt's lymphoma cells. J Immunol. 2002;169:1593–603. doi: 10.4049/jimmunol.169.3.1593. [DOI] [PubMed] [Google Scholar]


