Abstract
B-cell activation factor of the tumour necrosis factor family (BAFF), an important regulator of B-cell survival, has recently been found to be expressed on the surface of murine and human macrophages and engagement with its receptor was shown to trigger induction of pro-inflammatory mediators and block phagocytic activity. In an effort to generate immunomodulatory agents that can regulate BAFF-mediated signal, decapeptides representing the intracellular immunoreceptor tyrosine-based inhibitory motifs (ITIMs) of immune receptor expressed on myeloid cells (IREM)-1, an inhibitory transmembrane protein expressed on myeloid cells, were synthesized in conjugation with HIV-transactivator of transcription (TAT)48–57, which facilitates the internalization of peptides into cells. Interestingly, all five of these synthetic peptides, representing the five ITIM-like sequences present in the cytoplasmic tail of IREM-1, exhibited inhibitory action against BAFF-mediated induction of matrix metalloproteinase-9 and interleukin-8 expression. Inhibitor assay and immunoprecipitation assay followed by Western blotting demonstrated that the inhibitory action was mediated by Src homology 2 (SH2)-containing tyrosine phosphatase (SHP)-1 and/or phosphoinositide 3-kinase (PI3K). ELISA-based nuclear factor-κB DNA binding assay observed that the synthetic peptides blocked the activation of nuclear factor-κB in an SHP-1 and phosphoinositide 3-kinase-dependent manner. Three of these synthetic peptides exhibited varying degrees of inhibitory action against BAFF-mediated blockage of phagocytosis in a SHP-1 and PI3K-dependent manner. These data indicate that the synthetic peptides are capable of blocking BAFF-mediated regulation of macrophage activities through the activation of SHP-1 and PI3K as well as inhibition of nuclear factor-κB activation.
Keywords: B-cell activation factor of the tumour necrosis factor family, CD300F, immune receptor expressed on myeloid cells-1, intracellular immunoreceptor tyrosine-based inhibitory motifs, phosphoinositide 3-kinase, Src homology 2-containing tyrosine phosphatase-1
Introduction
The immune receptor expressed on myeloid cells-1 (IREM-1, CD300F) is a member of CD300 family of immunomodulators. Expression of IREM-1 has been detected on the surfaces of macrophages, dendritic cells, granulocytes, mast cells and a subset of B cells.1–4 Cross-linking of IREM-1 with monoclonal antibody (mAb) triggers the generation of IREM-1-mediated inhibitory signalling, which regulates biological processes such as FcR-mediated cytokine induction in mast cells1,5 and inflammatory activation of macrophages.6 The inhibitory action of IREM-1 was also demonstrated in experimental autoimmune encephalomyelitis, where its expression was detected on tissue-infiltrating myeloid cells. Selective depletion of its expression resulted in an increase in inflammation as well as aggravation of clinical symptoms.7 These inhibitory activities of IREM-1 are known to be mediated by SHP-1, a protein tyrosine phosphatase with multiple inhibitory actions against various signalling adapters in lymphocytes and myeloid cells.8,9 SHP-1 interacts with immunoreceptor tyrosine-based inhibitory motifs (ITIMs) of IREM-1 through its SH2 domain.1–3 All five ITIM-like sequences (YADL, YVTM, YASL, YCNM and YSTI) contain a tyrosine residue (Y205, Y236, Y249, Y263 and Y284, respectively) and two of them encompassing Y205 (YADL) and Y249 (YASL) were found to be required for the IREM-1-mediated inhibitory effects10 with Y205 (YADL) as the major docking site for SHP-1.1 In addition to its interaction with SHP-1, IREM-1 has also been shown to interact with phosphoinositide 3-kinase (PI3K). Two ITIM-like sequences encompassing Y236 (YVTM) and Y263 (YCNM) interact with the regulatory p85 subunit of PI3K and the interaction is required for the degranulation of rat basophilic leukaemia cells.10 Recently, intracellular introduction of a synthetic peptide that represents one of the ITIM sequences of IREM-1 [amino acids 201–210 (AA201–210)] was shown to mimic the inhibitory actions of IREM-1 in THP-1 cells that were stimulated by B-cell activation factor of the tumour necrosis factor (TNF) family (BAFF) or Fas ligand.6,11
As a member of the TNF superfamily, BAFF (TALL-1/THANK/BlyS/TNFSF13b/zTNF-4) is expressed in stromal cells, myeloid cells and osteoclasts.12–14 BAFF is involved in various immune reactions including B-cell survival and pathogenesis of autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis and Sjögren syndrome.14–19 BAFF can be recognized by three receptors found mainly in B cells, myeloid cells and certain subsets of T cells: transmembrane activator and a calcium-modulating cyclophilin ligand interactor (TACI), B-cell maturation antigen (BCMA) and BAFF receptor (BAFF-R/BR3).13,20 In addition to its role as a B-cell survival factor, a membrane-bound form of BAFF has been detected on the surfaces of various cells of monocyte/macrophage lineage.21–23 Stimulation of this membrane-bound form of BAFF in macrophages induces so-called ‘reverse signalling’, a signal initiated from a molecule that is considered to be a ligand of a cellular receptor. Stimulation of BAFF results in the expression of inflammatory mediators through activation of mitogen-activated protein kinase and nuclear factor (NF-κB); additionally, BAFF-mediated signalling blocks phagocytosis of macrophages.
In a previous report, triggering of IREM-1 using specific mAb resulted in the inhibition of BAFF-mediated inflammatory changes in a human macrophage-like cell line THP-1. In addition, an ITIM-containing synthetic peptide (TAT-YADL) encompassing Y205 (AA201–210) was found to mimic the inhibitory action of IREM-1.6 However, other ITIM-like sequences present in IREM-1 were not tested for their potential inhibitory action. The downstream mediators required for inhibition were not investigated in detail either. In this study, we tried to address these unanswered questions, making two unexpected observations. All five ITIM-containing peptides exhibited inhibitory activity toward BAFF-mediated production of pro-inflammatory mediators and the inhibitory action of these peptides was mediated by not only SHP-1 but also PI3K. The implications of these findings as well as the potential applications of these immunomodulatory peptides in the regulation of inflammation are discussed.
Materials and methods
Cell culture, antibodies and reagents
The mAb for BAFF (clone 148725) was purchased from R&D Systems (Minneapolis, MN); mouse IgG1 was from BD Pharmingen (HI111; San Jose, CA); polyclonal antibody against phospho-AKT, polyclonal antibody against AKT and mAb against phospho-tyrosine (clone P-Tyr-100) were purchased from Cell Signaling Technology Inc. (Danvers, MA); antibodies against p65 (F-6), RelB (C-19) and p50 (N-19) were purchased from Santa Cruz (Santa Cruz, CA); antibodies against p100/p52 (4882) and phospho-p65 (93H1) were purchased from Cell Signaling; SHP-1-specific mAb (clone PTY13) was purchased from Abcam (Cambridge, MA); protein tyrosine phosphatase (PTP) inhibitor III was purchased from Santa Cruz; LY294002 was purchased from Calbiochem International Inc. (La Jolla, CA); sodium pervanadate, sodium deoxycholate, proteinG–Sepharose and lipopolysaccharide were purchased from Sigma (St Louis, MO). Fusion peptides containing ITIM of IREM-1 (AA201–210) and HIV-TAT48–57 (TAT-YADL) were custom-designed and synthesized by Peptron Inc. (Daejeon, Korea). The human monocytic leukaemia cell line THP-1 was obtained from and cultured as suggested by the American Type Culture Collection (Rockville, MD) in a 5% CO2 incubator.
Gelatin zymogram and ELISA
Cells were activated by adding 1 μg/ml of anti-BAFF mAb into the RPMI-1640 medium supplemented with 0·1% fetal bovine serum (Wellgene, Daegu, Korea) containing 1 × 106/ml THP-1 cells. Culture supernatants were collected 24 hr after activation, and the concentrations of interleukin-8 (IL-8) were measured by sandwich ELISA following the manufacturer's instructions (R&D Systems Inc.). The detection limit was < 10 pg/ml. The matrix metalloproteinase (MMP) activity in the culture supernatant was determined via substrate gel electrophoresis, as described previously.13
Immunoprecipitation and Western blot analysis
For immunoprecipitation of SHP-1, THP-1 cells (5 × 106/well in six-well plate) were incubated with 1 mm sodium pervanadate (Sigma) for 15 min at 37° and treated with 5–10 μm of synthetic peptides for 3–30 min, followed by lysis with Nonidet P-40 buffer [150 mm NaCl, 50 mm Tris–HCl (pH 7·5), 5 mm EDTA, 1% Nonidet P-40 (IGEPAL, CA-630; Sigma), 0·5% sodium deoxycholate (Sigma) and 1% of a protease inhibitor cocktail (Calbiochem)]. Cellular debris was removed by centrifugation at 22 000 g for 15 min at 4° and the supernatants were pre-cleared with 30 μl protein G–Sepharose beads (Sigma) for 1 hr at 4°. Immunoprecipitation with 1 μg/ml of anti-SHP-1 mAb was performed overnight at 4°. Then, 50 μl protein G–Sepharose beads was added and incubated for 1 hr at 4°. After washing twice with lysis buffer, the beads were mixed with 50 μl of SDS–PAGE loading buffer. Western blot analysis was performed as described previously.22,23
ELISA-based NF-κB DNA binding activity
Binding activity of NF-κB was measured following a previously described method.24 Briefly, 96-well culture plates were coated with streptavidin overnight by incubation with 5 μg/ml streptavidin in PBS (both Sigma), followed by washing three times with PBS. The streptavidin-coated 96-well culture plates were then used to immobilize biotin-labelled, double-stranded oligonucleotides containing a consensus NF-κB binding site (5′-cacagttgaggggactttcccaggc-3′) (0·02 nm/well). Oligonucleotides were synthesized by Bioneer (Seoul, Korea). Whole cell lysates (40 μg/well) or nuclear lysates (10 μg/well) were then added to the plates and incubated at room temperature for 1 hr with mild agitation in 100 μl/well of PBS. The plates were then sequentially incubated with antibodies specific to NF-κB subunits, HRP-labelled goat anti-mouse IgG (Cell Signaling), and tetramethylbenzidine (Chromogen). Absorbance (450–540 nm) was measured, after which the values were normalized by subtracting the background values. The results were essentially the same between whole cell lysates and nuclear lysates, and the results with cell lysates are shown. For blocking, lysates were pre-incubated with 1·0 nm/sample of double-stranded oligonucleotides containing wild-type NF-κB binding sequence or a mutant sequence (5′-cacagttgaggccactttcccaggc-3′) before being added to the plates with immobilized oligonucleotides.
Phagocytosis
Zymosan opsonization and measurement of phagocytic activity were performed as described previously.21 Briefly, zymosan tagged with Alexa Fluor 594 (Invitrogen, Carlsbad, CA) was incubated with a 1/10 volume of zymosan A opsonizing reagent (Invitrogen) at 37° for 1 hr. THP-1 cells were pre-treated for 30 min with 5 μm of TAT peptides and then incubated with 30 mg/ml of opsonized zymosan for 3 hr. The percentage of cells that had phagocytosed zymosan was measured by flow cytometry analysis. Flow cytometry was performed using the FACScalibur system (Becton-Dickinson, Mountain View, CA). For background fluorescence, cells were analysed without treatment with opsonized zymosan. The fluorescence profiles of 1 × 104 cells were collected and analysed.
Statistical analysis
All data are presented as mean values ± SD, with the number of independent experiments indicated in the figure legends. All analyses were performed using spss software with one-way analysis of variance Mann–Whitney U-test, or the paired or unpaired Student's t-test, as appropriate. Differences were considered significant at P< 0·05.
Results and discussion
ITIM-containing synthetic peptides block BAFF-mediated induction of MMP-9 and IL-8 expression in THP-1 cells
To determine whether macrophage stimulation by membrane-bound BAFF is influenced by the ITIM-like region of IREM-1, THP-1 cells were incubated with BAFF-specific mAbs in the presence or absence of synthetic peptides representing ITIM-like sequences. THP-1 cells responded to anti-BAFF mAb treatment with the induction of pro-inflammatory mediators including MMP-9 and IL-8. Expression of MMP-2 was not induced by stimulation with BAFF. Five synthetic peptides, each representing an ITIM-like region in the cytoplasmic tail of IREM-1, were synthesized in conjugation with HIV-TAT48–57 (Table 1). As controls, a peptide containing only HIV-TAT48–57 (named TAT) and a tyrosine-to-phenylalanine substitution mutant of TAT-YADL (named TAT-FADL) were used. Internalization of the peptides was detected as early as 10 min after treatment (see Supplementary material, Data S1).6,11 When THP-1 cells were stimulated with anti-BAFF mAb in the presence of these peptides, induction of MMP-9 and IL-8 expression was inhibited by all the ITIM-containing peptides (Fig. 1a). Pre-treatment with TAT or TAT-FADL did not affect BAFF-mediated induction of the pro-inflammatory mediators.
Table 1.
Synthetic peptides used in the experiments
| Name | Sequence | Source |
|---|---|---|
| TAT-YADL | GRKKRRQRRR-GDLCYADLTL | IREM-1 (AA 201–210) |
| TAT-FADL | GRKKRRQRRR-GDLCFADLTL | IREM-1 (AA 201–210, Y205F) |
| TAT-YVTM | GRKKRRQRRR-VEVEYVTMAS | IREM-1 (AA 232–241) |
| TAT-YASL | GRKKRRQRRR-EDISYASLTL | IREM-1 (AA 245–254) |
| TAT-YCNM | GRKKRRQRRR-QEPTYCNMGH | IREM-1 (AA 259–268) |
| TAT-YSTI | GRKKRRQRRR-EPTEYSTISR | IREM-1 (AA 280–289) |
| TAT-YMNM | GRKKRRQRRR-LHSDYMNMTP | CD28 (AA 187–196) |
| TAT | GRKKRRQRRR | – |
Figure 1.
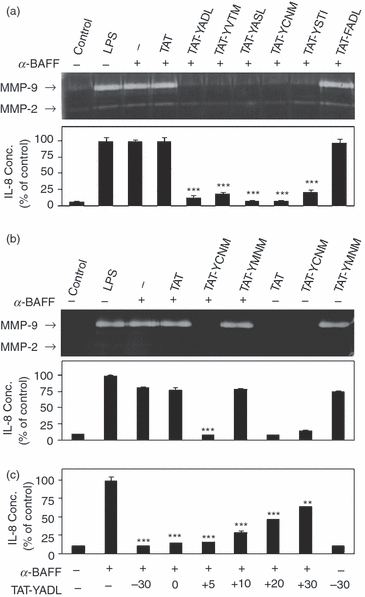
Synthetic peptides containing immunoreceptor tyrosine-based inhibitory motif (ITIM) -like domains of immune receptor expressed on myeloid cells (IREM-1) block the B-cell activation factor of the tumour necrosis factor family (BAFF) -mediated induction of matrix metalloproteinase 9 (MMP-9) and interleukin-8 (IL-8) expression in THP-1 cells. (a) THP-1 cells were pretreated with 5 μm of synthetic peptides for 30 min before stimulation with 1 μg/ml of anti-BAFF monoclonal antibody (α-BAFF). Cells were stimulated with 1 μg/ml of lipopolysaccharide (LPS) as a positive control. Culture supernatants were collected 24 hr after stimulation for measurement of MMP-9/MMP-2 activity using gelatin zymogram (a) and IL-8 concentration using double sandwich ELISA b. (b) THP-1 cells were treated as in (a) and culture supernatant was used for the gelatin zymogram and IL-8 ELISA. (c) THP-1 cells were stimulated with 1 μg/ml of anti-BAFF mAb. TAT-YADL was added at a concentration of 5 μm 30 min before (−30), simultaneously with anti-BAFF mAb (0) or 5, 10, 20 and 30 min after (+5, +10, +20 and +30, respectively). Culture supernatants were collected 24 hr after activation for IL-8 ELISA (n = 3, **P < 0·01 and ***< 0·001 when compared with samples treated with only anti-BAFF mAb).
The occurrence of an inhibitory effect in all of the tyrosine-containing synthetic peptides raises the possibility that any peptide containing a tyrosine may have inhibitory activity. To test this possibility, another synthetic peptide (TAT-YMNM) containing tyrosine in the context of the cytoplasmic tail of CD28 was synthesized. The YMNM-containing sequence has been implicated in the co-stimulatory function of CD28 in T cells through its association with various intracellular signalling adapters.25 When the effect of TAT-YMNM was compared with that of TAT-YCNM, inhibitory activity was detected only with TAT-YCNM whereas addition of TAT-YMNM had a stimulatory effect on the expression of MMP-9 and IL-8 (Fig. 1b). These data indicate that inhibition by the ITIM-containing peptides was specific and dependent on not only the presence of tyrosine but also the flanking amino acid sequences.
Addition of the synthetic peptides before stimulation with mAb may block the interaction between mAb and BAFF. To exclude this possibility, TAT-YADL was added simultaneously with anti-BAFF mAb or at various time-points (5, 10, 20 and 30 min) after. Addition of TAT-YADL simultaneously with anti-BAFF mAb or 5 min after resulted in similar inhibitory activity, which gradually decreased at later time-points (Fig. 1c). This indicates that the synthetic peptides exerted inhibitory activity without affecting the interaction between anti-BAFF mAb and its antigen.
Inhibition of BAFF-mediated expression of inflammatory molecules by all of the ITIM-containing peptides is an unexpected finding. According to a previous report that used Tyr-to-Phe substitution mutation, only ITIMs encompassing Y205 (YADL) and Y249 (YASL) displayed inhibitory activity and Y205 directly interacted with SHP-1.10 However, all of the synthetic peptides representing each of the tyrosine-containing ITIM-like sequences exhibited an inhibitory effect. These data indicate that the use of individual ITIM-like sequences in isolation with each other unveiled their hidden functions, which were not distinct in the context of IREM-1 cytoplasmic tail. It is likely that the multiple ITIMs compete with each other for binding with downstream adapter molecules. It is also possible that the three-dimensional positioning of some of the ITIM-like sequences blocks their interaction with downstream adapter molecules because of steric hindrance.
SHP-1 and PI3K are differentially involved in inhibitory activity of the synthetic peptides
The signalling adaptors responsible for the inhibitory actions of the synthetic peptides were investigated next. As SHP-1 has been reported to interact with the ITIMs encompassing Y205 (YADL) and Y249 (YASL),1,10 the involvement of SHP-1 was tested. In addition, the effect of PI3K inhibition was also tested because the tyrosine-to-phenylalanine substitution at Y236 (YVTM) or Y263 (YCNM) abolished the interaction with the p85 subunit of PI3K.10 Even though PI3K has not been implicated in the inhibitory action of IREM-1 previously, PI3K has been shown to have inhibitory action toward cellular activation in certain cases.26–30
The activity of SHP-1 was inhibited using a specific inhibitor, PTP inhibitor III, which has been shown to interact with the catalytic domain of SHP-1.31 As shown in Fig. 2, PTP inhibitor III attenuated the inhibitory activities of TAT-YADL, TAT-YASL and TAT-YSTI. For inhibition of PI3K, a well known PI3K-specific inhibitor, LY294002, was used. LY294002 attenuated the inhibitory activities of TAT-YADL, TAT-YVTM and TAT-YCNM. This experiment revealed two additional unexpected findings. First, both SHP-1 and PI3K mediate the inhibitory activity of the ITIM-containing peptides. Second, the inhibitory action of the synthetic peptides is differentially mediated by SHP-1 and PI3K. Specifically, the inhibitory activity of TAT-YADL was abrogated by either SHP-1 or PI3K inhibitor, whereas those of TAT-YVTM/TAT-YCNM and TAT-YASL/TAT-YSTI were blocked by PI3K and SHP-1 inhibitor alone, respectively.
Figure 2.

Inhibitory action of the synthetic peptides is inhibited by PTP inhibitor III and/or LY294002. THP-1 cells were sequentially pretreated with 1 mm of PTP inhibitor III or 20 μm of LY294002 for 30 min followed by 5 μm of TAT (T), TAT-YADL (1), TAT-YVTM (2), TAT-YASL (3), TAT-YCNM (4), or TAT-YATI (5) for another 30 min. As a vehicle control (VC), 0·2% DMSO was used. Finally cells were stimulated with 1 μg/ml of anti-B-cell activation factor of the tumour necrosis factor family (BAFF) monoclonal antibody (mAb). Culture supernatants were collected 24 hr after stimulation for the measurement of interleukin-8 (IL-8) concentration (n = 3, **P < 0·01 and ***< 0·001 when compared with samples treated with corresponding TAT peptides and anti-BAFF mAb).
The fact that inhibitors of SHP-1 or PI3K blocked the inhibitory activity of the synthetic peptides indicates that intracellular introduction of these peptides would result in the activation of SHP-1 and/or PI3K activity. Activation of SHP-1 leads to phosphorylation of its tyrosine residue.1 On the other hand, activation of PI3K leads to phosphorylation of its main substrate, AKT. To test whether the ITIM-containing peptides induce activation of SHP-1 and/or PI3K, THP-1 cells were treated with TAT-YADL and the phosphorylation levels of SHP-1 and AKT were measured. In the case of SHP-1, the proteins were immunoprecipitated with specific mAb and the phosphorylation levels of SHP-1 in the precipitate were analysed by Western blotting using a phospho-tyrosine-specific mAb. In the case of PI3K, the phosphorylation levels of AKT were measured by Western blot analysis using a phospho-AKT-specific mAb. As shown in Fig. 3, TAT-YADL induced phosphorylation of both SHP-1 and AKT within 3 min up to 30 min. As expected, TAT and TAT-FADL were not effective in the induction of SHP-1 or AKT phosphorylation. These data indicate that ITIM-containing peptides exert their inhibitory function through the activation of SHP-1 and PI3K. The SH2 domain of SHP-1 is known to directly interact with ITIM sequences through Y205 (YADL),1,10 so it is likely that TAT-YADL, TAT-YASL and TAT-YSTI directly interact with SHP-1.
Figure 3.
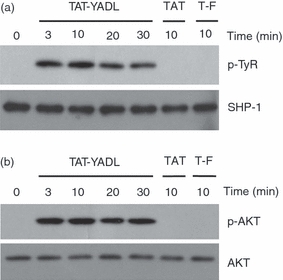
TAT-YADL treatment induces phosphorylation of SHP-1 and AKT. (a) THP-1 cells were treated with 5 μm of TAT, TAT-YADL or TAT-FADL (T-F) for the indicated times. Cell lysates were immunoprecipitated with anti-SHP-1 monoclonal antibody (mAb) and the resulting precipitates were subjected to Western blot analysis using anti-SHP-1 mAb or anti-phospho-tyrosine mAb. (b) Cells were stimulated as in (a). Total cell lysates were subjected to Western blot analysis using antibodies against AKT and phospho-AKT.
Signalling involving PI3K/AKT is known to be involved in the suppression of inflammation in certain cases. Activation of the PI3K/AKT pathway limits the Toll-like receptor-mediated inflammatory activation of human monocytes and neutrophils through modulation of mitogen-activated protein kinase activity.26,27 The PI3K activity has also been shown to be associated with the inhibition of lipopolysaccharide-induced or cytokine-induced expression of inducible nitric-oxide synthase through suppression of NF-κB activity in macrophages, glial cells and endothelial cells.28–30 These previous observations are in agreement with findings that showed the ITIMs derived from IREM-1 could inhibit BAFF-mediated signalling through the activation of PI3K activity. The PI3Ks are a family of heterodimeric enzymes that consist of a regulatory subunit (p85) and a catalytic subunit (p110). As the p85 subunit has an SH2 domain that can interact with phospho-tyrosine residues present on cellular receptors or cytoplasmic proteins,32,33 it is possible that TAT-YADL, TAT-YVTM and TAT-YCNM directly interact with the p85 subunit of PI3K. This is in partial agreement with a previous report with respect to the interaction of PI3K with Y236 (YVTM) and Y263 (YCNM).10
It is interesting that SHP-1 and PI3K differentially mediate the inhibitory action of the synthetic peptides. It is possible that the minor changes in the amino acid sequences of these peptides affect the preference for SHP-1 or p85 subunit in such a way that TAT-YADL can interact with both SHP-1 and PI3K, whereas other peptides interact with only one of them.
BAFF-mediated activation of NF-κB is inhibited by the synthetic peptide in SHP-1 and PI3K-dependent manner
The expression of most of the pro-inflammatory cytokines is regulated by NF-κB, the major transcription factor associated with inflammation. In most cases, NF-κB exists in a heterodimeric form composed of p65 (or RelB) and p50 (or p52). The homodimeric form containing p50 has also been observed. To identify the NF-κB dimers that are activated by BAFF stimulation, ELISA-based NF-κB DNA binding assay was performed in THP-1 cells after anti-BAFF mAb treatment. As shown in Fig. 4, stimulation of BAFF resulted in induction of the DNA binding activity of NF-κB containing p65, RelB, p50 and/or p52 subunits. When the assay was performed with mAb against the p65 subunit of NF-κB, the binding activity transiently increased at 30 min after treatment. This indicates that the NF-κB dimer containing the p65 subunit was most highly activated during this time period (Fig. 4a). Likewise, the binding activity of NF-κB dimers containing RelB (Fig. 4b) and p52 (Fig. 4d) also transiently increased 10 and 30 min after stimulation, respectively. In contrast, the binding activity of NF-κB containing a p50 subunit reached its peak at 10 min after activation and continued for up to 90 min after activation (Fig. 4c). These results show that NF-κB dimers containing the p50 subunit were the major form, whereas NF-κB dimers containing other subunits are transiently activated at different time-points after BAFF stimulation. Binding was inhibited by pre-incubation of cell lysates with consensus NF-κB binding sequences but not with mutant sequences, indicating that the interaction was specific.
Figure 4.

Stimulation of B-cell activation factor of the tumour necrosis factor family (BAFF) induces activation of nuclear factor-κB (NF-κB). THP-1 cells were stimulated with 1 μg/ml of anti-BAFF monoclonal antibody (mAb) for the indicated times. Cell lysates were obtained for the measurement of NF-κB DNA-binding activities using ELISA-based assay with antibodies specific to NF-κB p65 (a), RelB (b), p50 (c) or p52 (d). For competition, cell lysates were pre-incubated with oligonucleotides containing either the wild-type (WT) or mutant (Mut) form of the consensus NF-κB-binding sequence. Binding activity was compared with that of zero time control, which was set as 1 (n = 3, *P< 0·05, **< 0·01 and ***< 0·001 when compared with zero time control; ###< 0·001 when compared as indicated).
To test whether the synthetic peptides affect the BAFF-induced activation of NF-κB, THP-1 cells were pre-treated with TAT-YADL and then stimulated with anti-BAFF mAb for ELISA-based NF-κB DNA-binding activity. The DNA-binding activity of p65 or p50 subunit-containing NF-κB was blocked by TAT-YADL but not by TAT (Fig. 5) or TAT-FADL (data not shown). Nuclear translocation of NF-κB containing the p50 subunit was also blocked by TAT-YADL but not by TAT (see Supplementary material, Data S2). Furthermore, BAFF-mediated phosphorylation of the p65 subunit, which is associated with activation of NF-κB,34,35 was blocked by TAT-YADL, but not by TAT (see Supplementary material, Data S3).
Figure 5.

SHP-1 and PI3K-specific inhibitors block the B-cell activation factor of the tumour necrosis factor family (BAFF) -mediated activation of nuclear factor-κB (NF-κB) DNA-binding activity. THP-1 cells were sequentially pretreated with 1 mm of PTP inhibitor III (P) or 20 μm of LY294002 (L) for 30 min, followed by 5 μm of synthetic peptides (TAT or TAT-YADL) for another 30 min. As a vehicle control (VC), 0·2% DMSO was used. Finally, cells were stimulated with 1 μg/ml of anti-BAFF monoclonal antibody (mAb). Cell lysates were obtained for the measurement of NF-κB DNA-binding activities using ELISA-based assay with antibodies specific to NF-κB p65 and p50 (n = 3, ***P< 0·001 when compared with samples treated with anti-BAFF mAb and TAT-YADL).
To test whether SHP-1 (or PI3K) is involved in the peptide-mediated inhibition of NF-κB activation, ELISA-based DNA binding assay was performed in the presence of a specific inhibitor of SHP-1 (or PI3K). TAT-TADL, which can activate both SHP-1 and PI3K, was used as a representative peptide. As shown in Fig. 5, pre-incubation with either PTP inhibitor III or LY294002 blocked the TAT-YADL-mediated inhibition of NF-κB activity. These data indicate that TAT-YADL blocked the BAFF-mediated activation of NF-κB through SHP-1 and/or PI3K. Other ITIM-containing peptides are expected to have similar effects on NF-κB activation because they require either SHP-1 or PI3K activity for their inhibitory activity.
BAFF-mediated blockage of phagocytic activity is inhibited by the synthetic peptides in an SHP-1- and PI3K-dependent manner
Stimulation of THP-1 cells with anti-BAFF mAb results in blockage of the phagocytic activity of the THP-1 cells against opsonized zymosan.21 As the synthetic peptides blocked BAFF-mediated induction of IL-8 expression, it was expected that they would also affect the BAFF-mediated blockage of phagocytic activity. The phagocytic activity of THP-1 cells was therefore tested by sequential treatment with synthetic peptide, anti-BAFF mAb and opsonized zymosan. As shown in Fig. 6, TAT-YADL completely inhibited the blocking action of anti-BAFF mAb, whereas TAT-YASL and TAT-YCNM exhibited partial inhibition. TAT-YVTM and TAT-YSTI did not exhibit any inhibitory effect.
Figure 6.

Some synthetic peptides block the B-cell activation factor of the tumour necrosis factor family (BAFF) -mediated inhibition of phagocytic activity. THP-1 cells were sequentially treated with 5 μm of TAT (T), TAT-YADL (YADL), TAT-YVTM (YVTM), TAT-YASL (YASL), TAT-YCNM (YCNM) or TAT-YSTI (YSTI) peptides for 30 min, 1 μg/ml of anti-BAFF monoclonal antibody (mAb) (B) for 30 min and then 30 μg/ml of fluorescence-labelled opsonized zymosan (OZ) for an additional 3 hr. Fluorescence profiles from Alexa Fluor 594-labelled zymosan were then measured using flow cytometry. (a) Background fluorescence profile (filled area) was compared with that of each sample (empty area). Numbers indicate the percentages of cells that phagocytosed opsonized zymosan. (b) Experiment in (a) was repeated three times and the percentages of zymosan-positive cells were compared (***P< 0·001 when compared with samples treated with anti-BAFF mAb and opsonized zymosan).
To discover whether the inhibition of BAFF-mediated phagocytosis blockage was mediated by the same downstream adaptors, THP-1 cells were sequentially treated with either PTP inhibitor III or LY294002, TAT-YADL, anti-BAFF mAb and opsonized zymosan. Both PTP inhibitor III and LY294002 blocked the inhibitory action of TAT-YADL (Fig. 7).
Figure 7.
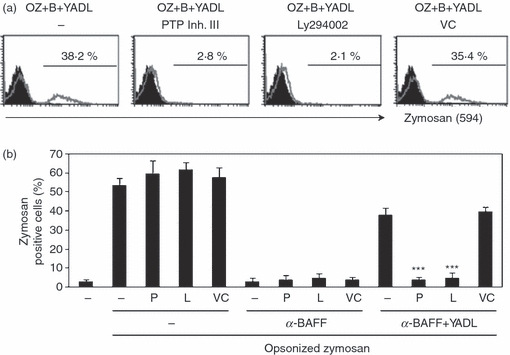
SHP-1 and PI3K-specific inhibitors can block B-cell activation factor of the tumour necrosis factor family (BAFF) -mediated inhibition of phagocytic activity. THP-1 cells were sequentially treated with 1 mM of PTP inhibitor III (P) or 20 μm of LY294002 (L) for 30 min, 5 μm of synthetic peptides TAT-YADL (YADL) for 30 min and 1 μg/ml of anti-BAFF monoclonal antibody (mAb) (B) for another 30 min. As a vehicle control (VC), 0·2% DMSO was used. Finally, cells were incubated with 30 μg/ml of fluorescence-labelled opsonized zymosan (OZ) for 3 hr. Fluorescence profiles from Alexa Fluor 594-labelled zymosan were then measured using flow cytometry. (a) Background fluorescence profile (filled area) was compared with that of each sample (empty area). Numbers indicate the percentages of cells that phagocytosed opsonized zymosan. (b) Experiment in (a) was repeated three times and the percentages of zymosan-positive cells were compared (***P< 0·001 when compared with samples treated with anti-BAFF mAb and opsonized zymosan).
In conclusion, isolated ITIM-like sequences were found to be capable of inhibiting BAFF-mediated inflammatory activation through inhibition of NF-κB activation in THP-1 cells. These peptides also inhibited BAFF-mediated blockage of the phagocytic process. The inhibitory actions of the synthetic peptides were made possible through the differential activation of SHP-1 and PI3K. These peptides are able to regulate inflammatory processes in the human macrophage-like cell line THP-1 so they are expected to be useful for the regulation of inflammation, especially that mediated by macrophages. Chronic inflammation is closely associated with the pathogenesis of many diseases. Atherosclerosis, for example, is a chronic inflammatory disease and macrophages play an essential role in the formation of fatty streak (the hallmark of atherosclerosis) and atherosclerotic plaques, rupture of plaque, and the formation of thrombi.36,37 In the case of cancer, chronic inflammation is well known to be associated with the development and metastasis of cancer.38–40 The synthetic peptides derived from ITIMs of IREM-1 could be used to control inflammation that is associated with these diseases.
Acknowledgments
This work was supported by a grant provided by the Korea Research Foundation Grant funded by the Korean Government (KRF-2008-313-C00647).
Disclosures
The authors have no conflict of interest or any relevant financial interest in any company or institution that might benefit from this publication.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Data S1. Synthetic peptides are internalized into THP-1 cells within 10 min after treatment.
Data S2. Stimulation with anti-BAFF monoclonal antibody induces nuclear translocation of nuclear factor-κB and it was blocked by TAT-YADL but not by TAT in THP-1 cells.
Data S3. Stimulation with anti-BAFF mAb induces phosphorylation of NF-κB and it was blocked by TAT-YADL but not by TAT in THP-1 cells.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Alvarez-Errico D, Aguilar H, Kitzig F, Brckalo T, Sayos J, Lopez-Botet M. IREM-1 is a novel inhibitory receptor expressed by myeloid cells. Eur J Immunol. 2004;34:3690–701. doi: 10.1002/eji.200425433. [DOI] [PubMed] [Google Scholar]
- 2.Sui L, Li N, Liu Q, et al. IgSF13, a novel human inhibitory receptor of the immunoglobulin superfamily, is preferentially expressed in dendritic cells and monocytes. Biochem Biophys Res Commun. 2004;319:920–8. doi: 10.1016/j.bbrc.2004.05.065. [DOI] [PubMed] [Google Scholar]
- 3.Chung DH, Humphrey MB, Nakamura MC, Ginzinger DG, Seaman WE, Daws MR. CMRF-35-like molecule-1, a novel mouse myeloid receptor, can inhibit osteoclast formation. J Immunol. 2003;171:6541–8. doi: 10.4049/jimmunol.171.12.6541. [DOI] [PubMed] [Google Scholar]
- 4.Shibuya A, Nakahashi-Oda C, Tahara-Hanaoka S. Regulation of immune responses by the activating and inhibitory myeloid-associate immunoglobuline-like receptors (MAIR) (CD300) Immune Netw. 2009;9:41–5. doi: 10.4110/in.2009.9.2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izawa K, Kitaura J, Yamanishi Y, et al. An activating and inhibitory signal from an inhibitory receptor LMIR3/CLM-1: LMIR3 augments lipopolysaccharide response through association with FcRgamma in mast cells. J Immunol. 2009;183:925–36. doi: 10.4049/jimmunol.0900552. [DOI] [PubMed] [Google Scholar]
- 6.Lee SM, Nam YP, Suk K, Lee WH. IREM-1 inhibits BAFF-mediated inflammatory regulation of THP-1 cells through modulation of the activities of ERK. Clin Exp Immunol. 2010;161:504–11. doi: 10.1111/j.1365-2249.2010.04211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xi H, Katschke KJ, Jr, Helmy KY, et al. Negative regulation of autoimmune demyelination by the inhibitory receptor CLM-1. J Exp Med. 2010;207:7–16. doi: 10.1084/jem.20091508. S1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol. 2007;22:1251–67. doi: 10.14670/hh-22.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorenz U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol Rev. 2009;228:342–59. doi: 10.1111/j.1600-065X.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvarez-Errico D, Sayos J, Lopez-Botet M. The IREM-1 (CD300f) inhibitory receptor associates with the p85alpha subunit of phosphoinositide 3-kinase. J Immunol. 2007;178:808–16. doi: 10.4049/jimmunol.178.2.808. [DOI] [PubMed] [Google Scholar]
- 11.Lee SM, Kim EJ, Suk K, Lee WH. Stimulation of FasL induces production of pro-inflammatory mediators through activation of mitogen activated protein kinases and nuclear factor-kappaB in THP-1 cells. Inflammation. 2011 doi: 10.1007/s10753-010-9283-3. in press. [DOI] [PubMed] [Google Scholar]
- 12.Gorelik L, Gilbride K, Dobles M, Kalled SL, Zandman D, Scott ML. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J Exp Med. 2003;198:937–45. doi: 10.1084/jem.20030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng LG, Mackay CR, Mackay F. The BAFF/APRIL system: life beyond B lymphocytes. Mol Immunol. 2005;42:763–72. doi: 10.1016/j.molimm.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 14.Dillon SR, Gross JA, Ansell SM, Novak AJ. An APRIL to remember: novel TNF ligands as therapeutic targets. Nat Rev Drug Discov. 2006;5:235–46. doi: 10.1038/nrd1982. [DOI] [PubMed] [Google Scholar]
- 15.Jonsson MV, Szodoray P, Jellestad S, Jonsson R, Skarstein K. Association between circulating levels of the novel TNF family members APRIL and BAFF and lymphoid organization in primary Sjögren's syndrome. J Clin Immunol. 2005;25:189–201. doi: 10.1007/s10875-005-4091-5. [DOI] [PubMed] [Google Scholar]
- 16.Pers JO, Daridon C, Devauchelle V, Jousse S, Saraux A, Jamin C, Youinou P. BAFF overexpression is associated with autoantibody production in autoimmune diseases. Ann N Y Acad Sci. 2005;1050:34–9. doi: 10.1196/annals.1313.004. [DOI] [PubMed] [Google Scholar]
- 17.Seyler TM, Park YW, Takemura S, Bram RJ, Kurtin PJ, Goronzy JJ, Weyand CM. BLyS and APRIL in rheumatoid arthritis. J Clin Invest. 2005;115:3083–92. doi: 10.1172/JCI25265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stohl W, Metyas S, Tan SM, et al. B lymphocyte stimulator overexpression in patients with systemic lupus erythematosus: longitudinal observations. Arthritis Rheum. 2003;48:3475–86. doi: 10.1002/art.11354. [DOI] [PubMed] [Google Scholar]
- 19.Mackay F, Schneider P, Rennert P, Browning J. BAFF and APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–64. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 20.Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17:282–9. doi: 10.1016/j.coi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Jeon ST, Kim WJ, Lee SM, et al. Reverse signaling through BAFF differentially regulates the expression of inflammatory mediators and cytoskeletal movements in THP-1 cells. Immunol Cell Biol. 2010;88:148–56. doi: 10.1038/icb.2009.75. [DOI] [PubMed] [Google Scholar]
- 22.Lee SM, Jeon ST, Kim WJ, Suk K, Lee WH. Macrophages express membrane bound form of APRIL that can generate immunomodulatory signals. Immunology. 2010;131:350–56. doi: 10.1111/j.1365-2567.2010.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SM, Kim WJ, Suk K, Lee WH. Cell to cell interaction can activate membrane-bound APRIL which are expressed on inflammatory macrophages. Immune Netw. 2010;10:173–80. doi: 10.4110/in.2010.10.5.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenau C, Emery D, Kaboord B, Qoronfleh MW. Development of a high-throughput plate-based chemiluminescent transcription factor assay. J Biomol Screen. 2004;9:334–42. doi: 10.1177/1087057103261446. [DOI] [PubMed] [Google Scholar]
- 25.Harada Y, Tokushima M, Matsumoto Y, et al. Critical requirement for the membrane-proximal cytosolic tyrosine residue for CD28-mediated costimulation in vivo. J Immunol. 2001;166:3797–803. doi: 10.4049/jimmunol.166.6.3797. [DOI] [PubMed] [Google Scholar]
- 26.Strassheim D, Asehnoune K, Park JS, et al. Phosphoinositide 3-kinase and Akt occupy central roles in inflammatory responses of Toll-like receptor 2-stimulated neutrophils. J Immunol. 2004;172:5727–33. doi: 10.4049/jimmunol.172.9.5727. [DOI] [PubMed] [Google Scholar]
- 27.Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277:32124–32. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- 28.Pahan K, Raymond JR, Singh I. Inhibition of phosphatidylinositol 3-kinase induces nitric-oxide synthase in lipopolysaccharide- or cytokine-stimulated C6 glial cells. J Biol Chem. 1999;274:7528–36. doi: 10.1074/jbc.274.11.7528. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Tupper JC, Bannerman DD, Winn RK, Rhodes CJ, Harlan JM. Phosphoinositide 3 kinase mediates Toll-like receptor 4-induced activation of NF-kappa B in endothelial cells. Infect Immun. 2003;71:4414–20. doi: 10.1128/IAI.71.8.4414-4420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diaz-Guerra MJ, Castrillo A, Martin-Sanz P, Bosca L. Negative regulation by phosphatidylinositol 3-kinase of inducible nitric oxide synthase expression in macrophages. J Immunol. 1999;162:6184–90. [PubMed] [Google Scholar]
- 31.Arabaci G, Guo XC, Beebe KD, Coggeshall KM, Pei D. α-Haloacetophenone derivatives as photoreversible covalent inhibitors of protein tyrosine phosphatases. J Am Chem Soc. 1999;121:5085–6. [Google Scholar]
- 32.Carpenter CL, Auger KR, Chanudhuri M, Yoakim M, Schaffhausen B, Shoelson S, Cantley LC. Phosphoinositide 3-kinase is activated by phosphopeptides that bind to the SH2 domains of the 85-kDa subunit. J Biol Chem. 1993;268:9478–83. [PubMed] [Google Scholar]
- 33.Street A, Macdonald A, Crowder K, Harris M. The hepatitis C virus NS5A protein activates a phosphoinositide 3-kinase-dependent survival signaling cascade. J Biol Chem. 2004;279:12232–41. doi: 10.1074/jbc.M312245200. [DOI] [PubMed] [Google Scholar]
- 34.Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell. 2002;9:625–36. doi: 10.1016/s1097-2765(02)00477-x. [DOI] [PubMed] [Google Scholar]
- 35.Yang F, Tang E, Guan K, Wang CY. IKK beta plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J Immunol. 2003;170:5630–5. doi: 10.4049/jimmunol.170.11.5630. [DOI] [PubMed] [Google Scholar]
- 36.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 37.Libby P. Atherosclerosis in inflammation. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 38.Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204–15. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 39.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 40.Maeda S, Omata M. Inflammation and cancer: role of nuclear factor-kappaB activation. Cancer Sci. 2008;99:836–42. doi: 10.1111/j.1349-7006.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


