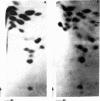
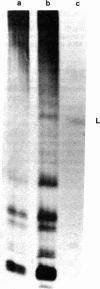
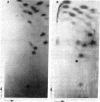
Abstract
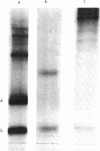
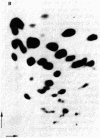
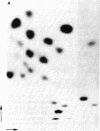
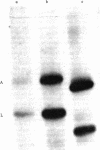
The DNA of the attenuator region of the histidine operon of Escherichia coli has been transcribed in a purified in vitro system and found to synthesize two major RNA transcripts. The first one, 180 nucleotides long, has been identified as the histidine-specific leader RNA. It contains the coding sequence for the leader peptide [Di Nocera, P. P., Blasi, F., Di Lauro, R., Frunzio, R. & Bruni, C. B. (1978) Proc. Natl. Acad. Sci. USA 75, 4276-4280] and is terminated at the attenuator site. Termination of transcription at this site is extremely efficient in the in vitro system. The leader RNA also has been detected in vivo in a minicell producer strain transformed with plasmids harboring the regulatory region of the histidine operon of E. coli. A second RNA molecule is synthesized in the in vitro system. It has a divergent direction of transcription with respect to the histidine leader RNA, but its role, if any, in the regulation of the histidine operon remains to be ascertained. The existence of the histidine leader RNA lends support to the regulatory mechanism which postulates that regulation of the histidine operon is dependent on the alternative secondary structures that the leader RNA may assume, depending on whether or not the histidine-rich leader peptide is translated.
Full text
PDF
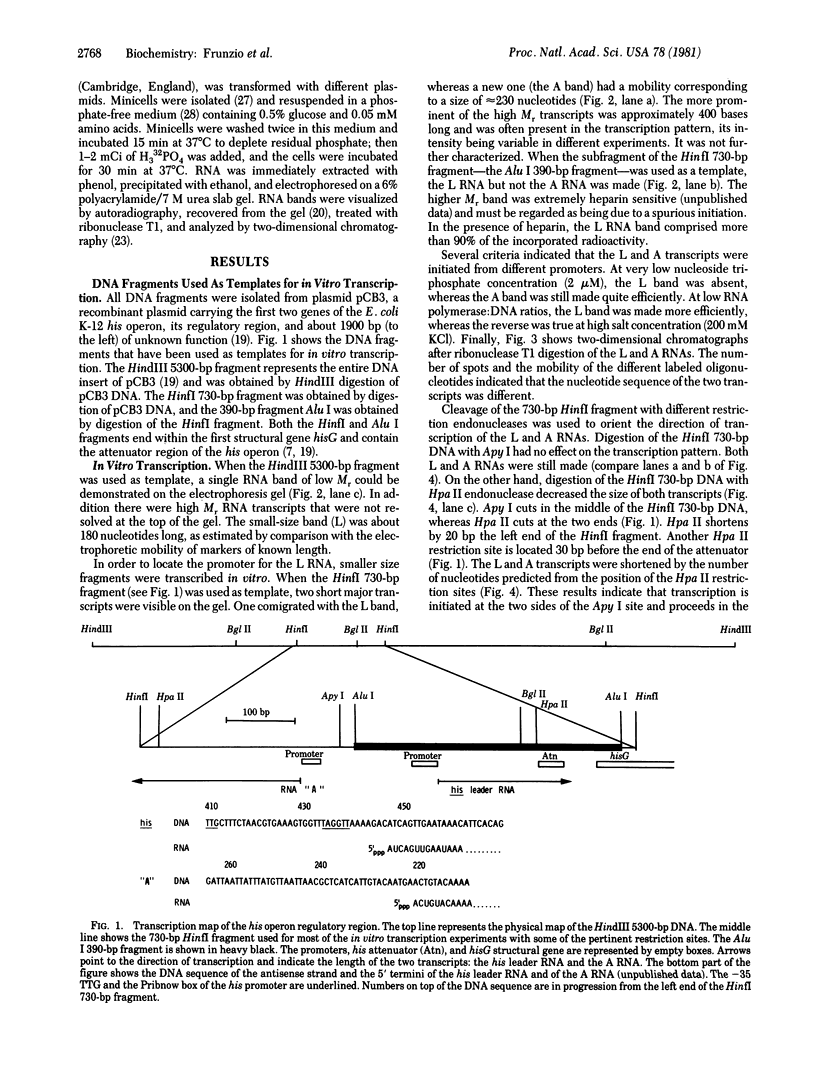


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artz S. W., Broach J. R. Histidine regulation in Salmonella typhimurium: an activator attenuator model of gene regulation. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3453–3457. doi: 10.1073/pnas.72.9.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes W. M. DNA sequence from the histidine operon control region: seven histidine codons in a row. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4281–4285. doi: 10.1073/pnas.75.9.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand K., Korn L., Lee F., Platt T., Squires C. L., Squires C., Yanofsky C. New features of the regulation of the tryptophan operon. Science. 1975 Jul 4;189(4196):22–26. doi: 10.1126/science.1094538. [DOI] [PubMed] [Google Scholar]
- Bertrand K., Squires C., Yanofsky C. Transcription termination in vivo in the leader region of the tryptophan operon of Escherichia coli. J Mol Biol. 1976 May 15;103(2):319–337. doi: 10.1016/0022-2836(76)90315-6. [DOI] [PubMed] [Google Scholar]
- Bickle T. A., Pirrotta V., Imber R. A simple, general procedure for purifying restriction endonucleases. Nucleic Acids Res. 1977 Aug;4(8):2561–2572. doi: 10.1093/nar/4.8.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni C. B., Colantuoni V., Sbordone L., Cortese R., Blasi F. Biochemical and regulatory properties of Escherichia coli K-12 hisT mutants. J Bacteriol. 1977 Apr;130(1):4–10. doi: 10.1128/jb.130.1.4-10.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni C. B., Musti A. M., Frunzio R., Blasi F. Structural and physiological studies of the Escherichia coli histidine operon inserted into plasmid vectors. J Bacteriol. 1980 Apr;142(1):32–42. doi: 10.1128/jb.142.1.32-42.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nocera P. P., Blasi F., Di Lauro R., Frunzio R., Bruni C. B. Nucleotide sequence of the attenuator region of the histidine operon of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4276–4280. doi: 10.1073/pnas.75.9.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. F. Regulation of the threonine operon: tandem threonine and isoleucine codons in the control region and translational control of transcription termination. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1706–1710. doi: 10.1073/pnas.76.4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmill R. M., Wessler S. R., Keller E. B., Calvo J. M. leu operon of Salmonella typhimurium is controlled by an attenuation mechanism. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4941–4945. doi: 10.1073/pnas.76.10.4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallewell R. A., Sherratt D. J. Isolation and characterization of Co1E2 plasmid mutants unable to kill colicin-sensitive cells. Mol Gen Genet. 1976 Aug 2;146(3):239–245. doi: 10.1007/BF00701246. [DOI] [PubMed] [Google Scholar]
- Horii T., Ogawa T., Ogawa H. Organization of the recA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):313–317. doi: 10.1073/pnas.77.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B. H., de Crombrugghe B., Rosenberg M. Transcription in vitro of bacteriophage lambda 4S RNA: studies on termination and rho protein. Nucleic Acids Res. 1977 Apr;4(4):827–842. doi: 10.1093/nar/4.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston H. M., Barnes W. M., Chumley F. G., Bossi L., Roth J. R. Model for regulation of the histidine operon of Salmonella. Proc Natl Acad Sci U S A. 1980 Jan;77(1):508–512. doi: 10.1073/pnas.77.1.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai T. Regulation of the expression of the histidine operon in Salmonella typhimurium. Nature. 1974 Jun 7;249(457):523–527. doi: 10.1038/249523a0. [DOI] [PubMed] [Google Scholar]
- Keller E. B., Calvo J. M. Alternative secondary structures of leader RNAs and the regulation of the trp, phe, his, thr, and leu operons. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6186–6190. doi: 10.1073/pnas.76.12.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Hatfield G. W. Effects of altered rho gene product on the expression of the Escherichia coli histidine operon. J Bacteriol. 1978 Dec;136(3):1201–1204. doi: 10.1128/jb.136.3.1201-1204.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Hatfield G. W. Multivalent translational control of transcription termination at attenuator of ilvGEDA operon of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1862–1866. doi: 10.1073/pnas.77.4.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. A., Ames B. N. Histidine regulation in Salmonella typhimurium. XI. The percentage of transfer RNA His charged in vivo and its relation to the repression of the histidine operon. J Mol Biol. 1972 Apr 28;66(1):131–142. doi: 10.1016/s0022-2836(72)80011-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miller J. S., Burgess R. R. Selectivity of RNA chain initiation in vitro. 1. Analysis of RNA initiations by two-dimensional thin-layer chromatography of 5'-triphosphate-labeled oligonucleotides. Biochemistry. 1978 May 30;17(11):2054–2059. doi: 10.1021/bi00604a005. [DOI] [PubMed] [Google Scholar]
- Nargang F. E., Subrahmanyam C. S., Umbarger H. E. Nucleotide sequence of ilvGEDA operon attenuator region of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1823–1827. doi: 10.1073/pnas.77.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxender D. L., Zurawski G., Yanofsky C. Attenuation in the Escherichia coli tryptophan operon: role of RNA secondary structure involving the tryptophan codon region. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5524–5528. doi: 10.1073/pnas.76.11.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Simoncsits A., Brownlee G. G., Brown R. S., Rubin J. R., Guilley H. New rapid gel sequencing method for RNA. Nature. 1977 Oct 27;269(5631):833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]
- Singer C. E., Smith G. R., Cortese R., Ames B. N. [Mutant tRNA His ineffective in repression and lacking two pseudouridine modifications]. Nat New Biol. 1972 Jul 19;238(81):72–74. doi: 10.1038/newbio238072a0. [DOI] [PubMed] [Google Scholar]
- Winkler M. E. Expression of the histidine operon in rho mutants of Escherichia coli. J Bacteriol. 1978 Aug;135(2):721–725. doi: 10.1128/jb.135.2.721-725.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler M. E., Roth D. J., Hartman P. E. Promoter- and attenuator-related metabolic regulation of the Salmonella typhimurium histidine operon. J Bacteriol. 1978 Feb;133(2):830–843. doi: 10.1128/jb.133.2.830-843.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Brown K., Killingly D., Yanofsky C. Nucleotide sequence of the leader region of the phenylalanine operon of Escherichia coli. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4271–4275. doi: 10.1073/pnas.75.9.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Elseviers D., Stauffer G. V., Yanofsky C. Translational control of transcription termination at the attenuator of the Escherichia coli tryptophan operon. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5988–5992. doi: 10.1073/pnas.75.12.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]