Abstract
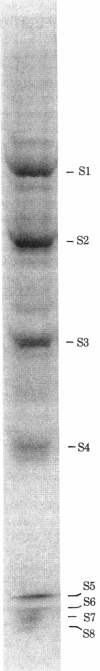
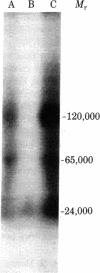
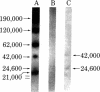
RNA polymerase I was purified to homogeneity from Morris hepatoma 3924A. Purified RNA polymerase I contained a protein kinase activity but comigrated with the polymerase in nondenaturing gels. RNA polymerase II, purified from the same hepatoma, lacked protein kinase activity. Analysis of the subunit composition of the RNA polymerase I showed the presence of eight polypeptides: S1, Mr 190,000; S2, Mr 120,000; S3, Mr 62,000; S4, Mr 42,000; S5, Mr 24,600; S6, Mr 21,000; S7, Mr 19,500; and S8, Mr 17,500. Antibodies prepared against purified polymerase I specifically inhibited RNA synthesis catalyzed by RNA polymerase I. When subunits of the enzyme were covalently linked to diazobenzyloxymethyl paper, complexes between the antibody preparation and S1-S6 were visualized. No immune complexes were formed between RNA polymerase I antibodies and RNA polymerase II subunits. The antibody preparation was able to inhibit both the protein phosphorylation catalyzed by RNA polymerase I and that catalyzed by a nuclear kinase (NII) purified from the same hepatoma. The two polypeptides of the nuclear kinase--Mr 42,000 and 24,600 (identical in size to S4 and S5 of polymerase I)--formed visible complexes with the RNA polymerase I antibodies. Both S4 and S5 of the polymerase contained an ATP binding site, a property associated with protein phosphorylation and also exhibited by the polypeptides of the purified kinase. These data suggest that polypeptides of Mr 42,000 and 24,600 associated with polymerase I are responsible for its kinase activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duceman B. W., Jacob S. T. Transcriptionally active RNA polymerases from Morris hepatomas and rat liver. Elucidation of the mechanism for the preferential increase in the tumour RNA polymerase I. Biochem J. 1980 Sep 15;190(3):781–789. doi: 10.1042/bj1900781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. I., Perriard J. C., Rutter W. J. Purification of rat liver and mouse ascites DNA-dependent RNA polymerase I. Biochemistry. 1977 Apr 19;16(8):1655–1665. doi: 10.1021/bi00627a021. [DOI] [PubMed] [Google Scholar]
- Hirsch J., Martelo O. J. Phosphorylation of rat liver ribonucleic acid polymerase I by nuclear protein kinases. J Biol Chem. 1976 Sep 10;251(17):5408–5413. [PubMed] [Google Scholar]
- Huet J., Buhler J. M., Sentenac A., Fromageot P. Characterization of ribonuclease H activity associated yeast RNA polymerase A. J Biol Chem. 1977 Dec 25;252(24):8848–8855. [PubMed] [Google Scholar]
- Jungmann R. A., Hiestand P. C., Schweppe J. S. Adenosine 3':5'-monophosphate-dependent protein kinase and the stimulation of ovarian nuclear ribonucleic acid polymerase activities. J Biol Chem. 1974 Sep 10;249(17):5444–5451. [PubMed] [Google Scholar]
- Kedinger C., Gissinger F., Chambon P. Animal DNA-dependent RNA polymerases. Molecular structures and immunological properties of calf-thymus enzyme AI and of calf-thymus and rat-liver enzymes B. Eur J Biochem. 1974 May 15;44(2):421–436. doi: 10.1111/j.1432-1033.1974.tb03500.x. [DOI] [PubMed] [Google Scholar]
- Kleinsmith L. J. Phosphorylation of non-histone proteins in the regulation of chromosome structure and function. J Cell Physiol. 1975 Apr;85(2 Pt 2 Suppl 1):459–475. doi: 10.1002/jcp.1040850412. [DOI] [PubMed] [Google Scholar]
- Kuehn G. D., Affolter H. U., Atmar V. J., Seebeck T., Gubler U., Braun R. Polyamine-mediated phosphorylation of a nucleolar protein from Physarum polycephalum that stimulates rRNA synthesis. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2541–2545. doi: 10.1073/pnas.76.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Renart J., Reiser J., Stark G. R. Transfer of proteins from gels to diazobenzyloxymethyl-paper and detection with antisera: a method for studying antibody specificity and antigen structure. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3116–3120. doi: 10.1073/pnas.76.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K. M., Jacob S. T. Phosphorylation of nuclear poly(A) polymerase. Comparison of liver and hepatoma enzymes. J Biol Chem. 1979 Oct 25;254(20):10256–10261. [PubMed] [Google Scholar]
- Rose K. M., Ruch P. A., Morris H. P., Jacob S. T. RNA polymerases from a rat hepatoma. Partial purification and comparison of properties with corresponding liver enzymes. Biochim Biophys Acta. 1976 Apr 15;432(1):60–72. doi: 10.1016/0005-2787(76)90041-1. [DOI] [PubMed] [Google Scholar]
- Sajdel E. M., Jacob S. T. Mechanism of early effect of hydrocortisone on the transcriptional process: stimulation of the activities of purified rat liver nucleolar RNA polymerases. Biochem Biophys Res Commun. 1971 Nov 5;45(3):707–715. doi: 10.1016/0006-291x(71)90474-8. [DOI] [PubMed] [Google Scholar]
- Todhunter J. A., Weissbach H., Brot N. Modification of rat liver RNA polymerase I after in vivo stimulation by hydrocortisone or methylisobutylxanthine. J Biol Chem. 1978 Jul 10;253(13):4514–4516. [PubMed] [Google Scholar]