Abstract
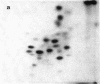
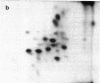
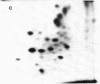
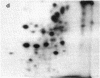
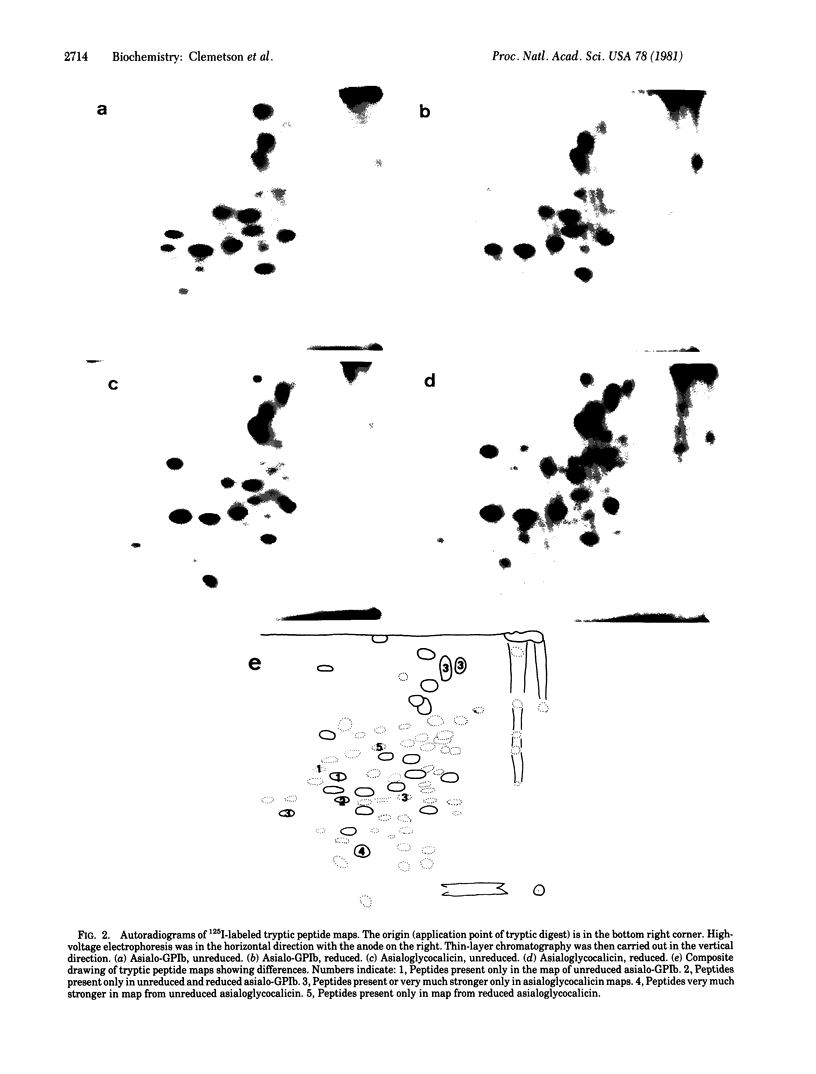
Asialoglycoprotein Ib and asialoglycocalicin have been isolated from the membranes and from the supernatant, respectively, after sonication of neuraminidase-treated platelets, by lectin affinity chromatography on peanut agglutinin. The isolated asialoglycoprotein Ib had an apparent molecular weight of 160,000 when not reduced and 150,000 when reduced, whereas the asialoglycocalicin had an apparent molecular weight of 150,000, both reduced and unreduced, on sodium dodecyl sulfate/polyacrylamide gel electrophoresis. Both preparations contained only trace amounts of impurities. The asialoglycoprotein Ib and asialoglycocalicin in both the unreduced and reduced states were separated by gel electrophoresis, radioiodinated in gel slices, and digested with trypsin, and the digests were analyzed by two-dimensional high-voltage electrophoresis and thin-layer chromatography followed by autoradiography. The tryptic peptide maps showed great similarities between glycocalicin and glycoprotein Ib, with the latter (both unreduced and reduced) containing additional peptides, supporting the idea that glycocalicin is derived from glycoprotein Ib. The unreduced glycoprotein Ib contained additional peptides compared to the reduced due to the disulfide-bond-linked beta component. There were also slight differences between unreduced and reduced glycocalicin, indicating that at least one intramolecular disulfide bond is present.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. C., Gahmberg C. G. Surface glycoproteins of human white blood cells. Analysis by surface labeling. Blood. 1978 Jul;52(1):57–67. [PubMed] [Google Scholar]
- BETTEX-GALLAND M., LUSCHER E. F. Studies on the metabolism of human blood platelets in relation to clot retraction. Thromb Diath Haemorrh. 1960 Mar 1;4:178–195. [PubMed] [Google Scholar]
- Clemetson K. J., Pfueller S. L., Luscher E. F., Jenkins C. S. Isolation of the membrane glycoproteins of human blood platelets by lectin affinity chromatography. Biochim Biophys Acta. 1977 Feb 4;464(3):493–508. doi: 10.1016/0005-2736(77)90025-6. [DOI] [PubMed] [Google Scholar]
- Cooper H. A., Clemetson K. J., Lüscher E. F. Human platelet membrane receptor for bovine von Willebrand factor (platelet aggregating factor): an integral membrane glycoprotein. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1069–1073. doi: 10.1073/pnas.76.3.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- George J. N. Studies on platelet plasma membranes. IV. Quantitative analysis of platelet membrane glycoproteins by (125I)-diazotized diiodosulfanilic acid labeling and SDS-polyacrylamide gel electrophoresis. J Lab Clin Med. 1978 Sep;92(3):430–446. [PubMed] [Google Scholar]
- Howard M. A., Hutton R. A., Hardisty R. M. Hereditary giant platelet syndrome: a disorder of a new aspect of platelet function. Br Med J. 1973 Jun 9;2(5866):586–588. doi: 10.1136/bmj.2.5866.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson G. A., Okumura T., Hasitz M. Structure and function of platelet glycocalicin. Thromb Haemost. 1980 Feb 29;42(5):1673–1678. [PubMed] [Google Scholar]
- Jenkins C. S., Phillips D. R., Clemetson K. J., Meyer D., Larrieu M. J., Lüscher E. F. Platelet membrane glycoproteins implicated in ristocetin-induced aggregation. Studies of the proteins on platelets from patients with Bernard-Soulier syndrome and von Willebrand's disease. J Clin Invest. 1976 Jan;57(1):112–124. doi: 10.1172/JCI108251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan R., Skutelsky E., Danon D., Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J Biol Chem. 1975 Nov 10;250(21):8518–8523. [PubMed] [Google Scholar]
- Marchesi S. L., Chasis J. A. Isolation of human platelet glycoproteins. Biochim Biophys Acta. 1979 Aug 23;555(3):442–459. doi: 10.1016/0005-2736(79)90398-5. [DOI] [PubMed] [Google Scholar]
- Massini P., Lüscher E. F. Some effects of ionophores for divalent cations on blood platelets. Comparison with the effects of thrombin. Biochim Biophys Acta. 1974 Nov 4;372(1):109–121. doi: 10.1016/0304-4165(74)90077-4. [DOI] [PubMed] [Google Scholar]
- Nachman R. L., Kinoshita T., Ferris B. Structural analysis of human platelet membrane glycoprotein I complex. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2952–2954. doi: 10.1073/pnas.76.6.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurden A. T. A peripheral high molecular weight glycoprotein located at the surface of human platelets. Experientia. 1977 Mar 15;33(3):331–332. doi: 10.1007/BF02002811. [DOI] [PubMed] [Google Scholar]
- Okumura T., Jamieson G. A. Platelet glycocalicin: a single receptor for platelet aggregation induced by thrombin or ristocetin. Thromb Res. 1976 May;8(5):701–706. doi: 10.1016/0049-3848(76)90250-4. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet plasma membrane glycoproteins. Evidence for the presence of nonequivalent disulfide bonds using nonreduced-reduced two-dimensional gel electrophoresis. J Biol Chem. 1977 Mar 25;252(6):2121–2126. [PubMed] [Google Scholar]
- Solum N. O., Hagen I., Filion-Myklebust C., Stabaek T. Platelet glycocalicin. Its membrane association and solubilization in aqueous media. Biochim Biophys Acta. 1980 Apr 10;597(2):235–246. doi: 10.1016/0005-2736(80)90102-9. [DOI] [PubMed] [Google Scholar]
- Solum N. O., Hagen I., Gjemdal T. Platelt membrane glycoproteins and the interaction between bovine factor VIII related protein and human platelets. Thromb Haemost. 1977 Dec 15;38(4):914–923. [PubMed] [Google Scholar]
- Solum N. O., Hagen I., Sletbakk T. Further evidence for glycocalicin being derived from a larger amphiphilic platelet membrane glycoprotein. Thromb Res. 1980 Jun 15;18(6):773–785. doi: 10.1016/0049-3848(80)90200-5. [DOI] [PubMed] [Google Scholar]
- Uy R., Wold F. 1,4-Butanediol diglycidyl ether coupling of carbohydrates to Sepharose: affinity adsorbents for lectins and glycosidases. Anal Biochem. 1977 Jul;81(1):98–107. doi: 10.1016/0003-2697(77)90602-9. [DOI] [PubMed] [Google Scholar]
- Wilchek M., Miron T. Polymers coupled to agarose as stable and high capacity spacers. Methods Enzymol. 1974;34:72–76. doi: 10.1016/s0076-6879(74)34008-6. [DOI] [PubMed] [Google Scholar]