Abstract
Purpose
To evaluate the safety and visual outcomes of two phakic intraocular lenses (IOLs) for correction of high myopia: Artisan and Visian ICL (ICL).
Patients and methods
In this retrospective study, a phakic IOL was implanted in 68 highly myopic eyes of 34 patients; 42 eyes received an Artisan IOL, and 26 eyes received ICL IOL.
Results
All patients completed a 1-year follow-up. The mean preoperative spherical equivalent (SEQ) was −12.89±3.78, and −12.44±4.15 diopters (D) for Artisan and ICL (P=0.078), respectively. The mean postoperative (1-year) uncorrected distance visual acuity was 0.39±0.13 and 0.41±0.15 logMAR for Artisan and ICL, respectively (P=0.268). The mean postoperative (1-year) corrected distance visual acuity was 0.36±0.12 and 0.31±0.12 logMAR for Artisan and ICL, respectively (P=0.128). The mean postoperative SEQ was −0.86±0.5 and −0.63±0.38 D for Artisan and ICL, respectively (P=0.67). Intraocular pressure change at 1 year was 0.64±2.7 and 1.88±0.6 mm Hg for Artisan and ICL, respectively (P=0.77).
Conclusion
Artisan and ICL showed equal and comparable safety, predictability, and efficacy.
Keywords: Artisan, Visian ICL, phakic IOLs
Introduction
Keratorefractive surgeries, such as photorefractive keratectomy and LASIK, have limitations when used for the correction of high refractive errors.1, 2, 3 Intraocular refractive procedures offer many potential advantages: a broader range of treatable ametropia, faster visual recovery, more stable refraction, and better visual quality.4, 5, 6 Two basic intraocular refractive procedures exist: phakic intraocular lens (pIOL) implantation, and clear lens extraction with lens implantation. Refractive lens exchange may increase the risk for retinal detachment,7 and is generally not considered in myopic pre-presbyopic patients who can still accommodate.
The risks and benefits of pIOL implantation in appropriate patients may be more favorable than other refractive surgery techniques. The pIOL is removable surgically, with fast visual recovery, and preserved accommodation. However, it is important to realize that complications relating to pIOLs can be more disabling than those from keratorefractive surgery.3 Several generations of both anterior and posterior pIOLs have been introduced in the past few years.
Multiple studies from different parts of the world addressed the visual outcomes of both procedures, but only few studies were conducted in the Middle East. The purpose of this assessment was to review the safety and outcomes of two pIOLs, which are currently approved by the FDA in our hospital. The Verisyse phakic IOL marketed internationally as the Artisan lens by Ophtec (Boca Raton, FL, USA; FDA approval 2004), and the Visian ICL (ICL), manufactured by STAAR Surgical Company (Monrovia, CA, USA; FDA approval 2005).
Patients and methods
Approval for the study was obtained from the hospital's ethical committee, and followed the tenets of the Declaration of Helsinki.
This retrospective comparative study was conducted in Al Nour Eye Hospital, searching files of all patients who underwent pIOL surgeries by either Artisan or ICL in the period from January 2007 to December 2009. This study included 68 eyes of 34 patients, 42 eyes were implanted with Artisan lens and 26 eyes were implanted with ICL. All patients completed a follow-up period of 1 year. The pIOL chosen for each patient was selected based only on the surgeons' preferences, and there were no specific parameters used to decide which pIOL for each patient.
Preoperative evaluation for implantation
The preoperative evaluation of patients consists of a complete ophthalmologic examination, including a manifest and, where appropriate, cycloplegic refraction, corrected distance visual acuity (CDVA), white to white (W–W) measurement by caliper, slit-lamp biomicroscopy, central corneal thickness measurement, endothelial cell count, keratometry, axial eye length measurement, tonometry, anterior chamber depth, measurement of mesopic pupil diameter, and indirect ophthalmoscopy. A thorough peripheral retinal examination is necessary to rule out retinal tears, especially in highly myopic eyes.
Exclusion criteria included the presence of hyperopia, cataract, glaucoma or ocular hypertension, history of retinal detachment, corneal affection, pupil abnormalities, endothelial cell count less than 2000 mm2, uveitis, less than 20 years old, or unstable refraction.
Operative technique
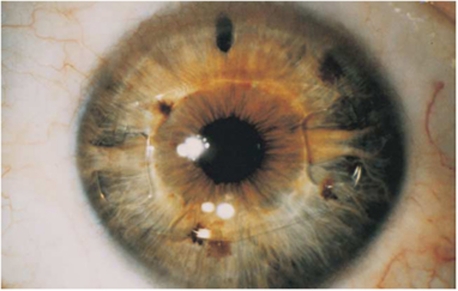
For eyes undergoing implantation of anterior-chamber, iris-fixated pIOLs, the pupil was constricted with miotic drops, and the procedure was performed under peribulbar anesthesia. Two paracenteses were created, and the anterior chamber was filled with viscoelastic. A limbal incision was made, usually in the steepest corneal meridian, which is approximately equal to the lens optic diameter. The pIOL was inserted and rotated into a horizontal position. A fold of the peripheral iris was then captured by the pincher-like lens haptics in a process called enclavation. A peripheral surgical iridotomy was performed. The incision was closed with an appropriate suture and the viscoelastic was then removed. Generally, the procedure on the other eye follows in 1 or 2 weeks (Figure 1).
Figure 1.
Artisan phakic intraocular lens postoperative.
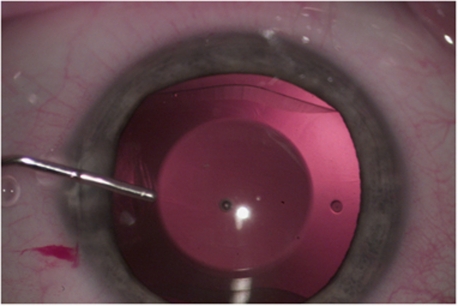
For eyes undergoing implantation of posterior-chamber pIOLs, the pupil was dilated with mydriatic drops, and the procedure was performed under peribulbar anesthesia. A 3.2-mm temporal clear corneal incision was created, as well as 1 or 2 paracenteses. The anterior chamber was filled with viscoelastic. The pIOLs was then injected into the anterior chamber, anterior and parallel to the iris plane, and allowed to unfold. Each corner of the footplates was gently tucked beneath the iris. Once the pIOL is well positioned, the viscoelastic was removed, a peripheral surgical iridotomy was performed, and the corneal wound was checked for integrity. Generally, the procedure on the other eye follows in 1 or 2 weeks (Figure 2).
Figure 2.
Visian ICL phakic intraocular lens intraoperative.
Postoperative management
Follow-up examinations are typically scheduled at 1 day, 1 week, 1 month, 3 months, 6 months, and 1 year after surgery. Postoperative examinations included slit-lamp biomicroscopy, keratometry, applanation tonometry, subjective and objective refraction, and measurement of uncorrected distance visual acuity (UDVA), and CDVA. Within the first six-postoperative weeks, the sutures were cut or removed if it has created undesirable corneal astigmatism in cases implanted with Artisan lens. Each procedure safety index and efficacy index were calculated as follows: safety index=mean postoperative CDVA/mean preoperative CDVA, and efficacy index=mean postoperative UDVA/mean preoperative CDVA (using decimals for visual acuity).
Data were statistically described in terms of range, mean±SD, and median when appropriate. Visual acuity was converted to logMAR for proper statistical analysis. Comparison between the study groups was done using Mann–Whitney U-test for independent samples. Correlation between various variables was done using Spearman rank correlation equation for non-normal variables. P-values <0.05 was considered statistically significant. All statistical calculations were done using computer programs Microsoft Excel 2007 (Microsoft Corporation, New York, NY, USA), and SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) version 15 for Microsoft Windows.
Results
Sixty-eight eyes of 34 patients were included in this retrospective study; 42 eyes received Artisan pIOLs, and 26 eyes received ICL pIOLs. All patients completed the follow-up period of one year. The mean age was 25.85 years (range 20–38), and 29.85 years (range 21–39) for the Artisan and ICL, respectively. The male to female ratio was 2 : 1 (n=14 : 7), and 3 : 1 (n=10 : 3) for the Artisan and ICL, respectively. Tables 1 and 2 show the baseline characteristics for both groups. The mean preoperative sphere, cylinder, spherical equivalent (SEQ) were −12.15±0.7, −1.69±0.8, and −12.89±3.78 D, and −11.41±2.0, −1.87±2.2, and −12.44±4.15 D for the Artisan and ICL (P=0.078), respectively. The mean preoperative CDVA was 0.42±0.23 and 0.40±0.24 logMAR for the Artisan and ICL (P=0.402), respectively. The mean anterior chamber depth was 3.45±0.41 and 3.4±0.3 mm for Artisan and ICL (P=0.675), respectively. The mean preoperative IOP was 15.4±4.0 (10–19) and 16.2±3.4 (11–18) mm Hg for the Artisan and ICL (P=0.247), respectively.
Table 1. The mean, SD, and range of the CDVA, UDVA, sphere, cylinder, SEQ, SEQ change, and IOP change for the Artisan group at baseline, and at 1 week, 1, 3, 6, and 12 months postoperative.
| Artisan | Sphere (diopters) | Cylinder (diopters) | SEQ (diopters) | CDVA (logMAR) | UDVA (logMAR) | IOP change (mm Hg) | Change in SEQ (diopters) |
|---|---|---|---|---|---|---|---|
| Pre-operative | −12.15±0.7 (−7.5 to−21) | −1.69±0.8 (−0.25 to −2.5) | −12.89±3.78 (−8 to −22) | 0.42±0.23 (0.22–1.3) | — | — | — |
| 1-week | −0.27±0.9 (+1.25 to −1.00) | −1.45±0.9 (−0.75 to −4.00) | −1.14±0.8 (+0.5 to −1.75) | 0.41±0.26 (0.22–0.9) | 0.43±0.12 (0.22–0.9) | 0.85±4.1 (0–8)a | 11.83±6.61 (7–20) |
| 1-month | −0.24±0.6 (+0.5 to −1.25) | −1.13±0.7 (−0.25 to −3.00) | −0.84±0.6 (+0.25 to −1.5) | 0.39±0.16 (0.22–0.8) | 0.43±0.12 (0.22–0.8) | 0.61±2.1 (0–5) | 12.83±3.61 (7.5–20.5) |
| 3-months | −0.23±0.3 (+0.5 to −1.5) | −1.17±0.4 (−0.5 to −1.75) | −0.89±0.7 (+0.75 to −1.5) | 0.36±0.15 (0.22–0.7) | 0.39±0.15 (0.22–0.7) | 0.64±2.7 (0–3) | 12.86±3.71 (7.5–20.5) |
| 6-months | −0.25±0.4 (+0.75 to −1.25) | −1.16±0.4 (−0.25 to −1.25) | −0.85±0.5 (+0.75 to −1.5) | 0.36±0.13 (0.22–0.7) | 0.39±0.14 (0.22–0.7) | 0.59±2.1 (0–7) | 12.83±3.61 (7.5–20.5) |
| 12-months | −0.26±0.4 (+0.75 to −1. 5) | −1.17±0.4 (−0.25 to −1.25) | −0.86±0.5 (+0.75 to −1.5) | 0.36±0.12 (0.22–0.7) | 0.39±0.13 (0.22–0.7) | 0.64±2.7 (0–6) | 12.86±3.71 (7.5–20.5) |
Abbreviations: CDVA, corrected distance visual acuity (in logMAR); UDVA, uncorrected distance visual acuity (in logMAR); SEQ, spherical equivalent.
IOP change without the patient with malignant glaucoma.
Table 2. The mean, SD, and range of the CDVA, UDVA, sphere, cylinder, SEQ, SEQ change, and IOP change for the ICL group at baseline, and at 1 week, 1, 3, 6, and 12 months postoperative.
| ICL | Sphere (diopters) | Cylinder (diopters) | SEQ (diopters) | CDVA (logMAR) | UDVA (logMAR) | IOP change (mm Hg) | Change in SEQ (diopters) |
|---|---|---|---|---|---|---|---|
| Pre-operative | −11.41±2.0 (−6 to −20) | −1.87±2.2 (−0.25 to −2.75) | −12.44±4.15 (−7 to −21) | 0.40±0.24 (0.15–1.3) | — | — | — |
| 1-week | −0.43±0.8 (+0.5 to −1.75) | −0.94±0.9 (−0.25 to −2.25) | −0.82±0.64 (+0.5 to −2.25) | 0.39±0.14 (0.1–0.6) | 0.41±0.26 (0.1–0.8) | 1.98±1.9 (0–7)a | 10.84±5.4 (6–18.5) |
| 1-month | −0.25±0.4 (+0.25 to −1. 5) | −0.71±0.5 (−0. 5 to −2.25) | −0.62±0.34 (+0.25 to −2.25) | 0.33±0.14 (0.1–0.6) | 0.42±0.16 (0.1–0.6) | 1.63±0.5 (0–5) | 11.14±3.4 (6–18.5) |
| 3-months | −0.21±0.5 (+0.25 to −1. 75) | −0.69±0.4 (−0.25 to −1.75) | −0.62±0.34 (+0.5 to −1.75) | 0.31±0.13 (0.1–0.5) | 0.41±0.16 (0.1–0.7) | 1.68±0.3 (0–7) | 11.63±5.4 (6–19) |
| 6-months | −0.23±0.3 (+0.25 to −1.75) | −0.74±0.3 (−0.25 to −2.00) | −0.62±0.34 (+0.25 to −2.00) | 0.31±0.13 (0.1–0.5) | 0.41±0.15 (0.1–0.7) | 1.68±0.4 (0–6) | 11.64±4.7 (6–19) |
| 12-months | −0.24±0.3 (+0.25 to −1.75) | −0.84±0.4 (−0.25 to −2.00) | −0.63±0.38 (+0.5 to −2.25) | 0.31±0.12 (0.1–0.5) | 0.41±0.15 (0.1–0.7) | 1.88±0.6 (0–5) | 11.69±4.2 (6.5–19) |
Abbreviations: CDVA, corrected distance visual acuity (in logMAR); UDVA, uncorrected distance visual acuity (in logMAR); SEQ, spherical equivalent.
IOP change without the patient with oversized pIOL.
Postoperative period (first 1–2 weeks)
For the Artisan group at 1 week (Table 1), the mean postoperative sphere, cylinder, and SEQ were −0.27±0.9, −1.45±0.9, and −1.14±0.8 respectively. The mean CDVA and UDVA were 0.41±0.26 and 0.43±0.12 logMAR, respectively. The mean change of SEQ was 11.83±6.6 D. There was one case of malignant glaucoma on the second postoperative day with IOP 46 mm Hg, anterior vitrectomy was done immediately, and the IOP dropped back to 16 mm Hg. The mean change in IOP in first week, when excluding this case, is 0.85±4.1 mm Hg. Pigment dispersion occurred in 12 eyes (28.6%).
For the ICL group at 1 week (Table 2), the mean postoperative sphere, cylinder, and SEQ were −0.43±0.8, −0.94±0.9, and −0.82±0.64, respectively. The mean CDVA and UDVA were 0.39±0.14 and 0.41±0.26 logMAR, respectively. The mean change of SEQ was 10.84±5.4 D. There was one case of increased IOP due to over sizing of the pIOL on the third postoperative day with IOP 35 mm Hg and it was replaced safely by another pIOL, and the IOP dropped back to 14 mm Hg. The mean change in IOP in the first week when excluding this case is 1.98±1.9 mm Hg. Pigment dispersion occurred in two eyes (15.38%).
Follow-up period
Tables 1 and 2 show the patients CDVA, UDVA, refraction, and IOP changes at 1, 3, 6, and 12 months. For the Artisan group at 1 month, the mean CDVA and UDVA were, 0.39±0.16 and 0.43±0.12 logMAR, respectively. At 3 months, the mean CDVA and UDVA were 0.36±0.15 and 0.39±0.15 logMAR, respectively. Displaced pIOL occurred in one eye after 6 months, and reenclavation was done safely. At 1 year, the mean postoperative sphere, cylinder, and SEQ were −0.26±0.4, −1.17±0.4, and −0.86±0.5 D, respectively. The mean CDVA and UDVA were, 0.36±0.12 and 0.39±0.13 logMAR, respectively. The mean difference in intraocular pressure (IOP) between baseline and 1-year follow-up was 0.64±2.7 mm Hg.
For the ICL group at 1 month, the mean CDVA and UDVA were 0.33±0.14 and 0.42±0.16 logMAR, respectively. At 3 months, the mean CDVA and UDVA were 0.31±0.13 and 0.41±0.16 logMAR, respectively. The mean postoperative (1-year) sphere, cylinder, SEQ were −0.24±0.3, −0.84±0.4, and −0.63±0.38 D, respectively. At 1 year, the mean CDVA and UDVA were 0.31±0.12 and 0.41±0.15 logMAR, respectively. The mean difference in IOP between baseline and 1-year follow-up was 1.88±0.6 mm Hg. There was no case of cataract, retinal detachment, or endophthalmitis in either group.
Between the two groups (Table 3), there was no statistically significant difference in age (P=0.578), preoperative SEQ (P=0.078), and preoperative CDVA (P=0.402). The mean change in SEQ was 12.86±3.71 in Artisan group, and 11.69±4.2 in ICL group, which was not statistically significant (P=0.17). There was no statistically significant difference between the two groups regarding postoperative UDVA, CDVA, and SEQ, and IOP change.
Table 3. Preoperative and 1-year postoperative: corrected and uncorrected distance visual acuity (CDVA, UDVA), spherical equivalent (SEQ), and change in intraocular pressures (IOP).
| Pre-SEQ (diopters) | Pre-CDVA (logMAR) | Post-UDVA (logMAR) | Post-CDVA (logMAR) | Post-SEQ (diopters) | Change in SEQ (diopters) | IOP change (mm Hg) | |
|---|---|---|---|---|---|---|---|
| Artisan | −12.89±3.78 | 0.42±0.23 | 0.39±0.13 | 0.36±0.12 | −0.86±0.5 | 12.86±3.71 | 0.64±2.7 |
| Visian ICL | −12.44±4.15 | 0.40±0.24 | 0.41±0.15 | 0.31±0.12 | −0.63±0.38 | 11.69±4.2 | 1.88±0.6 |
| P-value | 0.078 | 0.402 | 0.268 | 0.128 | 0.67 | 0.17 | 0.77 |
Between-groups statistical analysis P-values.
The safety index was 1.02 in Artisan group, and 1.18 in ICL group. In the Artisan group (mean difference in CDVA was 0.038±0.09) six eyes (14.28%) gained two lines of CDVA, nine eyes (21.42%) gained one line, one eye (2.38%) lost two lines, and there was no change in CDVA in 25 eyes (59.52%). In the ICL group (mean difference in CDVA was 0.075±0.19), two eyes (7.69%) gained three lines of CDVA, nine eyes (34.61%) gained two lines, three eyes (11.53%) gained one line, five eyes (19.23%) lost one line, one eye (3.85%) lost two lines, and there was no change in CDVA in six eyes (23.08%). The efficacy index was 0.95 in Artisan group and also in ICL group. In the Artisan group, 13 eyes (56.52%) were within 1 D SEQ, compared with seven eyes (53.84%) in the ICL group.
Discussion
Phakic IOL surgery is an efficacious technique for correcting refractive error in patients who would otherwise be poor candidates for corneal refractive surgery, and can provide immediate improvement in UDVA, an increase in CDVA, and preservation of accommodation.8
In this study, there was no statistically significant difference between the two groups as regards the postoperative CDVA and UDVA. Although most studies showed comparable outcomes between both lenses like ours, Boxer et al9 reported that binocular UDVA was better in the ICL group, and Menezo et al10 found slightly better visual results with the Artisan than with the Visian ICL.
In our study, there was one case of malignant glaucoma in the Artisan group that required anterior vitrectomy in the first postoperative day; there was also a case of angle closure glaucoma in the ICL group, which was related to oversizing of the IOL that required explantation and replacement of the IOL. There have been case reports of malignant glaucoma,11 and intractable elevation of IOP requiring filtration surgery after ICL implantation.12
We did not report any case of cataract after implantation of either Artisan or ICL, but this may be attributed to the relatively short follow-up period. Menezo et al10 found two basic cataract types: anterior subcapsular opacification (in cases of ICL), and nuclear cataract (in cases of Artisan). The mean time to nuclear cataract appearance after Artisan IOL implantation was 54.83±22.12, and ICL implantation was 20±1 month.
In our study, pigment dispersion occurred in 12 eyes (28.6%) in the Artisan group and in two eyes (15.38%) in the ICL group. Increased flare has also been found after the implantation of a posterior chamber pIOL.13, 14 We did not report any case of retinal detachment or endophthalmitis after implantation of either Artisan or ICL. One report showed that the risk of retinal detachment in pIOL cases was lower than in clear lens extraction cases.15 Although there were no cases of retinal detachment or endophthalmitis, however, the number of subjects studied was too low to adequately detect a retinal detachment or endophthalmitis.
We did not find any clinical or statistical difference in the visual outcomes between Artisan and ICL after 1-year follow-up, and the short-term rates of complications and loss of CDVA are acceptable. However, a weakness of the study is the retrospective design, and that the ICL group is significantly smaller (26) than the Artisan group (42). Comprehensive preoperative evaluation and long-term postoperative follow-up examinations are needed to monitor for and prevent serious complications, and to establish long-term safety.
Future research should be directed at studying of the long-term (10 years) efficacy and complications of pIOLs.

The authors declare no conflict of interest.
References
- Schallhorn SC, Farjo AA, Huang D, Boxer Wachler BS, Trattler WB, Tanzer DJ, Ophthalmic Technology Assessment Committee Refractive Management/Intervention Panel et al. Wavefront-guided LASIK for the correction of primary myopia and astigmatism: a report by the American Academy of Ophthalmology. Ophthalmology. 2008;115:1249–1261. doi: 10.1016/j.ophtha.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Varley GA, Huang D, Rapuano CJ, Schallhorn S, Boxer Wachler BS, Sugar A, Ophthalmic Technology Assessment Committee Refractive Surgery Panel LASIK for hyperopia, hyperopic astigmatism, and mixed astigmatism: a report by the American Academy of Ophthalmology. Ophthalmology. 2004;111:1604–1617. doi: 10.1016/j.ophtha.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Huang D, Schallhorn SC, Sugar A, Farjo AA, Majmudar PA, Trattler WB, Ophthalmic Technology Assessment et al. Phakic intraocular lens implantation for the correction of myopia: a report by the American Academy of Ophthalmology. Ophthalmology. 2009;116:2244–2258. doi: 10.1016/j.ophtha.2009.08.018. [DOI] [PubMed] [Google Scholar]
- El Danasoury MA, El Maghraby A, Gamali TO. Comparison of iris-fixed Artisan lens implantation with excimer laser in situ keratomileusis in correcting myopia between −9.00 and −19.50 diopters: a randomized study. Ophthalmology. 2002;109:955–964. doi: 10.1016/s0161-6420(02)00964-8. [DOI] [PubMed] [Google Scholar]
- Sanders DR. Matched population comparison of the Visian implantable collamer lens and standard LASIK for myopia of −3.00 to −7.88 diopters. J Refract Surg. 2007;23:537–553. doi: 10.3928/1081-597X-20070601-02. [DOI] [PubMed] [Google Scholar]
- Schallhorn S, Tanzer D, Sanders DR, Sanders ML. Randomized prospective comparison of Visian toric implantable collamer lens and conventional photorefractive keratectomy for moderate to high myopic astigmatism. J Refract Surg. 2007;23:853–867. doi: 10.3928/1081-597X-20071101-01. [DOI] [PubMed] [Google Scholar]
- Colin J, Robinet A, Cochener B. Retinal detachment after clear lens extraction for high myopia: seven-year follow-up. Ophthalmology. 1999;106:2281–2284. doi: 10.1016/S0161-6420(99)90526-2. [DOI] [PubMed] [Google Scholar]
- Kohnen T. Searching for the perfect phakic intraocular lens. J Cataract Refract Surg. 2000;26:1261–1262. doi: 10.1016/s0886-3350(00)00665-9. [DOI] [PubMed] [Google Scholar]
- Boxer Wachler BS, Scruggs RT, Yuen LH, Jalali S. Comparison of the Visian ICL and Verisyse phakic intraocular lenses for myopia from 6.00 to 20.00 diopters. J Refract Surg. 2009;25 (9:765–770. doi: 10.3928/1081597X-20090813-02. [DOI] [PubMed] [Google Scholar]
- Menezo JL, Peris-Martinez C, Cisneros AL, Martinez-Costa R. Phakic intraocular lenses to correct high myopia: Adatomed, Staar, and Artisan. J Cataract Refract Surg. 2004;30:33–44. doi: 10.1016/j.jcrs.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Kodjikian L, Gain P, Donate D, Rouberol F, Burillon C. Malignant glaucoma induced by a phakic posterior chamber intraocular lens for myopia. J Cataract Refract Surg. 2002;28:2217–2221. doi: 10.1016/s0886-3350(02)01213-0. [DOI] [PubMed] [Google Scholar]
- Sanchez-Galeana CA, Zadok D, Montes M, Cortés MA, Chayet AS. Refractory intraocular pressure increase after phakic posterior chamber intraocular lens implantation. Am J Ophthalmol. 2002;134:121–123. doi: 10.1016/s0002-9394(02)01414-9. [DOI] [PubMed] [Google Scholar]
- Jiménez-Alfaro I, Benítez del Castillo JM, García-Feijoó J, Gil de Bernabé JG, Serrano de La Iglesia JM. Safety of posterior chamber phakic intraocular lenses for the correction of high myopia: anterior segment changes after posterior chamber phakic intraocular lens implantation. Ophthalmology. 2001;108:90–99. doi: 10.1016/s0161-6420(00)00403-6. [DOI] [PubMed] [Google Scholar]
- Lackner B, Pieh S, Schmidinger G, Simader C, Franz C, Dejaco-Ruhswurm I, et al. Long-term results of implantation of phakic posterior chamber intraocular lenses. J Cataract Refract Surg. 2004;30:2269–2276. doi: 10.1016/j.jcrs.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Arne JL. Phakic intraocular lens implantation versus clear lens extraction in highly myopic eyes of 30- to 50-year-old patients. J Cataract Refract Surg. 2004;30:2092–2096. doi: 10.1016/j.jcrs.2004.02.082. [DOI] [PubMed] [Google Scholar]