Abstract
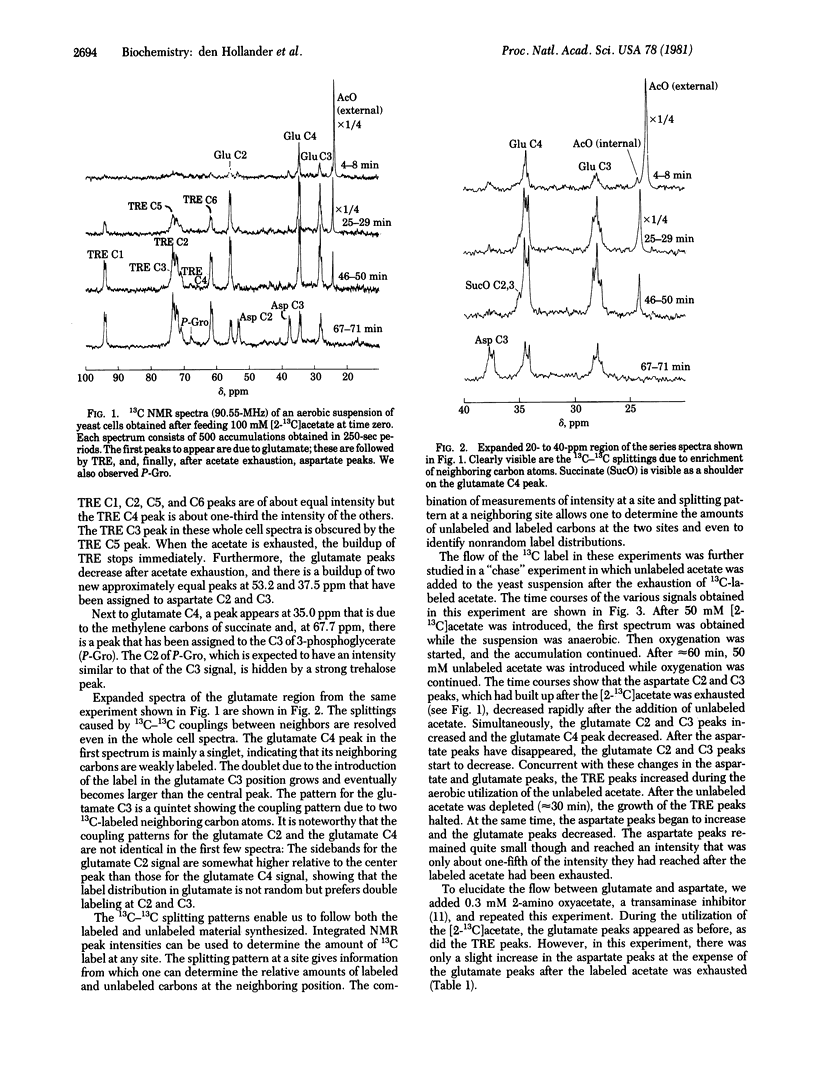
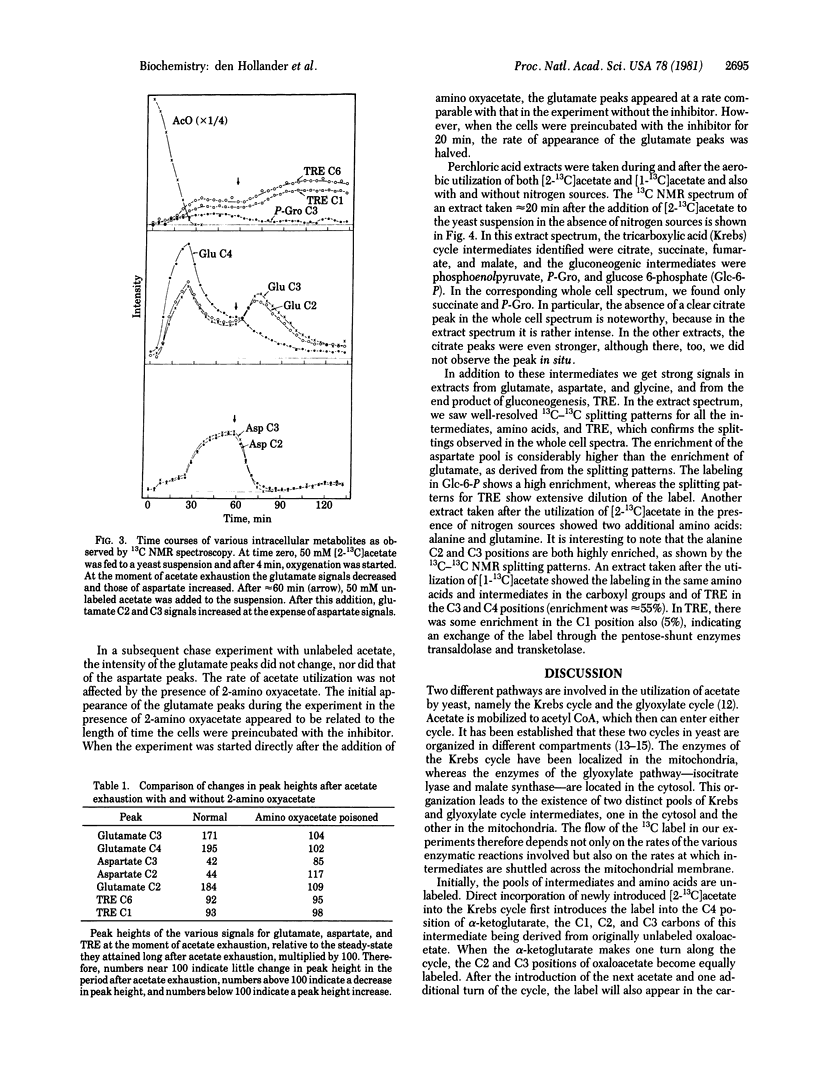
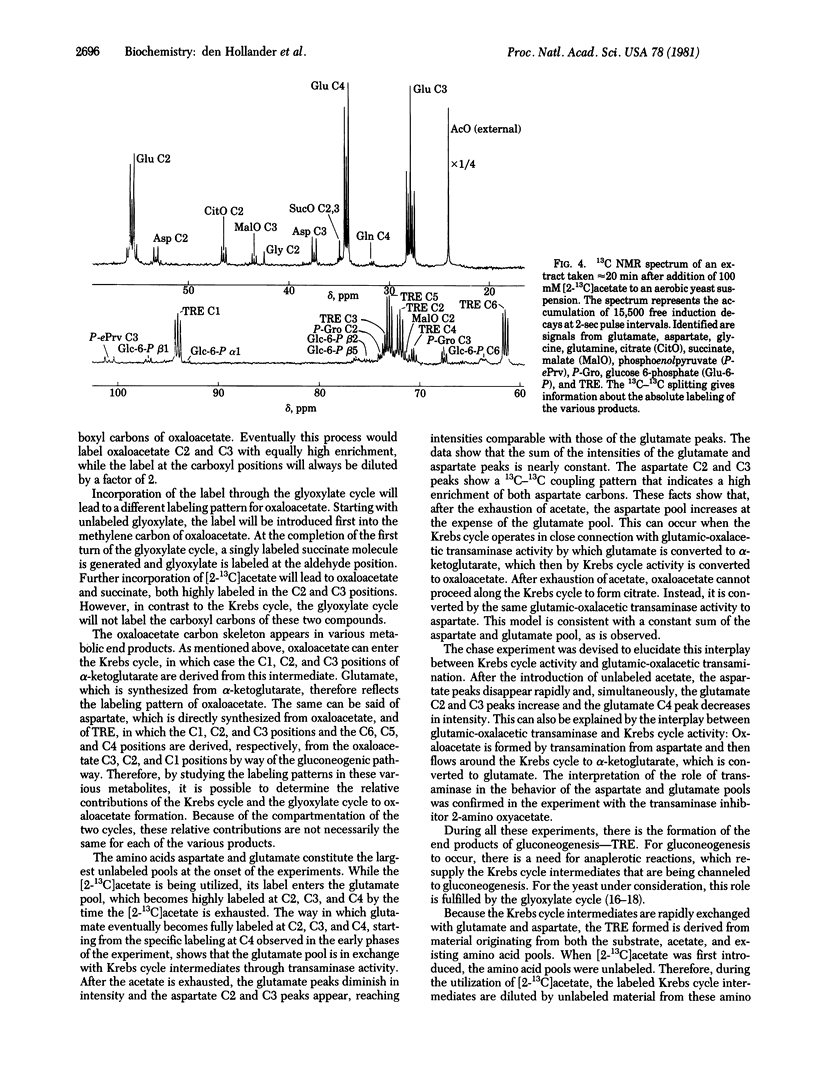
13C NMR was used to follow the metabolism of [2- 13C]acetate and [1- 13C]acetate in aerobic suspensions of Saccharomyces cerevisiae. In the experiment with [2- 13C]acetate, the 13C label appeared first in glutamate C4 and subsequently in glutamate C2 and C3. After exhaustion of the acetate, the glutamate signals diminished and the aspartate C2 and C3 peaks increased. During a subsequent chase experiment with unlabeled acetate, the aspartate peaks decreased and the glutamate C2 and C3 peaks increased in intensity. These observations are interpreted in terms of an interplay between the glutamic-oxalacetic transaminase and Krebs cycle activity. This interpretation was confirmed by an experiment with the transaminase inhibitor 2-amino oxyacetate. During all of these experiments, we observed the formation of trehalose. The NMR gives a direct measurement of the label distribution and from that information it followed that the flows through the glyoxylate and the Krebs cycles are comparable. The intermediates citrate, succinate, fumarate, malate, phosphoenolpyruvate, 3-phosphoglycerate, and glucose 6-phosphate were identified in a 13C NMR spectrum of a perchloric acid extract taken during the metabolism of [2- 13C]acetate. Enrichment of the glutamate C5 position shows the existence of a futile cycle in which phosphoenolpyruvate, formed from oxaloacetate, returns to the Krebs cycle through pyruvate and acetyl CoA
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen S. M., Ogawa S., Shulman R. G. 13C NMR studies of gluconeogenesis in rat liver cells: utilization of labeled glycerol by cells from euthyroid and hyperthyroid rats. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1603–1609. doi: 10.1073/pnas.76.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. M., Shulman R. G., McLaughlin A. C. Effects of ethanol on alanine metabolism in perfused mouse liver studied by 13C NMR. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4808–4812. doi: 10.1073/pnas.76.10.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duntze W., Neumann D., Gancedo J. M., Atzpodien W., Holzer H. Studies on the regulation and localization of the glyoxylate cycle enzymes in Saccharomyces cerevisiae. Eur J Biochem. 1969 Aug;10(1):83–89. doi: 10.1111/j.1432-1033.1969.tb00658.x. [DOI] [PubMed] [Google Scholar]
- Haarasilta S., Oura E. On the activity and regulation of anaplerotic and gluconeogenetic enzymes during the growth process of baker's yeast. The biphasic growth. Eur J Biochem. 1975 Mar 3;52(1):1–7. doi: 10.1111/j.1432-1033.1975.tb03966.x. [DOI] [PubMed] [Google Scholar]
- KORNBERG H. L. THE ROLE OF ACETATE IN ISOCITRATE LYASE INDUCTION. Biochim Biophys Acta. 1963 Jul 9;73:517–519. doi: 10.1016/0006-3002(63)90456-6. [DOI] [PubMed] [Google Scholar]
- Klein H. P., Jahnke L. Variations in the localization of acetyl-coenzyme A synthetase in aerobic yeast cells. J Bacteriol. 1971 May;106(2):596–602. doi: 10.1128/jb.106.2.596-602.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L. The role and control of the glyoxylate cycle in Escherichia coli. Biochem J. 1966 Apr;99(1):1–11. doi: 10.1042/bj0990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon G., Shulman R. G., Yamane T., Eccleshall T. R., Lam K. B., Baronofsky J. J., Marmur J. Phosphorus-31 nuclear magnetic resonance studies of wild-type and glycolytic pathway mutants of Saccharomyces cerevisiae. Biochemistry. 1979 Oct 16;18(21):4487–4499. doi: 10.1021/bi00588a006. [DOI] [PubMed] [Google Scholar]
- Perlman P. S., Mahler H. R. Intracellular localization of enzymes in yeast. Arch Biochem Biophys. 1970 Jan;136(1):245–259. doi: 10.1016/0003-9861(70)90348-6. [DOI] [PubMed] [Google Scholar]
- Rognstad R. Futile hydrogen cycling in liver cells from triiodothyronine treated rats. Biochem Biophys Res Commun. 1977 Oct 10;78(3):881–888. doi: 10.1016/0006-291x(77)90505-8. [DOI] [PubMed] [Google Scholar]
- Shilo B., Shilo V., Simchen G. Cell-cycle initiation in yeast follows first-order kinetics. Nature. 1976 Dec 23;264(5588):767–770. doi: 10.1038/264767a0. [DOI] [PubMed] [Google Scholar]
- Shulman R. G., Brown T. R., Ugurbil K., Ogawa S., Cohen S. M., den Hollander J. A. Cellular applications of 31P and 13C nuclear magnetic resonance. Science. 1979 Jul 13;205(4402):160–166. doi: 10.1126/science.36664. [DOI] [PubMed] [Google Scholar]
- Ugurbil K., Brown T. R., den Hollander J. A., Glynn P., Shulman R. G. High-resolution 13C nuclear magnetic resonance studies of glucose metabolism in Escherichia coli. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3742–3746. doi: 10.1073/pnas.75.8.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander J. A., Brown T. R., Ugurbil K., Shulman R. G. 13C nuclear magnetic resonance studies of anaerobic glycolysis in suspensions of yeast cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6096–6100. doi: 10.1073/pnas.76.12.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]


