Abstract
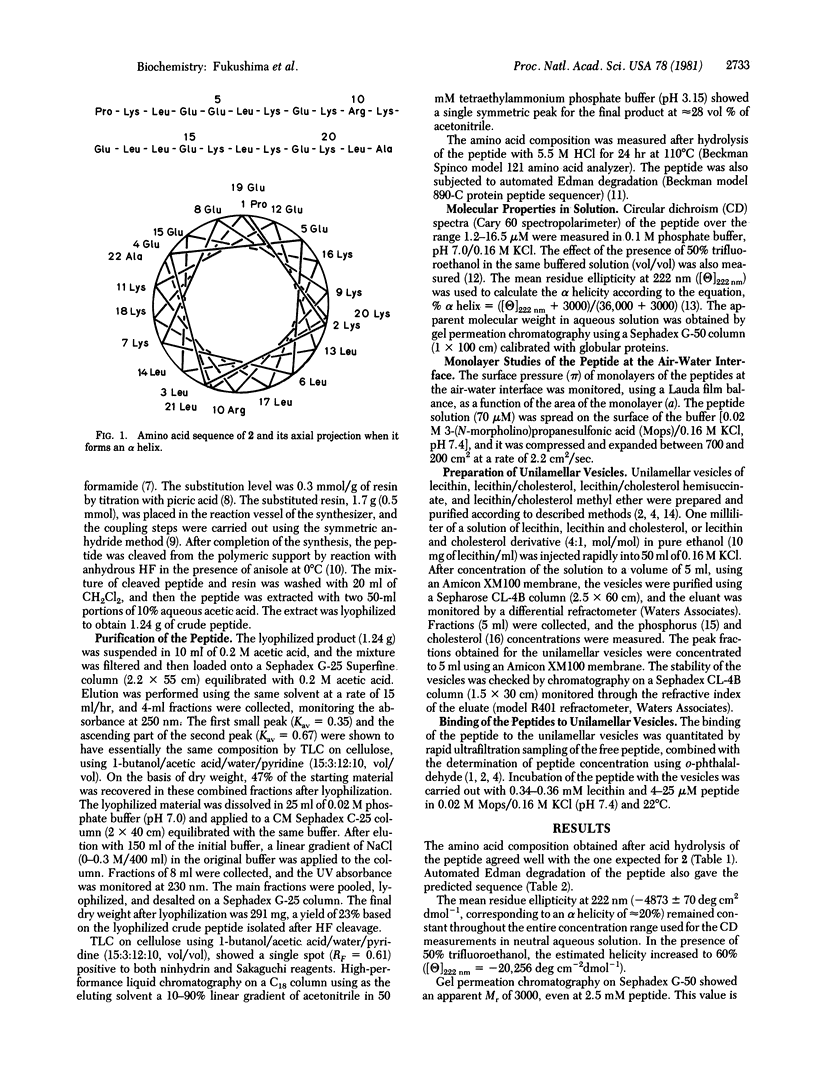
In earlier studies, we prepared a docosapeptide, 1, designed with minimum homology as an amphiphilic alpha-helical model of apolipoprotein A-I (apo A-I) and described its lipid-binding characteristics, surface properties, and enzyme-activating ability. Although the affinity of 1 for egg lecithin unilamellar vesicles was comparable with that for the binding of apo A-I, the affinity of 1 for mixed lecithin/cholesterol (4:1 mol/mol) vesicles was less than that of apo A-I. It appeared possible that the 3-hydroxyl group of cholesterol may have a deleterious interaction with the hydrophobic portion of the amphiphilic helix of 1 that is inserted into the vesicles. Examination of the amphiphilic alpha-helical segments of apo A-I suggested that the preferential interaction of apo A-I with the mixed vesicles might be due to the presence of polar arginine residues in the otherwise hydrophobic regions of two of the helices. Therefore, we synthesized a model docosapeptide, 2, corresponding to the sequence of 1 but containing arginine rather than leucine at position 10 in the hydrophobic region of the alpha helix to assess the role of the alcohol function of cholesterol in protein--cholesterol interactions. The results of studies on the binding of 2 to unilamellar vesicles containing lecithin only, lecithin/cholesterol, lecithin/cholesterol hemisuccinate, or lecithin/cholesterol methyl ether were consistent with the postulate that the major role of cholesterol in the binding of proteins to phospholipid surfaces is the creation of free space between the phospholipid head groups that can accommodate the amphiphilic peptide chains at the interface.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Batzri S., Korn E. D. Single bilayer liposomes prepared without sonication. Biochim Biophys Acta. 1973 Apr 16;298(4):1015–1019. doi: 10.1016/0005-2736(73)90408-2. [DOI] [PubMed] [Google Scholar]
- COURCHAINE A. J., MILLER W. H., STEIN D. B., Jr Rapid semi-micro procedure for estimating free and total cholesterol. Clin Chem. 1959 Dec;5:609–614. [PubMed] [Google Scholar]
- Edelstein C., Kézdy F. J., Scanu A. M., Shen B. W. Apolipoproteins and the structural organization of plasma lipoproteins: human plasma high density lipoprotein-3. J Lipid Res. 1979 Feb;20(2):143–153. [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Gisin B. F. The monitoring of reactions in solid-phase peptide synthesis with picric acid. Anal Chim Acta. 1972 Jan;58(1):248–249. doi: 10.1016/S0003-2670(00)86882-8. [DOI] [PubMed] [Google Scholar]
- Lala A. K., Buttke T. M., Bloch K. On the role of the sterol hydroxyl group in membranes. J Biol Chem. 1979 Nov 10;254(21):10582–10585. [PubMed] [Google Scholar]
- Morrisett J. D., David J. S., Pownall H. J., Gotto A. M., Jr Interaction of an apolipoprotein (apoLP-alanine) with phosphatidylcholine. Biochemistry. 1973 Mar 27;12(7):1290–1299. doi: 10.1021/bi00731a008. [DOI] [PubMed] [Google Scholar]
- Tan N. H., Kaiser E. T. Studies on the solid-phase synthesis of bovine pancreatic trypsin inhibitor (Kunitz) and the characterization of the synthetic material. J Org Chem. 1976 Aug 20;41(17):2787–2793. doi: 10.1021/jo00879a001. [DOI] [PubMed] [Google Scholar]
- Yokoyama S., Fukushima D., Kupferberg J. P., Kézdy F. J., Kaiser E. T. The mechanism of activation of lecithin:cholesterol acyltransferase by apolipoprotein A-I and an amphiphilic peptide. J Biol Chem. 1980 Aug 10;255(15):7333–7339. [PubMed] [Google Scholar]


