Abstract
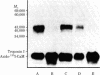
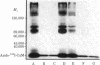
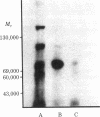
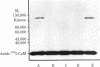
A photoaffinity label for calmodulin-binding proteins was prepared from 125I-labeled calmodulin (125I-calmodulin) and methyl-4-azidobenzimidate. Azidocalmodulin containing one azido group per calmodulin retained its ability to stimulate the CA2+-sensitive phosphodiesterase purified from bovine heart muscle. The concentrations of calmodulin and azidocalmodulin required for half-maximal stimulation of phosphodiesterase activity were 170 and 230 pM, respectively. Azido-125I-calmodulin was used to photoaffinity label troponin I, myosin light chain kinase, and the Ca2+-sensitive phosphodiesterase. Formation of crosslinked complexes required the presence of Ca2+ or Mn2+ and was inhibited by excess unmodified calmodulin. The calmodulin-binding subunits all formed 1:1 complexes with calmodulin, and the molecular weights of the crosslinked products obtained with troponin I, the phosphodiesterase, and myosin light chain kinase were 43,000, 79,000, and 116,000, respectively. Photolysis experiments using azido-125I-calmodulin and bovine cerebral cortex membranes or detergent-solubilized membranes resulted in formation of a limited number of specifically labeled polypeptides. Azido-calmodulin appears to be an appropriate photoaffinity label for the identification and characterization of calmodulin-binding subunits.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amphlett G. W., Vanaman T. C., Perry S. V. Effect of the troponin C-like protein from bovine brain (brain modulator protein) on the Mg2+-stimulated ATPase of skeletal muscle actinomyosin. FEBS Lett. 1976 Dec 15;72(1):163–168. doi: 10.1016/0014-5793(76)80836-8. [DOI] [PubMed] [Google Scholar]
- Blumenthal D. K., Stull J. T. Activation of skeletal muscle myosin light chain kinase by calcium(2+) and calmodulin. Biochemistry. 1980 Nov 25;19(24):5608–5614. doi: 10.1021/bi00565a023. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Huang Y. C., Breckenridge B. M., Wolff D. J. Identification of a calcium-binding protein as a calcium-dependent regulator of brain adenylate cyclase. Proc Natl Acad Sci U S A. 1975 Jan;72(1):64–68. doi: 10.1073/pnas.72.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Demonstration of an activator. Biochem Biophys Res Commun. 1970 Feb 6;38(3):533–538. doi: 10.1016/0006-291x(70)90747-3. [DOI] [PubMed] [Google Scholar]
- Cohen P., Burchell A., Foulkes J. G., Cohen P. T., Vanaman T. C., Nairn C. Identification of the Ca2+-dependent modulator protein as the fourth subunit of rabbit skeletal muscle phosphorylase kinase. FEBS Lett. 1978 Aug 15;92(2):287–293. doi: 10.1016/0014-5793(78)80772-8. [DOI] [PubMed] [Google Scholar]
- Dabrowska R., Sherry J. M., Aromatorio D. K., Hartshorne D. J. Modulator protein as a component of the myosin light chain kinase from chicken gizzard. Biochemistry. 1978 Jan 24;17(2):253–258. doi: 10.1021/bi00595a010. [DOI] [PubMed] [Google Scholar]
- Gopinath R. M., Vincenzi F. F. Phosphodiesterase protein activator mimics red blood cell cytoplasmic activator of (Ca2+-Mg2+)ATPase. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1203–1209. doi: 10.1016/s0006-291x(77)80107-1. [DOI] [PubMed] [Google Scholar]
- Jarrett H. W., Penniston J. T. Partial purification of the Ca2+-Mg2+ ATPase activator from human erythrocytes: its similarity to the activator of 3':5' - cyclic nucleotide phosphodiesterase. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1210–1216. doi: 10.1016/s0006-291x(77)80108-3. [DOI] [PubMed] [Google Scholar]
- Ji T. H. A novel approach to the identification of surface receptors. The use of photosensitive hetero-bifunctional cross-linking reagent. J Biol Chem. 1977 Mar 10;252(5):1566–1570. [PubMed] [Google Scholar]
- Keller C. H., LaPorte D. C., Toscano W. A., Jr, Storm D. R., Westcott K. R. Ca2+ regulation of cyclic nucleotide metabolism. Ann N Y Acad Sci. 1980;356:205–219. doi: 10.1111/j.1749-6632.1980.tb29612.x. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Richman P. G. Calmodulin. Annu Rev Biochem. 1980;49:489–515. doi: 10.1146/annurev.bi.49.070180.002421. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Krinks M. H. Purification of cyclic 3',5'-nucleotide phosphodiesterase inhibitory protein by affinity chromatography on activator protein coupled to Sepharose. Biochemistry. 1978 Jan 10;17(1):120–126. doi: 10.1021/bi00594a017. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Storm D. R. Detection of calcium-dependent regulatory protein binding components using 125I-labeled calcium-dependent regulatory protein. J Biol Chem. 1978 May 25;253(10):3374–3377. [PubMed] [Google Scholar]
- LaPorte D. C., Toscano W. A., Jr, Storm D. R. Cross-linking of iodine-125-labeled, calcium-dependent regulatory protein to the Ca2+-sensitive phosphodiesterase purified from bovine heart. Biochemistry. 1979 Jun 26;18(13):2820–2825. doi: 10.1021/bi00580a021. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Wierman B. M., Storm D. R. Calcium-induced exposure of a hydrophobic surface on calmodulin. Biochemistry. 1980 Aug 5;19(16):3814–3819. doi: 10.1021/bi00557a025. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Teshima Y., Kakiuchi S. Mechanism of stimulation of Ca2+ plus Mg2+ -dependent phospodiesterase from rat cerebral cortex by the modulator protein and Ca2+. Biochem Biophys Res Commun. 1974 Jan 23;56(2):489–495. doi: 10.1016/0006-291x(74)90869-9. [DOI] [PubMed] [Google Scholar]
- Walsh M., Stevens F. C. Chemical modification studies on the Ca2+-dependent protein modulator of cyclic nucleotide phosphodiesterase. Biochemistry. 1977 Jun 14;16(12):2742–2749. doi: 10.1021/bi00631a024. [DOI] [PubMed] [Google Scholar]
- Wang J. H., Desai R. Modulator binding protein. Bovine brain protein exhibiting the Ca2+-dependent association with the protein modulator of cyclic nucleotide phosphodiesterase. J Biol Chem. 1977 Jun 25;252(12):4175–4184. [PubMed] [Google Scholar]
- Wang J. H., Teo T. S., Ho H. C., Stevens F. C. Bovine heart protein activator of cyclic nucleotide phosphodiesterase. Adv Cyclic Nucleotide Res. 1975;5:179–194. [PubMed] [Google Scholar]
- Watterson D. M., Vanaman T. C. Affinity chromatography purification of a cyclic nucleotide phosphodiesterase using immobilized modulator protein, a troponin C-like protein from brain. Biochem Biophys Res Commun. 1976 Nov 8;73(1):40–46. doi: 10.1016/0006-291x(76)90494-0. [DOI] [PubMed] [Google Scholar]
- Westcott K. R., La Porte D. C., Storm D. R. Resolution of adenylate cyclase sensitive and insensitive to Ca2+ and calcium-dependent regulatory protein (CDR) by CDR-sepharose affinity chromatography. Proc Natl Acad Sci U S A. 1979 Jan;76(1):204–208. doi: 10.1073/pnas.76.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson J. M. The preparation and properties of the components of troponin B. Biochim Biophys Acta. 1974 Aug 8;359(2):379–388. doi: 10.1016/0005-2795(74)90238-4. [DOI] [PubMed] [Google Scholar]
- Wolff D. J., Poirier P. G., Brostrom C. O., Brostrom M. A. Divalent cation binding properties of bovine brain Ca2+-dependent regulator protein. J Biol Chem. 1977 Jun 25;252(12):4108–4117. [PubMed] [Google Scholar]