Abstract
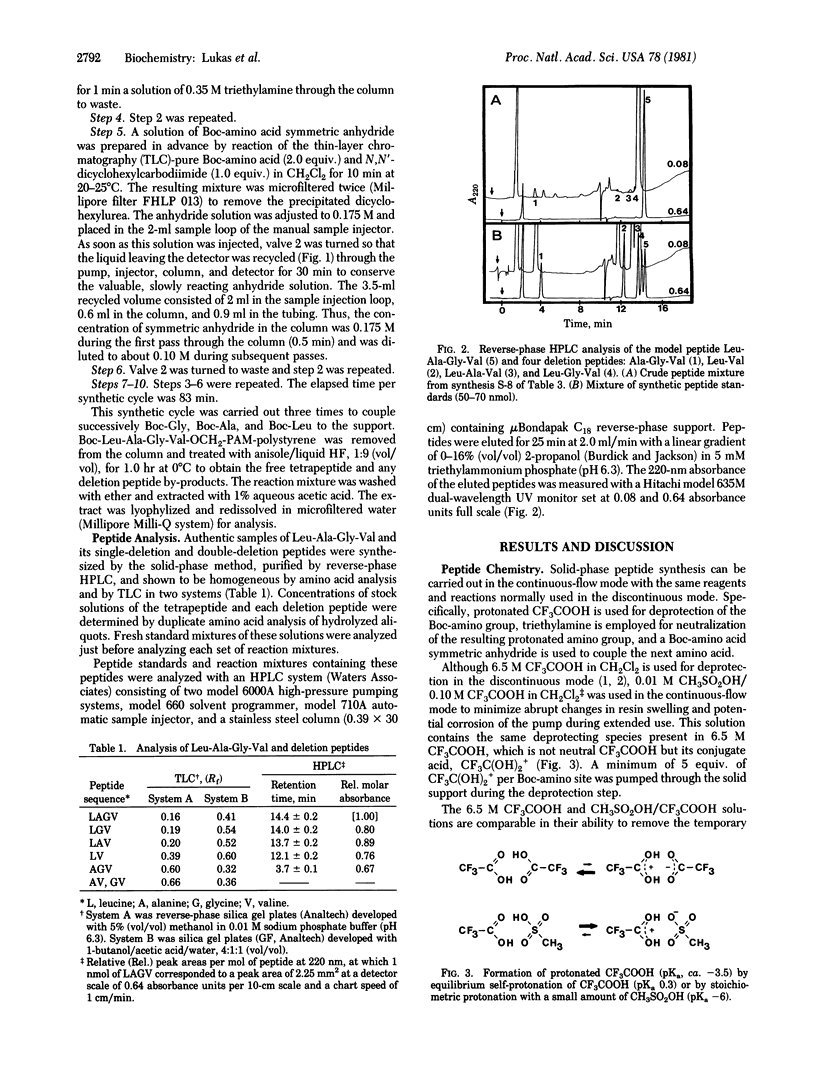
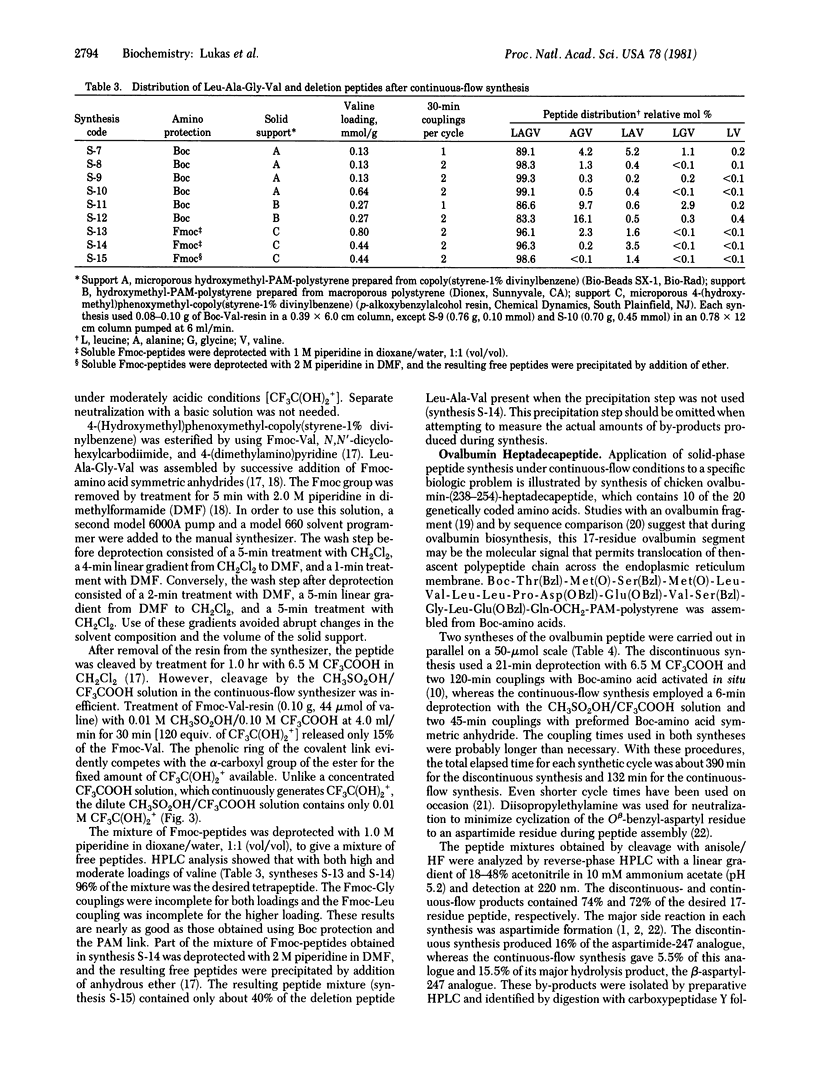
A system is described for solid-phase synthesis of peptides under continuous-flow conditions with liquid chromatographic equipment, conventional polystyrene supports, and well-defined chemistry. The model tetrapeptide Leu-Ala-Gly-Val was assembled in 99.3% purity in about 4 hr on microporous copoly(styrene-1% divinylbenzene). During coupling, the preformed symmetric anhydrides were conserved by being recycled. Relative yields of the peptide products were determined quantitatively in 20 min by reverse-phase high-pressure liquid chromatography. This rapid assay system was used to examine the influence on product yields of (i) the time and number of couplings per cycle, (ii) microporous versus macroporous polystyrene, and (iii) tert-butoxycarbonyl (Boc) group versus 9-fluorenylmethoxycarbonyl for amine protection. Use of microporous polystyrene and two 30-min couplings of Boc-amino acids per cycle gave the best results. This continuous-flow system provides a rapid and efficient approach to solid-phase peptide synthesis. A 17-residue peptide from chicken ovalbumin was obtained in similar purity and yield from a discontinuous synthesis and from a continuous-flow synthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer E., Jun G., Halász I., Sebestian I. A new support for polypeptide synthesis in columns. Tetrahedron Lett. 1970 Nov;(51):4503–4505. doi: 10.1016/s0040-4039(01)83961-7. [DOI] [PubMed] [Google Scholar]
- Corley L., Sachs D. H., Anfinsen C. B. Rapid solid-phase synthesis of bradykinin. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1353–1359. doi: 10.1016/0006-291x(72)90221-5. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Blobel G. Chicken ovalbumin contains an internal signal sequence. Nature. 1979 Sep 13;281(5727):117–121. doi: 10.1038/281117a0. [DOI] [PubMed] [Google Scholar]
- Meienhofer J., Waki M., Heimer E. P., Lambros T. J., Makofske R. C., Chang C. D. Solid phase synthesis without repetitive acidolysis. Preparation of leucyl-alanyl-glycyl-valine using 9-fluorenylmethyloxycarbonylamino acids. Int J Pept Protein Res. 1979 Jan;13(1):35–42. [PubMed] [Google Scholar]
- Merrifield R. B., Mitchell A. R., Clarke J. E. Detection and prevention of urethane acylation during solid-phase peptide synthesis by anhydride methods. J Org Chem. 1974 Mar 8;39(5):660–668. doi: 10.1021/jo00919a018. [DOI] [PubMed] [Google Scholar]
- Mitchell A. R., Erickson B. W., Ryabtsev M. N., Hodges R. S., Merrifield R. B. Tert-butoxycarbonylaminoacyl-4-(oxymethyl)-phenylacetamidomethyl-resin, a more acid-resistant support for solid-phase peptide synthesis. J Am Chem Soc. 1976 Nov 10;98(23):7357–7362. doi: 10.1021/ja00439a041. [DOI] [PubMed] [Google Scholar]
- Scott R. P., Zolty S., Chan K. K. An automatic apparatus for the synthesis of peptides using resin coated glass beads in the form of a packed bed. J Chromatogr Sci. 1972 Jun 10;10(6):384–391. doi: 10.1093/chromsci/10.6.384. [DOI] [PubMed] [Google Scholar]
- Wang S. S. p-alkoxybenzyl alcohol resin and p-alkoxybenzyloxycarbonylhydrazide resin for solid phase synthesis of protected peptide fragments. J Am Chem Soc. 1973 Feb 21;95(4):1328–1333. doi: 10.1021/ja00785a602. [DOI] [PubMed] [Google Scholar]
- Wong T. W., Merrifield R. B. Solid-phase synthesis of thymosin alpha 1 using tert-butyloxycarbonylaminoacyl-4-(oxymethyl)phenylacetamidomethyl-resin. Biochemistry. 1980 Jul 8;19(14):3233–3238. doi: 10.1021/bi00555a021. [DOI] [PubMed] [Google Scholar]



