Abstract
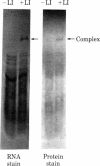
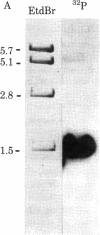
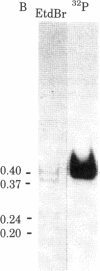
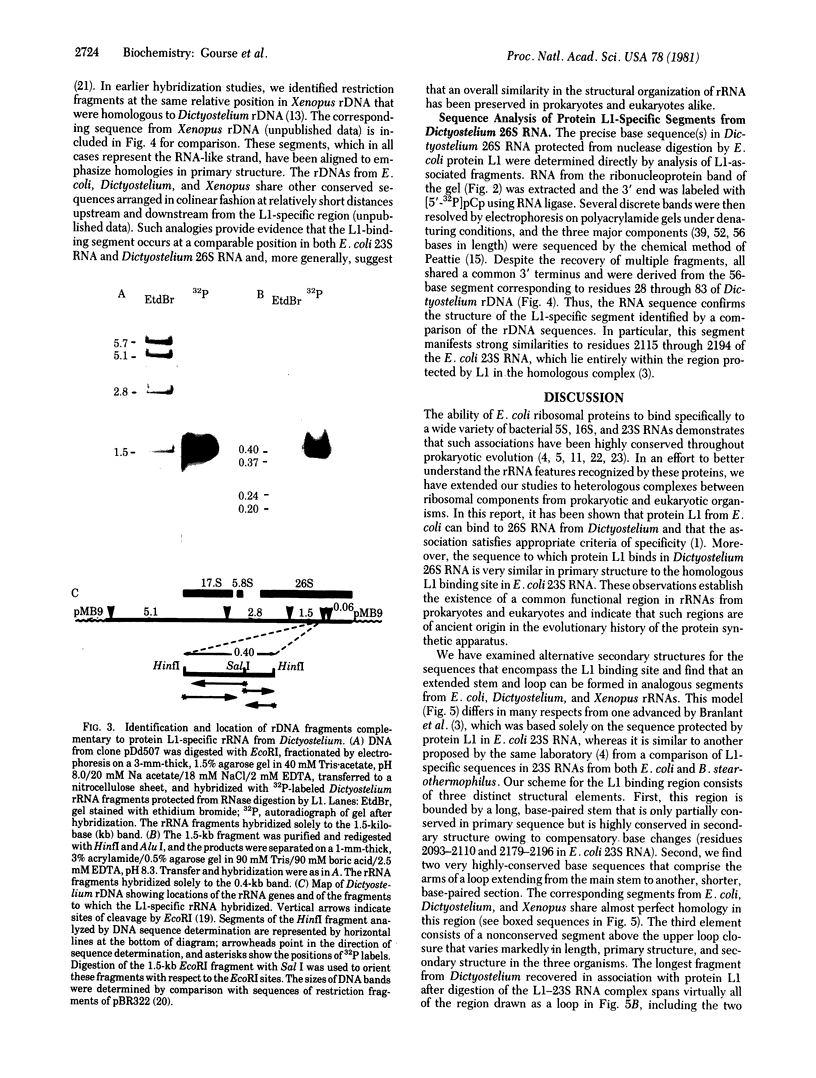
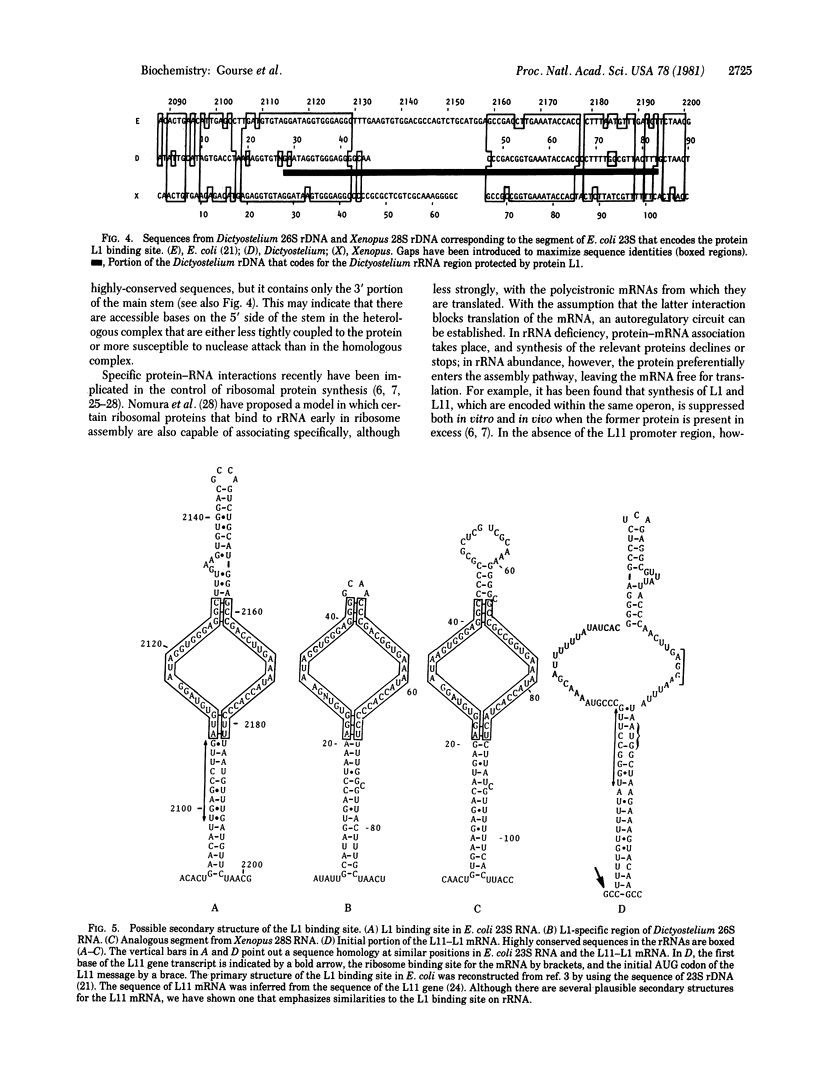
Ribosomal protein L1 from the prokaryote Escherichia coli has been shown to form a specific complex with 26S ribosomal RNA from the eukaryote Dictyostelium discoideum. The segment of Dictyostelium rRNA protected from ribonuclease digestion by L1 and the corresponding region in Dictyostelium rDNA were investigated by nucleotide sequence analysis, and an analogous section in rDNA from Xenopus laevis was identified. When the L1-specific segments from eukaryotic rRNA were compared with those from prokaryotic rRNA, striking similarities in both primary and secondary structure were apparent. These conserved features suggest a common structural basis for protein recognition and indicate that such regions became fixed at a very early stage in rRNA evolution. In addition, certain structural elements of the L1 binding sites in rRNA are also found in the initial segment of the polycistronic L11-L1 mRNA, providing support for the hypothesis that L1 participates in the regulation of ribosomal protein synthesis by specific interaction with its own mRNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branlant C., Korobko V., Ebel J. P. The binding site of protein L1 on 23-S ribosomal RNA from Escherichia coli. 3. Nucleotide sequence. Eur J Biochem. 1976 Nov 15;70(2):471–482. doi: 10.1111/j.1432-1033.1976.tb11038.x. [DOI] [PubMed] [Google Scholar]
- Branlant C., Krol A., Machatt A., Ebel J. P. The secondary structure of the protein L1 binding region of ribosomal 23S RNA. Homologies with putative secondary structures of the L11 mRNA and of a region of mitochondrial 16S rRNA. Nucleic Acids Res. 1981 Jan 24;9(2):293–307. doi: 10.1093/nar/9.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Noller H. F. Complete nucleotide sequence of a 23S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):201–204. doi: 10.1073/pnas.77.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daya-Grosjean L., Geisser M., Stöffler G., Garret R. A. Heterologous protein-RNA interactions in bacterial ribosomes. FEBS Lett. 1973 Nov 15;37(1):17–20. doi: 10.1016/0014-5793(73)80416-8. [DOI] [PubMed] [Google Scholar]
- Dean D., Nomura M. Feedback regulation of ribosomal protein gene expression in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3590–3594. doi: 10.1073/pnas.77.6.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke J., Kessin R. A defined minimal medium for axenic strains of Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1977 May;74(5):2157–2161. doi: 10.1073/pnas.74.5.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel G., Cockburn A. F., Kindle K. L., Firtel R. A. Organization of the ribosomal RNA genes of Dictyostelium discoideum. Mapping of the transcribed region. J Mol Biol. 1977 Feb 5;109(4):539–558. doi: 10.1016/s0022-2836(77)80090-9. [DOI] [PubMed] [Google Scholar]
- Garret R. A., Müller S., Spierer P., Zimmermann R. A. Letter: Binding of 50 S ribosomal subunit proteins to 23 S RNA of Escherichia coli. J Mol Biol. 1974 Sep 15;88(2):553–557. doi: 10.1016/0022-2836(74)90503-8. [DOI] [PubMed] [Google Scholar]
- Geisser M., Mackie G. A. The binding site of Escherichia coli ribosomal protein S4 on 16-S ribosomal RNA from different bacterial species. Eur J Biochem. 1976 Nov 1;70(1):159–170. doi: 10.1111/j.1432-1033.1976.tb10966.x. [DOI] [PubMed] [Google Scholar]
- Gourse R. L., Gerbi S. A. Fine structure of ribosomal RNA. III. Location of evolutionarily conserved regions within ribosomal DNA. J Mol Biol. 1980 Jun 25;140(2):321–339. doi: 10.1016/0022-2836(80)90109-6. [DOI] [PubMed] [Google Scholar]
- Gourse R. L., Gerbi S. A. Fine structure of ribosomal RNA. IV. Extraordinary evolutionary conservation in sequences that flank introns in rDNA. Nucleic Acids Res. 1980 Aug 25;8(16):3623–3637. doi: 10.1093/nar/8.16.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn L. J., Queen C. L., Wegman M. N. Computer analysis of nucleic acid regulatory sequences. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4401–4405. doi: 10.1073/pnas.74.10.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl L., Zengel J. M. Operon-specific regulation of ribosomal protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6542–6546. doi: 10.1073/pnas.76.12.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Morrison C. A., Garrett R. A., Zeichhardt H., Stöffler G. Proteins occurring at, or near, the subunit interface of E. coli ribosomes. Mol Gen Genet. 1973 Dec 31;127(4):359–368. doi: 10.1007/BF00267106. [DOI] [PubMed] [Google Scholar]
- Nomura M., Yates J. L., Dean D., Post L. E. Feedback regulation of ribosomal protein gene expression in Escherichia coli: structural homology of ribosomal RNA and ribosomal protein MRNA. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7084–7088. doi: 10.1073/pnas.77.12.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post L. E., Strycharz G. D., Nomura M., Lewis H., Dennis P. P. Nucleotide sequence of the ribosomal protein gene cluster adjacent to the gene for RNA polymerase subunit beta in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1697–1701. doi: 10.1073/pnas.76.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M. L., Olsen G. J. Identification and mapping of a 60 bp EcoRI fragment in the Dictyostelium discoideum ribosomal DNA. Gene. 1980 Feb;8(3):231–238. doi: 10.1016/0378-1119(80)90001-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanley J., Sloof P., Ebel J. P. The binding site of ribosomal protein L1 from Escherichia coli on the 23-S ribosomal RNA from Bacillus stearothermophilus. A possible base-pairing scheme differing from that proposed for Escherichia coli. Eur J Biochem. 1978 Apr;85(1):309–316. doi: 10.1111/j.1432-1033.1978.tb12240.x. [DOI] [PubMed] [Google Scholar]
- Subramanian A. R., Dabbs E. R. Functional studies on ribosomes lacking protein L1 from mutant Escherichia coli. Eur J Biochem. 1980 Nov;112(2):425–430. doi: 10.1111/j.1432-1033.1980.tb07222.x. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlow D. L., Zimmermann R. A. Conservation of ribosomal protein binding sites in prokaryotic 16S RNAs. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2859–2863. doi: 10.1073/pnas.75.6.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. L., Arfsten A. E., Nomura M. In vitro expression of Escherichia coli ribosomal protein genes: autogenous inhibition of translation. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1837–1841. doi: 10.1073/pnas.77.4.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. L., Nomura M. E. coli ribosomal protein L4 is a feedback regulatory protein. Cell. 1980 Sep;21(2):517–522. doi: 10.1016/0092-8674(80)90489-4. [DOI] [PubMed] [Google Scholar]
- Zengel J. M., Mueckl D., Lindahl L. Protein L4 of the E. coli ribosome regulates an eleven gene r protein operon. Cell. 1980 Sep;21(2):523–535. doi: 10.1016/0092-8674(80)90490-0. [DOI] [PubMed] [Google Scholar]
- Zimmermann R. A. Protein-RNA interactions in the bacterial ribosome. Methods Enzymol. 1979;59:551–583. doi: 10.1016/0076-6879(79)59113-7. [DOI] [PubMed] [Google Scholar]