Abstract
Objective
To compare and quantify the retinal vascular changes induced by non-intentional pressure contact by digital handheld camera during retinopathy of prematurity (ROP) imaging by means of a computer-based image analysis system, Retinal Image multiScale Analysis.
Methods
A set of 10 wide-angle retinal pairs of photographs per patient, who underwent routine ROP examinations, was measured. Vascular trees were matched between ‘compression artifact' (absence of the vascular column at the optic nerve) and ‘not compression artifact' conditions. Parameters were analyzed using a two-level linear model for each individual parameter for arterioles and venules separately: integrated curvature (IC), diameter (d), and tortuosity index (TI).
Results
Images affected with compression artifact showed significant vascular d (P<0.01) changes in both arteries and veins, as well as in artery IC (P<0.05). Vascular TI remained unchanged in both groups.
Conclusions
Non-adverted corneal pressure with the RetCam lens could compress and decrease intra-arterial diameter or even collapse retinal vessels. Careful attention to technique is essential to avoid absence of the arterial blood column at the optic nerve head that is indicative of increased pressure during imaging.
Keywords: ROP, telemedicine, retinopathy of prematurity
Introduction
During the 21st century, telemedicine has been established as a reliable tool in retinopathy of prematurity (ROP) screening. Because of a lack of ophthalmologists specialized in ROP eye care, retinal imaging with a wide-field digital system (RetCam) has become the standard in many Neonatal Intensive Care Units (NICUs). RetCam imaging has demonstrated many advantages during ROP screening as the premature babies are examined within the American Academy of Pediatrics schedule recommendations, preventing examination delays and unnecessary patient transfer outside NICUs. Furthermore, the use of computer-based image analysis has shown the potential to provide quantifiable and objective measurements to support the diagnosis of plus disease.1
The RetCam can be operated by ophthalmologists, as well as by trained nurses and ophthalmic technicians. To date, there are few ocular adverse events related to RetCam operation.2 Caution should be taken, especially when a handheld camera is placed over the cornea. Previous papers have reported masking of ROP type 1 because of vessels collapsing secondary to corneal overpressure,3, 4 yet to our knowledge no previous studies have attempted to compare and quantify the differences on vessel parameters due to non-adverted pressure during digital contact imaging.
The purpose of this paper is to compare and quantified whether significant retinal vascular changes can be induced by non-intentional handheld camera pressure during RetCam imaging using a computer-based image analysis system, Retinal Image multiScale Analysis (RISA).5, 6
Materials and methods
This study was reviewed and approved by the Ethics Committee of the Hospital Civil de Guadalajara. Parents signed informed consent forms. All patients were followed up by a pediatric ophthalmologist weekly until resolution of ROP due to full retinal vascularization.
Image acquisition
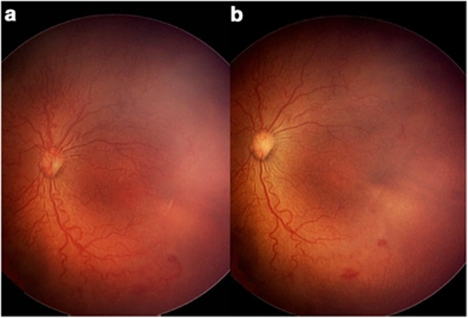
Clinical pairs of images of 10 patients who underwent routine ROP screening at Hospital Civil de Guadalajara were selected for this study (10 pairs, N=20 images). These infants had been born at 27–32 (29.4±1.71) weeks, 700–1642 (1164.7±282.97) g, and were 35- to 41 (35.83±2.56)-week old when images were taken. The 10 cases were selected because the same session of photographs recorded during the RetCam examination for each patient showed subjectively different diameters of the vessels, and different expression of the plus disease, the difference being more noticeable when the vascular column at the optic nerve was absent (Figure 1). The first image of every series was selected to show vascular abnormalities in the posterior pole sufficient to be called plus disease; the second image was selected because of the presence of compression artifact (absence of the optic nerve vascular column).
Figure 1.
Pairs of images per patient. (a) Left eye fundus image of a premature infant born at 31.3 weeks, weighing 1150 g, and examined at the fourth week of the extra uterine life, showing aggressive posterior ROP with dilatation and tortuosity of venules and arterioles in the four quadrants, multiple shunts, and circumferential demarcation vessel. (b) Same session picture with signs of compression artifact that shows tortuosity and diminished dilatation of venules and arterioles, circumferential vessel is not evident. Note the absence of blood column at the optic disc and whitening of the underlying retina.
The two set of images selected per patient were taken by one of the authors (LCZR) using digitalized imaging system RetCam II (Clarity Medical Systems, Pleasanton, CA, USA); the handheld camera was placed on a bed of carboxymethylcellulose and hypromellose eye ointment (Gen Teal gel, Novartis Pharmaceutical Corporation, East Hanover, NJ, USA) trying to avoid inadverted corneal touch. To examine the babies, the infant's pupils were previously dilated with two separated doses of tropicamide 0.5% combined with phenylephrine 2.5% eye drops. Vital signs were closely monitored during all eye examinations by an experienced neonatologist.
Computer-based image analysis
Each image is prepared for analysis by cropping to select the vessel trees of interest. RISA meets the following steps: segmentation, skeleton construction, selection of vessel root, and tracking. Segmentation involves extracting the vessels of interest from the background. Skeleton construction involves reducing the segmented vessel to a one-pixel-width tree; terminal, crossing, and bifurcation points are marked. The skeleton is then tracked from the optic disc, and each portion of the vessel between a terminal and a bifurcation or a bifurcation and a bifurcation is assigned a unique identifier. Each portion is termed a vessel segment. RISA operates on vascular trees and requires at least one bifurcation.5, 6
Three system parameters were calculated for each vessel in each image: integrated curvature (IC), diameter (d), and tortuosity index (TI). IC (radians/pixel) is defined as the sum of angles along the skeleton, normalized by length of the vessel; diameter (pixels) is the total area of the vessel divided by its length; and TI is the length of the vessel divided by the length of a line segment connecting its end points. For further details refer to Gelman et al1 and Martinez-Perez et al.6
Images have a resolution of 640 × 480 pixels and 24-bit RGB color. The average diameter of the optic nerve head in these images was 40.6 pixels. If the physical diameter of the optic nerve head is assumed to be 1.015 mm at <40 week gestation,8 then one pixel in these images represents an average of 25.00 μm physical distance.
The same vascular trees were selected from each patient's pair of pictures to measure and compare the parameters IC, d, and TI with and without compression artifact. Trees were paired only where vessel segments matched.
Statistical model
To compare vascular changes induced by compression artifact, we fitted a two-level linear model for each variable (IC, d, and TI), separating arteries from veins using as levels the subjects and their vascular segments. We used as an explanatory variable the compression artifact (CA). Gestation age and weight at birth, as well as the age at which photographs were taken, were considered as possible covariates.
This model allows us to control the fact that within subject the observations are not independent, and to take into account that the same vessel segment length was measured with and without compression artifact, given the fact that the exact amount of pressure cannot be determined. Regression model analysis was performed using Spotfire S+ software (TIBCO Software Inc., Somerville, MA, USA).
Results
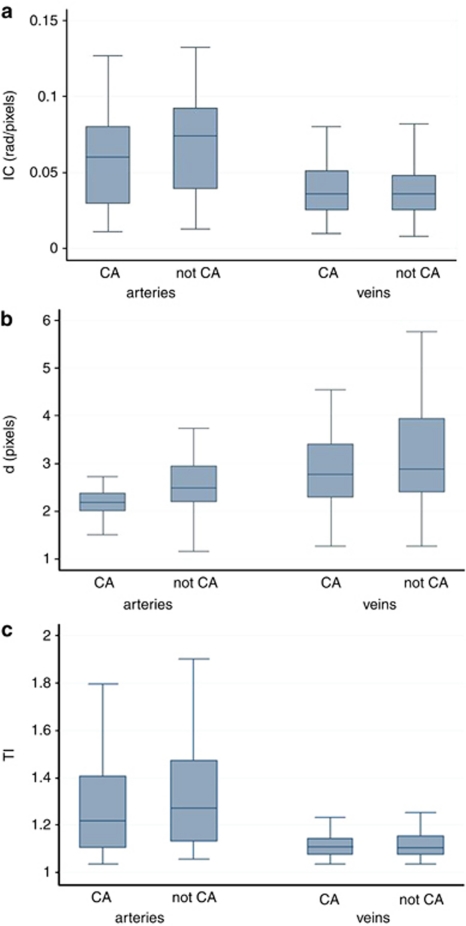
Only 2 out of 69 arterioles could not be measured because of collapse in the compression artifact pictures. Compression artifact induced during RetCam imaging produced significant vascular d (P<0.01) changes in both arteries and veins, as well as artery IC (P<0.05). The vascular TI remained unchanged in both groups. Table 1 summarizes the statistical results of the two-level linear model. Figure 2 shows that the mean and percentile values of each individual system parameter, with the exception of TI, were significantly higher in ‘not-compression artifact' images than ‘compression artifact' ones.
Table 1. Two-level linear model for each parameter.
|
Arteries |
Veins |
|||
|---|---|---|---|---|
| Difference | P-value | Difference | P-value | |
| IC | −0.0055 | 0.0438 | 0.0003 | 0.9286 |
| d | −0.1879 | <0.0001 | −0.1354 | 0.0070 |
| TI | −0.0228 | 0.0831 | 0.0017 | 0.5880 |
Abbreviations: IC, integrated curvature; d, diameter; TI, tortuosity index. The table reports the differences in IC, d and TI when the compression artifact is present. We found that this difference is significantly different than zero in the case of d for both arteries and veins, and significantly different in the case of IC only for arteries. The difference is marginally significant for TI, again only in the case of arteries.
Bold values represent significant differences P<0.05.
Figure 2.
Box plots of computer-based system parameter values in images with ‘not compression artifact' (not-CA) compared with ‘compression artifact' (CA). Individual parameters of (a) integrated curvature, IC, (b) diameter, d, and (c) tortuosity index, TI, for arterioles and venules are displayed. Whiskers represent 10th and 90th percentile values.
No RetCam imaging had to be aborted because of cardiovascular or respiratory changes during examination.
Discussion
The growing lack of ophthalmologists available for examining premature patients at risk of ROP is well known. Telemedicine strategies focussing on ROP screening could in part solve this problem. RetCam imaging performed by certified personnel has demonstrated satisfactory results in telemedicine identification of ROP.7 However, RetCam operators should be notified of this potential image misinterpretation during RetCam imaging, because vascular changes induced by unnoticed eye overpressure can mask plus disease. Variations in pressure induced by inadvertent indentation with the RetCam lens could compress or decrease intra-arterial diameter or even collapse retinal vessels. Evaluation of images of plus disease may then be misinterpreted giving a false-negative result, with a subsequent delay in detection of serious retinopathy. Careful attention to the imaging technique is essential to eliminate blanching of the choroid or absence of the arterial blood column at the optic nerve head, which might indicate excessive pressure during imaging. Images showing signs of compression artifact should be avoided as a document of the ROP vascular status, as this could prevent accurate interpretation and diagnosis of plus disease.
The authors declare no conflict of interest.
References
- Gelman R, Martinez-Perez ME, Vanderveen DK, Moskowitz A, Fulton AB. Diagnosis of plus disease in retinopathy of prematurity using Retinal Image multiScale Analysis. Invest Ophthalmol Vis Sci. 2005;46:4734–4738. doi: 10.1167/iovs.05-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams GGW, Clark BJ, Fanh BJ, Hill M. Retinal haemorrhages in an infant following RetCam screening for retinopathy of prematurity. Eye. 2004;18:652–653. doi: 10.1038/sj.eye.6700728. [DOI] [PubMed] [Google Scholar]
- Zepeda-Romero LC, Martinez-Perez ME, Ramírez-Ortiz MA, Gutierrez-Padilla JA. RetCam compression artifact can mask plus disease. Eye. 2009;23:2266–2267. doi: 10.1038/eye.2009.12. [DOI] [PubMed] [Google Scholar]
- Koreen S, Lopez R, Jokl DH, Flynn JT, Chiang MF. Variation in appearance of severe zone 1 retinopathy of prematurity during wide-angle contact photography. Arch Ophtalmol. 2008;126 (5:736–737. doi: 10.1001/archopht.126.5.736. [DOI] [PubMed] [Google Scholar]
- Martinez-Perez ME, Hughes AD, Thom SA, Bharath AA, Parker KH. Segmentation of Blood Vessels from Red-free and Fluorescein Retinal Images. Med Image Anal. 2007;11 (1:47–61. doi: 10.1016/j.media.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Martinez-Perez ME, Hughes AD, Stanton AV, Thom SA, Chapman N, Bharath AA, Parker KH. Retinal vascular tree morphology: a semi-automatic quantification. IEEE Trans Biomed Eng. 2002;49:912–917. doi: 10.1109/TBME.2002.800789. [DOI] [PubMed] [Google Scholar]
- Photographic Screening for Retinopathy of Prematurity Cooperative Group The Photographic Screening for Retinopathy of Prematurity Study (Photo ROP): Primary Outcomes. Retina. 2008;28 (3:S47–S54. doi: 10.1097/IAE.0b013e31815e987f. [DOI] [PubMed] [Google Scholar]
- Rimmer S, Keating C, Chou T, Farb MD, Christenson PD, Foos RY, Bateman JB. Growth of the human optic disk and nerve during gestation, childhood, and early adulthood. Am J Ophthalmol. 1993;116:748–753. doi: 10.1016/s0002-9394(14)73476-2. [DOI] [PubMed] [Google Scholar]