Abstract
Natural killer T (NKT) cells are a newly identified T-cell population with potential immunomodulatory functions. Several studies have shown modulating effects of NKT cells activated by α-galactosylceramide, a model antigen, on NK cell function. We here report a differential modulating effect of NKT cells on the interferon-γ (IFN-γ) production and cytolytic function of NK cells in a chlamydial infection model, using NKT-cell-deficient mice and antibody blocking (anti-CD1d monoclonal antibody) approaches. Our results showed that both NKT and NK cells became activated and produced IFN-γ following Chlamydia muridarum infection in vitro and in vivo. The NK cells in NKT-cell-deficient mice and CD1d-blocked mice showed decreased CD69 expression, cellular expansion and IFN-γ production but surprisingly showed increased cytolytic activity (degranulation) of immature and more mature NK cell subsets, suggesting an inhibitory role of NKT cells on NK cell killing activity. The results suggest that NKT cells preferentially promote IFN-γ production but are inhibitory for the cytotoxic function of NK cells in this infection model. Furthermore, the differential modulating effect of NKT cells on the IFN-γ production and cytotoxicity of NK cells was observed in immature and mature NK cell subsets, although it was more dramatic in the relatively mature CD11bhigh CD27high NK cell subset. This finding demonstrates the complexity of innate cell interactions in infection and the possible differential impact of NKT cells on the variable functional aspects of other cell(s) even in one infection setting.
Keywords: Chlamydia, cytotoxicity, interferon-γ, natural killer cell, natural killer T cell
Introduction
Natural killer T (NKT) cells are a unique subset of T lymphocytes that also express typical NK cell markers, such as NK1.1 (or NKR-P1C) and the molecules belonging to the Ly49 killer-inhibitor receptor family.1,2 The majority of NKT cells in mice express an invariant Vα14-Jα18 T-cell receptor (TCR) α-chain (Vα24-Jα18 in human) and a biased set of Vβ8, Vβ7 and Vβ2 TCR β-chains (Vβ11 in humans).3,4 After their TCRs are stimulated by CD1d-presented glycolipid antigen, NKT cells can be rapidly activated to secrete different types of cytokines such as interferon-γ (IFN-γ). Because of their capability to rapidly respond to stimulation, NKT cells are considered to play a promoting or regulatory role in a broad range of disease conditions including infections, allergy, atherosclerosis, autoimmune diseases, tumours and allograft rejection.5–7 The NKT cells have been implicated in the modulation of the function of dendritic cells, macrophages, B cells, conventional T cells as well as NK cells.8–11 At the same time, NKT cells can also play a role as effector cells, as shown by their cytolytic function against tumour cells.12–14
Natural killer cells, as large granular lymphocytes, play an important role in the early stage of immune responses by their two major functions: cytotoxicity and cytokine production, especially of IFN-γ. Activated NK cells can kill infectious cells and tumour cells without prior sensitization. In addition, NK cells can also produce cytokines that regulate subsequent adaptive immune responses against infectious pathogens. The NK cells are phenotypically and functionally heterogeneous. Although inconsistent findings have been reported, humans NK cells can be grouped into two subsets according to the levels of surface CD56 expression, which are different in phenotype, tissue distribution and function.15 Murine NK cells are grouped into four subpopulations based on CD11b and CD27 expression: CD11blow CD27low, CD11blow CD27high, CD11bhigh CD27high and CD11bhigh CD27low16–18 Some studies suggest that the four subsets may represent different stages of NK cell maturation, successively from the immature CD11blow CD27low subset to the terminally mature CD11bhigh CD27low subset.17 Like human NK cell subsets, the murine NK cell subsets appeared different in phenotype and function, particularly in cytokine production, cytotoxicity and migratory capacity.16 Notably, most of the data on NK cell subsets were generated from studies that activated NK cells in ex vivo cell cultures through adding exogenous cytokines or cross-linking of NK cell activating receptors.16,18,19 The major mechanisms, by which NK cells play their role in host defence against infections and tumours, are thought to be related to their cytokine, especially IFN-γ, production and cytotoxicity. For killing cells by cytotoxicity, NK cells release lytic granules by exocytosis leading to CD107a mobilization onto the cell surface, which is easily detectable by flow cytometry.20,21 Therefore, examining CD107a expression on NK cells (degranulation) following co-culture with target cells (such as Yac-1) has become a widely used method for measuring NK cell cytotoxicity.
A promoting effect of NKT cells on NK cells has been shown in several studies using α-galactosylceramide (α-GalCer), a soluble model lipid antigen initially isolated from marine sponge. Indeed, following the stimulation by α-GalCer, NKT cells rapidly activate NK cells to produce IFN-γ.8 Injection of α-GalCer in vivo induced NK cell proliferation and cytotoxicity through a CD1d/NKT-cell-dependent mechanism.22 Activation of human NKT cells through TCR stimulation also increased NK cell cytotoxicity against tumour cell lines showing enhanced interleukin-2 and IFN-γ production.14 On the other hand, a recent study showed that the NK cell activation and protection in Trypanosoma cruzi infection occurred independently of NKT cell responses.23 Therefore, the influence of NKT cells on NK cells is probably different in different disease settings. Further study on the interaction of NKT cells and NK cells especially from the angle of NK cell functions in real infection settings becomes an important topic in understanding the mechanism in immune regulation.
Chlamydia is an obligate intracellular bacterial pathogen causing a wide range of human diseases including ocular, respiratory and reproductive tract infections. Previous studies have shown NK and NKT cell responses in various types of chlamydial infections.24–27 However, the relationship between these two important innate immune cells in chlamydial infections has not been reported. In the present study, using a mouse Chlamydia muridarum respiratory tract infection model, we addressed the question of the possible modulating effect of NKT cells on NK cell activation, expansion and function in this intracellular bacterial infection setting. We found that NKT cells could enhance the activation, expansion and maturation of NK cells in chlamydial infection. We also found a differential modulating effect for NKT cells on the functions of NK cells regarding their IFN-γ production and cytotoxicity. Specifically, the C. muridarum-activated NKT cells promoted IFN-γ production by NK cells but showed an inhibitory effect on the cytolytic function of NK cells. The data provide new insights into the modulating effect of NKT cells on the function of NK cells and the complexity of the functional interaction between innate immune cells in real infection settings.
Methods
Mice
Female C57BL/6 mice were maintained in a specific-pathogen-free animal facility at the University of Manitoba. Breeding pairs of Jα18 gene knockout (Jα18−/−) mice, which lacked invariant NKT cells in B6 background were kindly provided by Dr Masaru Taniguchi (RIKEN Research Center for Allergy and Immunology) and bred at the animal care facility of the University of Manitoba. All mice used were between 6 and 12 weeks old. All experiments were conducted in accord with the guidelines of the Canadian Council on Animal Care and the protocol was approved by the ethical committee of the University of Manitoba.
Chlamydia
Chlamydia muridarum was propagated, purified and quantified as previously described.10 Briefly, C. muridarum was grown in HeLa 229 cells in Eagle's minimum essential medium containing 10% fetal bovine serum (FBS) and 2 mm l-glutamine. After 48 hr culture, infected cells were harvested. Elementary bodies (EBs) were purified by discontinuous density gradient centrifugation. The purified EBs were measured by immunostaining and stored at −80°. For in vitro experiments, cells were treated with live EBs at a multiplicity of infection of 3. For mouse infection, 2 × 103 inclusion-forming units (IFUs) of C. muridarum in 40 μl final volume of PBS were used to inoculate mice intranasally.10 The same seed stock of EBs was used throughout the study.
Lung mononuclear cell preparation
Mice were killed at specific time-points following C. muridarum infection. The lungs were cleared of blood by perfusion using ice-cold PBS, cut into small pieces and digested for 1 hr at 37° using 2 mg/ml collagenase XI (Sigma-Aldrich, Oakville, Ontario, Canada) and 100 μg/ml DNase I (Sigma-Aldrich). After digestion, the tissue fragments were transferred into 15-ml tubes and then centrifuged. The cell pellets were resuspended in 5 ml of 35% (volume/volume) Percoll (Pharmacia, Uppsala, Sweden) and centrifuged at 700 g for 15 min at room temperature. Red blood cells were lysed with ACK lysis buffer followed by two washes in RPMI-1640 with 10% FBS and resuspended in complete RPMI-1640 medium (RPMI-1640 supplemented with 10% FBS, 2 mm l-glutamine, 25 g/ml gentamicin and 5 × 10−5 m 2-mercaptoethanol) for further analysis.
Antibodies
Fluorescently labelled monoclonal antibodies (mAbs) and corresponding isotype controls were purchased from eBiosciences or Biolegend. Staining for iNKT (invariant NKT) cells was carried out using phycoerythrin (PE) -conjugated PBS-57 glycolipid–CD1d tetramer (kindly provided by the National Institute of Allergy and Infectious Disease MHC Tetramer Core Facility, Atlanta, GA). For analysis of NK subsets by flow cytometry, Anti-CD3ε-PE-Cy7, anti-NK1.1-PE, anti-NK1.1-allophycocyanin (APC), anti-CD69-PE, anti-CD11b-APC, anti-NK1.1-Pacific Blue, anti-CD27-FITC, anti-CD11b-APC-Cy7 were used. For CD107a degranulation assay, anti-CD107a-PE or anti-CD107a-FITC (Biolegend) mAbs were used. For CD1d blocking experiments, functional grade purified anti-mouse CD1d antibody (eBioscience) and isotype control antibody (Rat IgG2bk Functional Grade; eBioscience) were used at a final concentration of 10 μg/ml.
Degranulation assay
The NK cell cytotoxicity (degranulation) was assessed by testing CD107a expression using flow cytometry, as described previously.20,21 Single splenocytes and lung mononuclear suspensions from wild-type (WT) and Jα18−/− mice were resuspended at 5 × 106 in RPMI-1640 containing 10% FBS, 2 mm l-glutamine, 25 g/ml gentamicin, and stimulated with YAC-1 cells in the presence of anti-CD107a antibody at an effector to target ratio of 5 : 1 in 96-well plates. Cells stimulated with PMA (2·5 μg/ml) and ionomycin (0·5 μg/ml) served as a positive control. Cells alone served as the negative control. After 1 hr of culture, monensin (6 μg/ml; eBioscience) was added and cells were incubated for an additional 3 hr at 37°. Thereafter, cells were stained with fluorescence-conjugated anti-NK1.1and anti-CD3e mAb, and analysed by flow cytometry. The frequency of CD107a-positive NK cells (NK1.1+ CD3e−) in the samples of different groups was calculated by subtracting the frequency of CD107a-positive NK cells in negative controls.
Surface marker and intracellular cytokine staining
For in vitro experiments, bulk ex vivo spleen cells from naive mice were prepared and cultured in the presence of live EBs. The cells were collected for surface staining of CD69 and intracellular IFN-γ staining at different time-points of culture and brefeldin A (eBioscience) was added to the culture 3 hr before cell collection. For in vivo experiments, the fresh lung mononuclear cells and splenocytes were stained with anti-CD3ε-PE-Cy7, anti-NK1.1-APC and anti-CD69-PE for CD69 expression. Some cells were stimulated with PMA (50 ng/ml) and ionomycin (1 μg/ml) and incubated in complete RPMI-1640 medium at 37°. After 3 hr of incubation, brefeldin A was added and cells were cultured for another 3 hr to accumulate cytokines intracellularly. Cells were subsequently washed and blocked for 10 min with anti-CD16/CD32 in FACS buffer (Dulbecco's PBS, 2% heat-inactivated FBS, 0·09% sodium azide) and then surface stained with the appropriate antibodies. Cells were fixed (IC fixation buffer; eBioscience), permeabilized (permeabilization buffer; eBioscience) and subsequently stained with APC-anti-IFN-γ for 30 min. Stained cells were washed and analysed using an LSR II flow cytometer (BD Bioscience, San Jose, CA). The data were subsequently analysed with FACS express software (De Novo Software, Los Angeles, CA).
Statistical analysis
Statistical analysis of the data was performed using unpaired Student's t-test (GraphPad Prism software, version 4, San Diego, CA). Values of P < 0·05 were considered significant.
Results
Infection of ex vivo mouse spleen cells with C. muridarum activate both NK and NKT cells which express activation markers and produce IFN-γ
Ex vivo spleen cells from naive C57BL/6 mice were cultured with live C. muridarum EB at a multiplicity of infection of 3. At different time-points after infection, we evaluated the activation of NKT cells (PBS-57 glycolipid–CD1d tetramer+ CD3e+) and NK cells (NK1.1+ CD3e−) by measuring surface CD69 expression using flow cytometry. We also measured IFN-γ production by these cells using intracellular cytokine staining. As shown in Fig. 1, C. muridarum infection rapidly increased CD69 expression on both NK and NKT cells, peaking at 12–24 hr, with about twofold increase of mean fluorescence intensity (Fig. 1c,d, left panel). Production of IFN-γ by NK and NKT cells was also rapidly enhanced although the peak of IFN-γ production by NKT cells appeared earlier than NK cells (12 hr versus 24 hr). The percentages of IFN-γ-producing NKT and NK cells were lower than the percentage of CD69+ cells (17–22%). The NKT cells and NK cells had similar kinetics of CD69 expression and IFN-γ production in response to C. muridarum infection in vitro (Fig. 1c,d). The results generated from the in vitro culture system suggest that chlamydial infection can up-regulate the activation and induce IFN-γ production of both NKT cells and NK cells.
Figure 1.
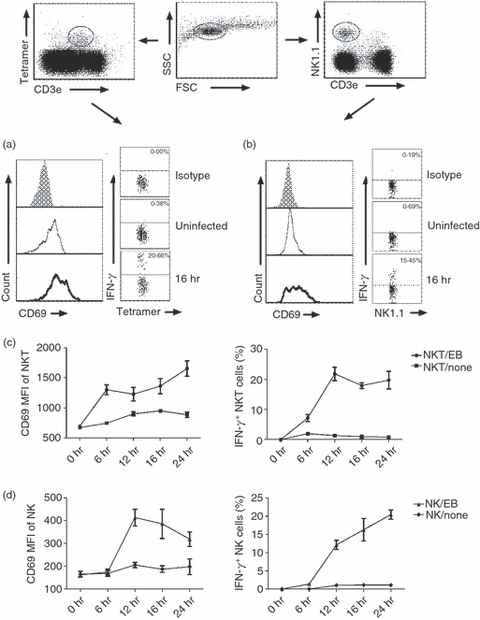
Natural killer (NK) and NKT cells were activated and produced interferon-γ (IFN-γ) following in vitro infection with Chlamydia muridorum. Ex vivo splenocytes isolated from naive C57BL/6 mice (three mice) were infected with live C. muridorum elementary bodies at a multiplicity of infection of 3 and cultured for 6–24 hr in complete culture medium. Control cells were cultured but not infected. Cells were analysed by multi-colour staining and flow cytometry at designated time-points. The different cell populations were gated as shown in dot plot and analysed for CD69 and intracellular IFN-γ. (a) Expression of activation marker CD69 on iNKT (PBS-57 glycolipid loaded CD1d tetramer+ CD3e+) cells and intracellular IFN-γ staining at 16 hr culture. Grey histograms show isotype control antibody staining, thin line shows uninfected control cells and thick line shows infected cells. (b) CD69 expression and IFN-γ production by NK (NK1.1+ CD3e−) cells at 16 hr culture. The mean fluorescence intensity (MFI) of CD69 expression and the percentage of IFN-γ production by iNKT cells (c) and NK cells (d) at different time-points after infection are represented. Data are shown as means of triplicate analysis ± SD and are representative of three independent experiments.
C. muridarum infection-activated NKT cells influence IFN-γ production by NK cells in the in vitro culture system
Previous data showed that NKT cells activated by α-GalCer can promote IFN-γ production by NK cells.8,22 To analyse whether NKT cells can influence NK cells in chlamydial infection, we compared NK cell responses, measured by CD69 expression and IFN-γ production, in the infection system of ex vivo spleen cells isolated from WT and NKT knockout (KO; Jα18−/−) mice. As shown in Fig. 2, NK cells from Jα18−/− mice showed dramatically lower, compared with WT mice, CD69 expression (40% versus 11%; mean fluorescence intensity 680 versus 350) and IFN-γ production (14% versus 5%) following infection. At most time-points, the percentage of IFN-γ-producing NK cells from Jα18−/− mice was less than half of those from WT mice. As the activation of NKT cells was mostly dependent on CD1d–TCR interactions, we also added CD1d-blocking mAb to the culture of WT spleen cells to confirm the effect of NKT activation on NK cells. As shown in Fig. 3, the blockade of CD1d–TCR interaction, and hence the activation of NKT cells, significantly inhibited CD69 expression and IFN-γ production by NK cells, again suggesting the influence of NKT cells activated by C. muridarum infection on NK cell activation. Together, the experiments, using both NKT KO mice and CD1d mAb blocking, confirmed the significant modulating effect of NKT cells on NK cell activation and cytokine production. However, it is also clear that NK activation (CD69 expression and IFN-γ production) was not dependent on NKT cells because significant NK activation was observed in the condition of NKT cell deficiency and CD1d blocking although the levels were significantly lower.
Figure 2.
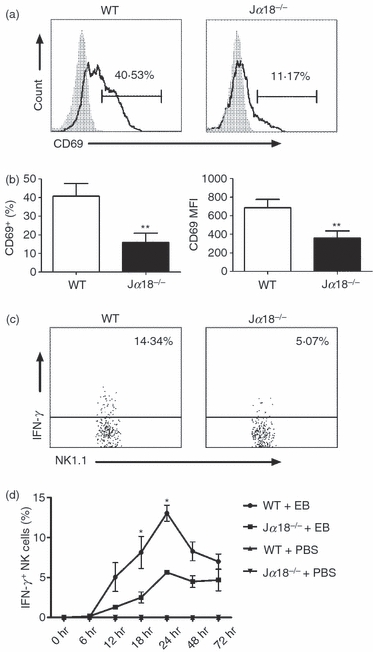
Natural killer (NK) cells from Jα18−/− mice show lower CD69 expression and interferon-γ (IFN-γ) production in response to live Chlamydia muridorum infection than those from wild-type (WT) mice. Ex vivo splenocytes from WT and Jα18−/− mice were infected by live elementary bodies at a multiplicity of infection of 3. (a) CD69 expression on NK (NK1.1+ CD3e−) cells was analysed at 12 hr after infection. Representative histograph is shown. Grey histograms, isotype control antibody staining; solid lines, anti-CD69 antibody staining. (b) Summary of the proportion and the mean fluorescence intensity (MFI) of CD69 on NK cells. Data represent mean ± SD for three to four mice. **P < 0·01. (c) Representative dot plot of intracellular IFN-γ staining at 24 hr culture is depicted. Cells were gated on NK1.1+ CD3e− cells. (d) Summary data on IFN-γ-producing NK cells at different time-points of infection. *P < 0·05. The results are shown as mean ± SD of three mice in each group and are representative of three independent experiments.
Figure 3.
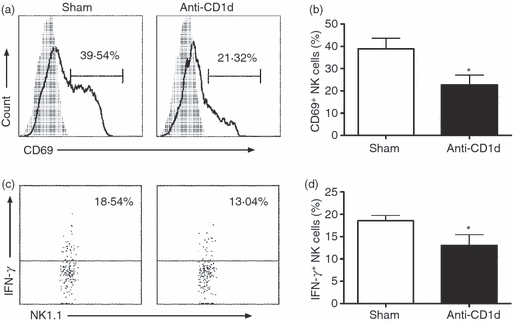
Natural killer (NK) cells show lower CD69 expression and interferon-γ (IFN-γ) production when NKT cells were blocked by anti-CD1d antibody. Splenocytes were infected by live elementary bodies (multiplicity of infection 3) in the presence of anti-CD1d antibody (10 μl/ml) or isotype control monoclonal antibodies (mAbs; sham). (a) CD69 expression on NK cells at 12 hr infection. Grey histograms, isotype control antibody staining; solid lines, anti-CD69 mAb staining. (b) Summary of CD69 expression on NK cells in difference culture conditions. (c) Intracellular IFN-γ staining of NK cells at 24 hr after infection. Cells were gated at NK1.1+ CD3e− cells. (d) Summary of intracellular IFN-γ staining of NK cells. Data are mean ± SEM of cumulative data from three separate experiments and three mice in each group.
NK and NKT cells become activated and expanded during in vivo C. muridarum infection
After showing activation of both NKT and NK cells by C. muridarum infection and the influence of NKT cells on NK cells in the in vitro system, we further tested NKT and NK cell responses in vivo. As shown in Fig. 4(a,c), both NKT cells (PBS-57 glycolipid–CD1d tetramer+ CD3e+) and NK cells (NK1.1+ CD3e−) expanded following C. muridarum lung infection. The increase in percentages of NK cells began to be observed at day 1 post-infection with significant increase at day 3 post-infection (Fig. 4a). However, the percentage of NKT cells only displayed a mild increase at day 3 (Fig. 4c). Considering the absolute number of infiltrating cells in the lung changes following infection, we also measured the absolute number of NK and NKT cells in the lung at different times following infection. As shown in Fig. 4(b,d), both cell types expanded following infection. In addition, we found increased CD69 expression on NKT (PBS-57 glycolipid–CD1d+ CD3e+) cells (Fig. 4e) and NK (NK1.1+ CD3e−) cells (Fig. 4f) following in vivo infection. Similar results were obtained with spleen cells (data not shown). The results indicate that C. muridarum lung infection can induce the activation and expansion of both NKT cells and NK cells, confirming the finding of in vitro experiments.
Figure 4.
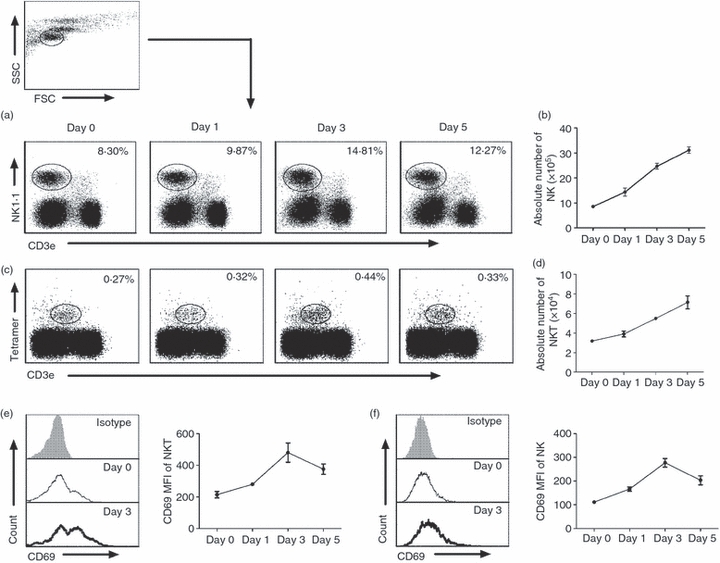
Natural killer (NK) and NKT cells expand and become activated following Chlamydia muridorum lung infection. Mice were infected with C. muridorum elementary bodies (2 × 103IFUs) intranasally and killed at various days after infection. Lung mononuclear cells were prepared as described in the Materials and methods and stained for PBS-57 glycolipid-loaded CD1d tetramer, NK1.1, CD3e and CD69. Representative dot plot of the percentage of NK (NK1.1+ CD3e−) cells (a) and iNKT (PBS-57 glycolipid loaded CD1d tetramer+ CD3e+) cells (c) among gated lymphocytes based on forward and side scatter (as shown in Fig. 1) are shown. Kinetics of the absolute number of NK cells (b) and iNKT cells (d) in lung after infection. The data represent one of four independent experiments and are shown as mean ± SD for three mice. (e) Left panel: representative histograph of CD69 expression by iNKT cells (PBS-57 glycolipid loaded CD1d tetramer+ CD3e+) at day 3 after infection: isotype control antibody staining (grey histograms), uninfected mice (solid thin lines), infected mice (solid thick line). Right panel: the mean fluorescence intensity (MFI) of CD69 expression by lung iNKT cells. Data are mean ± SD of three mice. Three independent experiments were carried out. (f) Left panel: representative histograph of CD69 on NK cells gated as NK1.1+ CD3e− at day 3 after infection: isotype control antibody staining (grey histograms), uninfected mice (solid thin lines), infected mice (solid thick line). Right panel: the MFI of CD69 by lung NK cells. The data represent one of three independent experiments and are shown at mean ± SD for three mice.
Induction of IFN-γ production and degranulation of NK cells by in vivo C. muridarum infection
Having shown the activation and expansion of NK cells in mice with C. muridarum lung infection, we further examined the function of NK cells on IFN-γ production and cytotoxicity following the infection. As shown in Fig. 5(a,c), lung NK cells (NK1.1+ CD3e−) from C. muridarum -infected mice showed quick enhancement of IFN-γ production even at day 1 post-infection. For cytotoxicity, NK cells were tested for degranulation, measured by CD107a expression following stimulation with YAC-1 cells. Similar to IFN-γ production, C. muridarum lung infection enhanced NK (NK1.1+ CD3e−) cell degranulation, and hence cytotoxicity, although the enhancement appeared later than that of IFN-γ (Fig. 5b,d). Analysis of splenic NK cells showed a similar kinetics of IFN-γ production and CD107a expression (data not show). Collectively, the results suggest that NK cells not only produce IFN-γ but also display cytotoxicity during C. muridarum lung infection.
Figure 5.
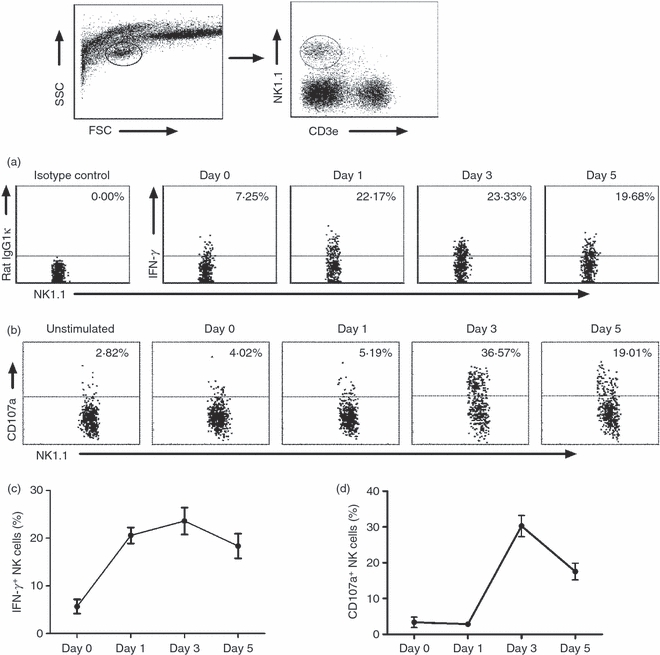
Induction of interferon-γ (IFN-γ) production and degranulation of natural killer (NK) cells following Chlamydia muridorum lung infection. Mice were killed at different days following intranasal infection with C. muridorum elementary bodies (2 × 103 IFUs) and the lung mononuclear cells were prepared for flow cytometry. The gating strategy for NK cells (NK1.1+ CD3e−) is shown on the top. (a) IFN-γ production by NK cells (NK1.1+ CD3e− cells) was assayed by intracellular cytokine staining. (b) CD107a expression by NK cells showing degranulation as described in the Materials and methods. Data are representative one of three independent experiments. (c) Summary data of the percentage of IFN-γ+ NK cells at different days after infection. (d) Summary data of the percentage of CD107a+ NK cells at different days after infection. Data represent one of three independent experiments and are shown as mean ± SD for three mice.
NKT cell deficiency leads to reduced expansion of NK cells with differential changes in IFN-γ production and degranulation of NK cells following C. muridarum infection
To further test the influence of NKT cells on NK cells in vivo following chlamydial infection, we first examined the expansion of NK cells in the spleen and lung following C. muridarum infection in the WT and Jα18−/− mice. As shown in Fig. 6(a–d), NK cells (CD3– NK1.1+) in the Jα18−/− mice was significantly reduced in both percentages and absolute numbers in the spleen (Fig. 6a,b) and lung (Fig. 6c,d) following C. muridarum lung infection. We then compared WT and Jα18−/− mice in IFN-γ production and degranulation by NK cells following C. muridarum lung infection to examine the impact of NKT cells on NK cell function. As shown in Fig. 6(e), at day 3 after infection, significantly lower percentages of splenic and pulmonary NK cells in Jα18−/− mice produced IFN-γ than in WT mice. In contrast, the percentages of degranulating splenic and pulmonary NK cells (measured by CD107a expression) in Jα18−/− mice were significantly higher than in WT mice (Fig. 6f). A similar finding was observed at day 5 post-infection (data not shown). Taken together, the results suggest that NKT cells have a positive effect on NK cell expansion but have differential effects on the NK cells’ IFN-γ production (promoting) and cytotoxicity (inhibitory) following C. muridarum lung infection.
Figure 6.
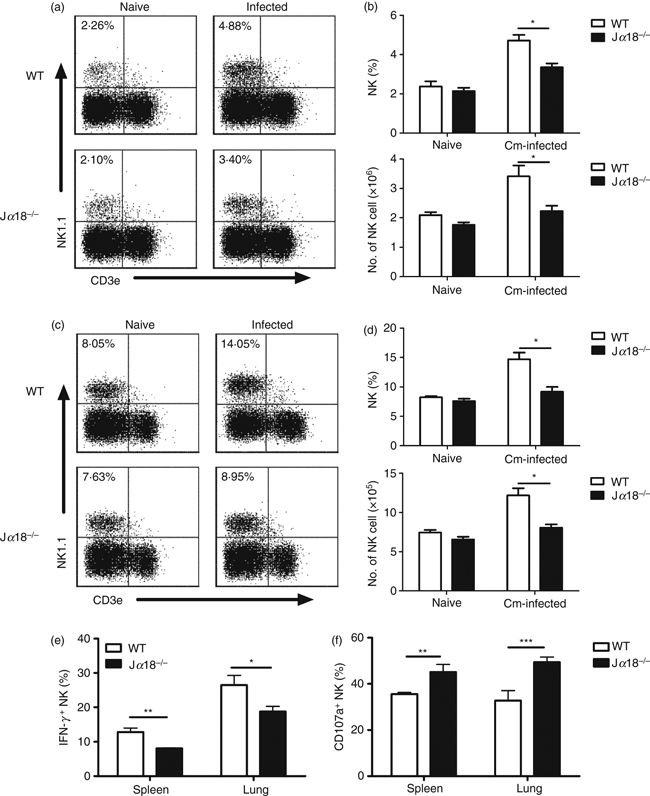
Reduced cellular expansion, interferon-γ (IFN-γ) production and enhanced surface CD107a expression of natural killer (NK) cells in Jα18−/− mice following Chlamydia muridorum (Cm) lung infection. Mice were intranasally infected with C. muridorum (2 × 103 IFUs) and examined at day 3 post-infection. Splenocytes and lung mononuclear cells from wild-type (WT) and Jα18−/− mice were prepared for flow cytometry analysis. Lower expansion of NK cells in Jα18−/− mice in spleen (a) and in lung (c). Representative dot plots are shown. (b) The percentage (upper) and the absolute number (lower) of NK cells in spleen. (d) The percentage (upper) and the absolute number (lower) of NK cells in lung. Data are shown as mean ± SEM of three independent experiments, each with three mice in one group. *P < 0·05. (e) Spleen and lung NK cells (NK1.1+ CD3e−) were analysed for intracellular IFN-γ and the percentage of IFN-γ-producing NK cells was summarized. (f) Summary of CD107a expression on the surface of NK cells obtained from spleen and lung following C. muridorum infection. The data are shown as mean ± SD (n = 3). One representative experiment of three independent experiments is shown.*P < 0·05; **P < 0·01; ***P < 0·001.
NKT cell deficiency leads to changes in NK cell subset expansion but the differential effect on IFN-γ production and cytotoxicity was not restricted to particular NK cell subsets
Having found the differential effect of NKT cells on the IFN-γ production and cytotoxicity of NK cells, we then decided to examine whether the differential effect was caused by the potential differential modulating effect of NKT cells on NK cell subsets, which might have different patterns for the two aspects of NK cell function. Although still controversial, some studies found that murine NK cells can be grouped into subsets based on CD11b and CD27 makers, especially for splenic NK cells.16,28 Accordingly, we first measured the percentage of different NK subsets in Jα18−/− and WT mice based on the surface expression of CD27 and CD11b on NK1.1+ CD3e− NK cells following C. muridarum lung infection. As shown in Fig. 7(a,b), the proportion of NK subsets was altered in the spleen of Jα18−/− mice following C. muridarum infection. Specifically, the subset of immature NK cells (CD11blow CD27high) was significantly higher in Jα18−/− mice than in WT mice (23·32 ± 1·531% versus 17·09 ± 0·289%) and the more mature CD11bhigh CD27high NK subset was significantly reduced in Jα18−/− mice compared with in WT mice (10·98 ± 1·75% versus 22·78 ± 1·52%). However, the proportion of terminal mature CD11bhigh CD27lowNK cells appeared to be comparable between the two types of mice. Notably, in the lung, virtually all the NK cells (> 97%) were mature (CD11bhigh) and no significant difference was observed in NK subsets between the Jα18−/− mice and WT mice (Fig. 7c,d). Moreover, there was no significant difference in proportion of NK subsets between naive WT and NKT KO mice (data not shown). The data suggest that the NKT cells do have some impact on the maturation of NK cells, and hence on the proportion of NK subsets, but this impact is minimal in infected tissues (lung).
Figure 7.
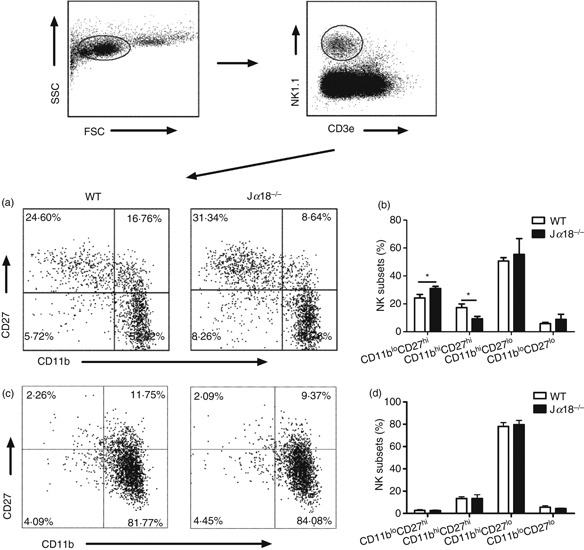
Natural killer T (NKT) cell-deficient mice show altered pattern of NK subsets. Splenocytes and lung mononuclear cells were isolated from wild-type (WT) and Jα18−/− mice at day 3 following Chlamydia muridorum lung infection (2 × 103 IFUs) and analysed by multi-colour flow cytometry. The gating strategy is depicted. (a–d) differential NK subsets in WT and Jα18−/− mice. (a, c) Representative dot plots showing NK subsets in spleen (a) and lung (c) based on CD27 and CD11b expression on gated NK1.1+ CD3e− cells. (b, d) Summary of the NK cell subsets in spleen (b) and lung (d). Data are mean ± SD of three mice in each group. Three independent experiments were performed and one representative experiment is shown. *P < 0·05.
We further examined IFN-γ production and surface CD107a expression on different NK subsets in spleen and lung by flow cytometry (Fig. 8). We found that although the different NK cell subsets in spleen (Fig. 8a–d) and lung (Fig. 8e–h) were different in IFN-γ production and surface CD107a expression following C. muridarum infection, CD27high CD11bhigh subset had the highest IFN-γ production and CD107a expression among the subsets. When the IFN-γ production by different NK subsets was compared between WT and Jα18−/− mice, it was found that although the immature splenic CD27high CD11blow NK cells in Jα18−/− and WT mice showed comparable levels of IFN-γ production, the two more mature NK subsets (CD27high CD11bhigh and CD27low CD11bhigh) in the Jα18−/− mice showed significantly lower production of IFN-γ than those in WT mice (Fig. 8a,b). In sharp contrast, when the surface CD107a expression was compared, both CD27high CD11blow and CD27high CD11bhigh NK subsets in Jα18−/− mice showed significantly higher CD107a expression than those in WT mice (Fig. 8c,d). Analysis of pulmonary NK cells. which were mostly mature CD11bhigh cells. showed a very similar pattern of distinction between IFN-γ production and CD107a expression by NK cells between WT and Jα18−/− mice (Fig. 8e–h). These results suggested that NKT cells promote IFN-γ production by NK cells, but are inhibitory for NK cell cytotoxicity following C. muridarum infection.
Figure 8.
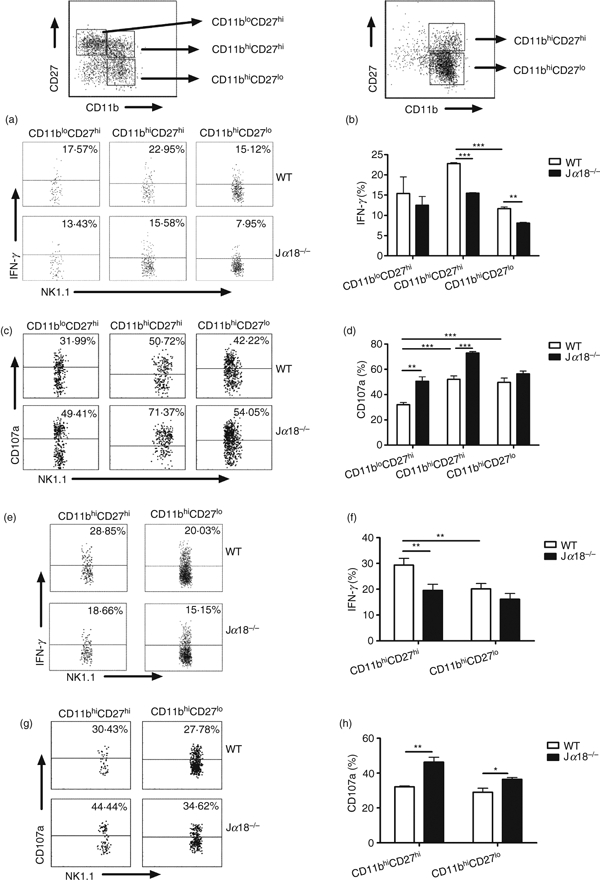
Natural killer T (NKT) cell-deficient mice show differential effect on interferon-γ (IFN-γ) production and cytotoxicity of NK subsets. Splenocytes and lung mononuclear cells were isolated from wild-type (WT) and Jα18−/− mice at day 3 following Chlamydia muridorum lung infection (2 × 103 IFUs) and analysed by multi-colour flow cytometry. NK subsets were gated based on the expression of CD11b and CD27, as shown. (a–h) Differential IFN-γ production and CD107a expression of NK subsets in the spleen (a–d) and lung (e–h) in WT and Jα18−/− mice are shown. (a, c) Representative dot plots of IFN-γ production (a) and CD107a expression(c) by NK subsets in spleen. (b, d) Summary data of IFN-γ-producing NK cells (b) and cell surface CD107a expression (d) in spleen. (e, g) Representative dot plots of IFN-γ production (e) and CD107a expression (g) by NK subsets in lung. (f, h) Summary data of IFN-γ-producing NK cells (f) and cell surface CD107a expression (h) in the lung. Data are presented as mean ± SEM of cumulative data from two independent experiments and three mice in each group. *P < 0·05, **P < 0·01, ***P < 0·001.
Discussion
In the present study, we sought to investigate whether NK cell responses were influenced by NKT cells in cell activation, expansion, subsets and functions during chlamydial infection. We used in vitro and in vivo infection models for this investigation. The results showed that both NKT cells and NK cells became activated (up-regulated CD69 expression) and produced IFN-γ following C. muridarum infection of bulk cultured ex vivo murine spleen cells and when mice were infected in vivo through intranasal inoculation. Moreover, the data showed that NKT cells preferentially promote CD27high CD11bhigh NK subsets which were also the major IFN-γ producers and most powerful cytotoxic cells. More importantly, we found differential influential effects of NKT cells on the functions of NK cells in IFN-γ production and cytotoxicity, enhancing IFN-γ production but inhibiting cytolytic function.
Our data, on the one hand, confirmed the promoting effect of NKT cells on NK cells reported in previous studies, which were mostly performed using model antigens rather than natural infections.8,14,22,29 But most interestingly, we found a differential effect of NKT cells on IFN-γ production (promoting) and cytotoxicity (inhibitory) of NK cells in chlamydial infection. This is the first study, to our knowledge, to show this kind of differential effect, especially in a real infection model system. Both IFN-γ production and cytotoxicity are the major functions of NK cells which are critically important for the cells to fight against infections and tumours. Notably, the differential capacity of NK cells in IFN-γ production and cytotoxicity has been reported in various model systems. For example, NK cells from hepatitis C virus-infected patients displayed enhanced degranulation but decreased IFN-γ production compared with healthy donor controls.30 The data in our study showing the differential effect of NKT cells on the different effector functions of NK cells suggests a contribution of NKT cells to the functional polarization of NK cells. This differential effect of NKT cells on the different aspects of NK cell function further emphasizes the importance of the existence of multi-layer cellular interactions in immune regulation in complicated pathogenic infections. However, the exact mechanism by which NKT cells polarize NK cell function remains unclear. In particular, the differential effect of NKT cells on the IFN-γ production and cytotoxicity of NK cells did not appear to be through modulating different NK cell subsets. Although NKT cells did show a preferential promoting effect on the expansion of the CD27high CD11bhigh (Fig. 7a,b) subset, the effect of NKT cells on the function of this NK subset regarding IFN-γ production and cytotoxicity was in sharp contrast, promoting IFN-γ production but inhibiting cytotoxicity (Fig. 8a–d). In addition, the differential effect on NK functions was also observed in other NK subsets including immature (CD27high CD11blow) and terminal mature (CD27low CD11bhigh) NK cells (Fig. 8a–d). One aspect to focus on in the next step of mechanistic studies may be the biochemical pathways. It has been reported that different biochemical pathways are probably involved in controlling the cytokine production and cytotoxicity function of NK cells. The enhanced degranulation of NK cells in hepatitis C virus-infected patients was associated with increased TRAIL expression.30 It was also reported that the signalling of KIR2DL4, a killer cell immunoglobulin-like receptor family member, induced strong IFN-γ production but not cytotoxicity by resting NK cells.31 The deficiency of phospholipase C (PLC)-γ2 led to defective degranulation of cytotoxic granules but had no effect on interleukin-12-induced IFN-γ production by NK cells.32 Moreover, NK cells from Vav-1-deficient mice had reduced capacity to kill tumour cells upon 2B4, CD16 and NK1.1 stimulation, but their IFN-γ production was normal.33 Therefore, it is possible that NKT cells can influence the signalling pathways in NK cells, hence modulating the function of NK cells depending on the biological context.
The exact implication of the differential effect of NKT cells on NK cells’ IFN-γ production and cytotoxicity in host defence against chlamydial infection per se remains to be investigated. Superficially, it is even puzzling in some degree. Specifically, because IFN-γ has been shown to be critically important for controlling chlamydial infection, the enhancing effect of NKT cells on NK cells’ IFN-γ production should theoretically have a positive impact on host defence against chlamydial infection. However, we recently reported that NKT-cell-deficient CD1d KO mice showed accelerated resolution of C. muridarum pulmonary infection and better protection than WT mice.34 We also showed that enhancing NKT cell function by injecting αGalCer led to more serious infection and pathological changes in C. muridarum-infected mice.34 The reported data would suggest a detrimental role of NKT cells in this infection model, which appeared to contradict the observed IFN-γ-promoting effect on NK cells. On the one hand, the data might suggest a less important contribution of IFN-γ production by NK cells than that by T cells in chlamydial infection. It is possible that the NKT-enhanced IFN-γ production by NK cells does not translate into enhanced IFN-γ production by T cells in this condition. Indeed, the IFN-γ production by T cells in the CD1d KO mice was not low compared with WT mice following C. muridarum infection.34 It is also true that there is no clear evidence that IFN-γ produced by NK cells per se plays an important role in controlling chlamydial infection, unlike the critical role of IFN-γ production by CD4 and CD8 T cells which has been shown in numerous studies. On the other hand, the data might suggest a relatively more important contribution of NK cell cytotoxic function in controlling chlamydial infection. The cytotoxic function of NK cells has also been shown to play a role in controlling chlamydial infection.24,35,36 The present study showed increased cytotoxic function of NK cells in the condition of NKT cell deficiency, which was parallel with the reported better protection in these mice, so the data appeared to support a relatively important role of cytotoxicity of NK cells. Hence, the reduced IFN-γ production of NK cells in the condition of NKT cell deficiency might not have significant negative impact on host defence whereas the enhanced NK cell cytotoxicity in these circumstances may have a positive impact. More importantly, the differential modulating effect of NKT cells on NK cells’ IFN-γ production and killing function has further impact on the next level of immune regulation, such as influencing dendritic cell function. Indeed, both the IFN-γ production and killing function of NK cells can influence dendritic cell function, which is critically important for immune regulation.37–39 In particular, NK cells were found to kill certain dendritic cell subsets that were related to T helper type 2 responses.39 Hence, NK cells may lead to promotion or inhibition of particular dendritic cell subsets, consequently influencing adaptive immunity in different circumstances. Obviously, further study in this area may enhance our understanding not only of host defence against chlamydial infections in particular, but also immune regulation in general. Moreover, further study on the modulating effect of NKT cells on NK cells in different infection and disease models particularly from the angle of combined examination of the effect on both cytokine production and cytotoxic functions will also be likely to enrich our understanding on the interaction between different types of innate and adaptive immune cells.
Acknowledgments
This work was supported by grants jointly funded by Canadian Institutes of Health Research to X.Y. (CCI 92213) and National Natural Sciences Foundation of China to W.Z. (30811120425). X. G. was a recipient of Manitoba Institute of Children Health Graduate Studentship. X. Y. is Canada Research Chair in Infection and Immunity.
Glossary
Abbreviations:
- Cm
Chlamydia muridarum
- DC
dendritic cell
- EB
Chlamydial elementary body
- IFU
inclusion forming unit
- KO
gene knockout
- MOI
multiplicity of infection
- NK cell
natural killer cell
- NKT cell
natural killer T cell
- SPG
sucrose phosphate glutamic acid buffer
- WT
wild-type
Disclosures
The authors declare no conflicts of interest.
References
- 1.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev. 2002;2:557–68. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 3.Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4–8–α/β T cells demonstrates preferential use of several Vβ genes and an invariant TCR α chain. J Exp Med. 1993;178:1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant Vα 24-Jα Q/Vβ 11 T cell receptor is expressed in all individuals by clonally expanded CD4–8– T cells. J Exp Med. 1994;180:1171–6. doi: 10.1084/jem.180.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh AK, Wilson MT, Hong S, et al. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1801–11. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Exley MA, Bigley NJ, Cheng O, et al. CD1d-reactive T-cell activation leads to amelioration of disease caused by diabetogenic encephalomyocarditis virus. J Leukoc Biol. 2001;69:713–8. [PubMed] [Google Scholar]
- 7.Smyth MJ, Thia KY, Street SE, et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–8. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–50. [PubMed] [Google Scholar]
- 9.Tonti E, Galli G, Malzone C, Abrignani S, Casorati G, Dellabona P. NKT-cell help to B lymphocytes can occur independently of cognate interaction. Blood. 2009;113:370–6. doi: 10.1182/blood-2008-06-166249. [DOI] [PubMed] [Google Scholar]
- 10.Joyee AG, Qiu H, Fan Y, Wang S, Yang X. Natural killer T cells are critical for dendritic cells to induce immunity in chlamydial pneumonia. Am J Respir Crit Care Med. 2008;178:745–56. doi: 10.1164/rccm.200804-517OC. [DOI] [PubMed] [Google Scholar]
- 11.Joyee AG, Uzonna J, Yang X. Invariant NKT cells preferentially modulate the function of CD8 α+ dendritic cell subset in inducing type 1 immunity against infection. J Immunol. 2010;184:2095–106. doi: 10.4049/jimmunol.0901348. [DOI] [PubMed] [Google Scholar]
- 12.Cui J, Shin T, Kawano T, et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science (New York, NY) 1997;278:1623–6. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 13.Kawano T, Cui J, Koezuka Y, et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Vα14 NKT cells. Proc Natl Acad Sci U S A. 1998;95:5690–3. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metelitsa LS, Naidenko OV, Kant A, Wu HW, Loza MJ, Perussia B, Kronenberg M, Seeger RC. Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J Immunol. 2001;167:3114–22. doi: 10.4049/jimmunol.167.6.3114. [DOI] [PubMed] [Google Scholar]
- 15.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–9. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–24. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 17.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113:5488–96. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 18.Walzer T, Chiossone L, Chaix J, et al. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat Immunol. 2007;8:1337–44. doi: 10.1038/ni1523. [DOI] [PubMed] [Google Scholar]
- 19.Gregoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–82. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Uhrberg M. The CD107 mobilization assay: viable isolation and immunotherapeutic potential of tumor-cytolytic NK cells. Leukemia. 2005;19:707–9. doi: 10.1038/sj.leu.2403705. [DOI] [PubMed] [Google Scholar]
- 22.Eberl G, MacDonald HR. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur J Immunol. 2000;30:985–92. doi: 10.1002/(SICI)1521-4141(200004)30:4<985::AID-IMMU985>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 23.Duthie MS, Kahn SJ. NK cell activation and protection occur independently of natural killer T cells during Trypanosoma cruzi infection. Int Immunol. 2005;17:607–13. doi: 10.1093/intimm/dxh239. [DOI] [PubMed] [Google Scholar]
- 24.Mavoungou E, Poaty-Mavoungou V, Toure FS, et al. Impairment of natural killer cell activity in Chlamydia trachomatis infected individuals. Trop Med Int Health. 1999;4:719–27. doi: 10.1046/j.1365-3156.1999.00479.x. [DOI] [PubMed] [Google Scholar]
- 25.Tseng CT, Rank RG. Role of NK cells in early host response to chlamydial genital infection. Infect Immun. 1998;66:5867–75. doi: 10.1128/iai.66.12.5867-5875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams DM, Grubbs BG, Schachter J, Magee DM. Gamma interferon levels during Chlamydia trachomatis pneumonia in mice. Infect Immun. 1993;61:3556–8. doi: 10.1128/iai.61.8.3556-3558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joyee AG, Qiu H, Wang S, Fan Y, Bilenki L, Yang X. Distinct NKT cell subsets are induced by different Chlamydia species leading to differential adaptive immunity and host resistance to the infections. J Immunol. 2007;178:1048–58. doi: 10.4049/jimmunol.178.2.1048. [DOI] [PubMed] [Google Scholar]
- 28.Hayakawa Y, Huntington ND, Nutt SL, Smyth MJ. Functional subsets of mouse natural killer cells. Immunol Rev. 2006;214:47–55. doi: 10.1111/j.1600-065X.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 29.Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007;178:2706–13. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- 30.Ahlenstiel G, Titerence RH, Koh C, et al. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-α-dependent manner. Gastroenterology. 2010;138:325–35 e1-2. doi: 10.1053/j.gastro.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajagopalan S, Fu J, Long EO. Cutting edge: induction of IFN-γ production but not cytotoxicity by the killer cell Ig-like receptor KIR2DL4 (CD158d) in resting NK cells. J Immunol. 2001;167:1877–81. doi: 10.4049/jimmunol.167.4.1877. [DOI] [PubMed] [Google Scholar]
- 32.Caraux A, Kim N, Bell SE, et al. Phospholipase C-γ2 is essential for NK cell cytotoxicity and innate immunity to malignant and virally infected cells. Blood. 2006;107:994–1002. doi: 10.1182/blood-2005-06-2428. [DOI] [PubMed] [Google Scholar]
- 33.Colucci F, Rosmaraki E, Bregenholt S, et al. Functional dichotomy in natural killer cell signaling: Vav1-dependent and -independent mechanisms. J Exp Med. 2001;193:1413–24. doi: 10.1084/jem.193.12.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bilenki L, Wang S, Yang J, Fan Y, Joyee AG, Yang X. NK T cell activation promotes Chlamydia trachomatis infection in vivo. J Immunol. 2005;175:3197–206. doi: 10.4049/jimmunol.175.5.3197. [DOI] [PubMed] [Google Scholar]
- 35.Hook CE, Telyatnikova N, Goodall JC, Braud VM, Carmichael AJ, Wills MR, Gaston JS. Effects of Chlamydia trachomatis infection on the expression of natural killer (NK) cell ligands and susceptibility to NK cell lysis. Clin Exp Immunol. 2004;138:54–60. doi: 10.1111/j.1365-2249.2004.02596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hook CE, Matyszak MK, Gaston JS. Infection of epithelial and dendritic cells by Chlamydia trachomatis results in IL-18 and IL-12 production, leading to interferon-γ production by human natural killer cells. FEMS Immunol Med Microbiol. 2005;45:113–20. doi: 10.1016/j.femsim.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev. 2002;2:957–64. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- 38.Ferlazzo G, Morandi B, D'Agostino A, Meazza R, Melioli G, Moretta A, Moretta L. The interaction between NK cells and dendritic cells in bacterial infections results in rapid induction of NK cell activation and in the lysis of uninfected dendritic cells. Eur J Immunol. 2003;33:306–13. doi: 10.1002/immu.200310004. [DOI] [PubMed] [Google Scholar]
- 39.Della Chiesa M, Vitale M, Carlomagno S, Ferlazzo G, Moretta L, Moretta A. The natural killer cell-mediated killing of autologous dendritic cells is confined to a cell subset expressing CD94/NKG2A, but lacking inhibitory killer Ig-like receptors. Eur J Immunol. 2003;33:1657–66. doi: 10.1002/eji.200323986. [DOI] [PubMed] [Google Scholar]


