Abstract
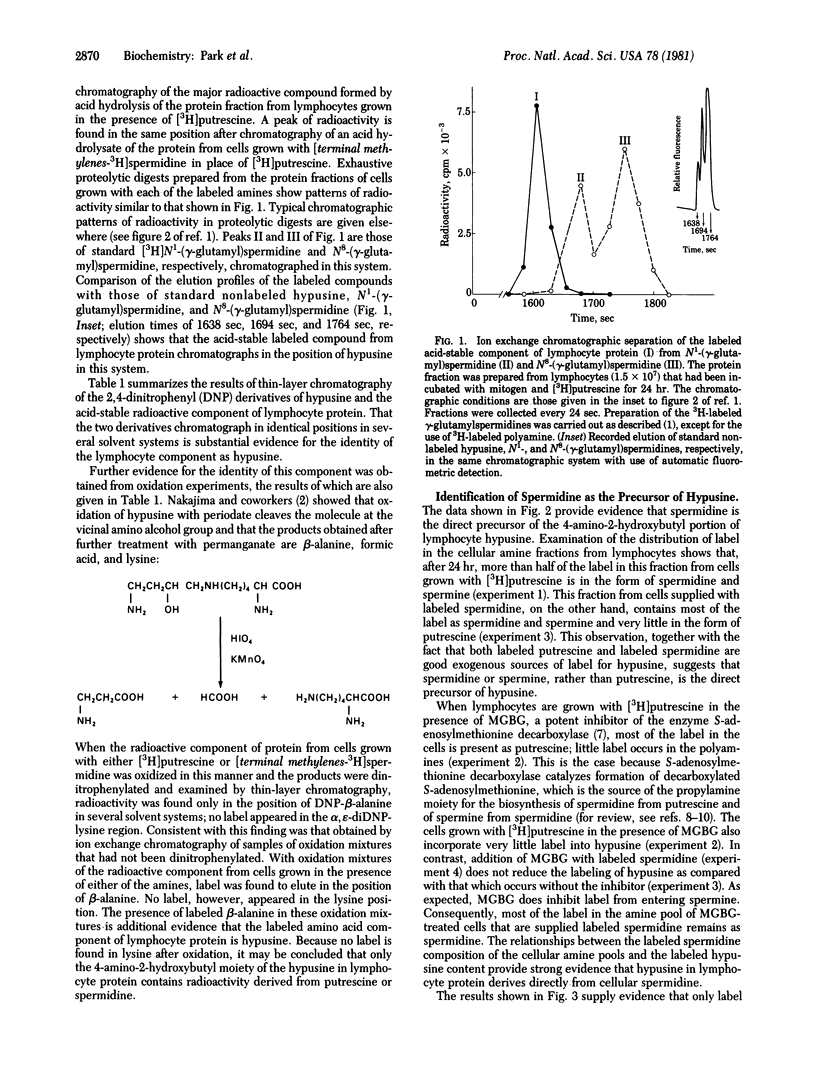
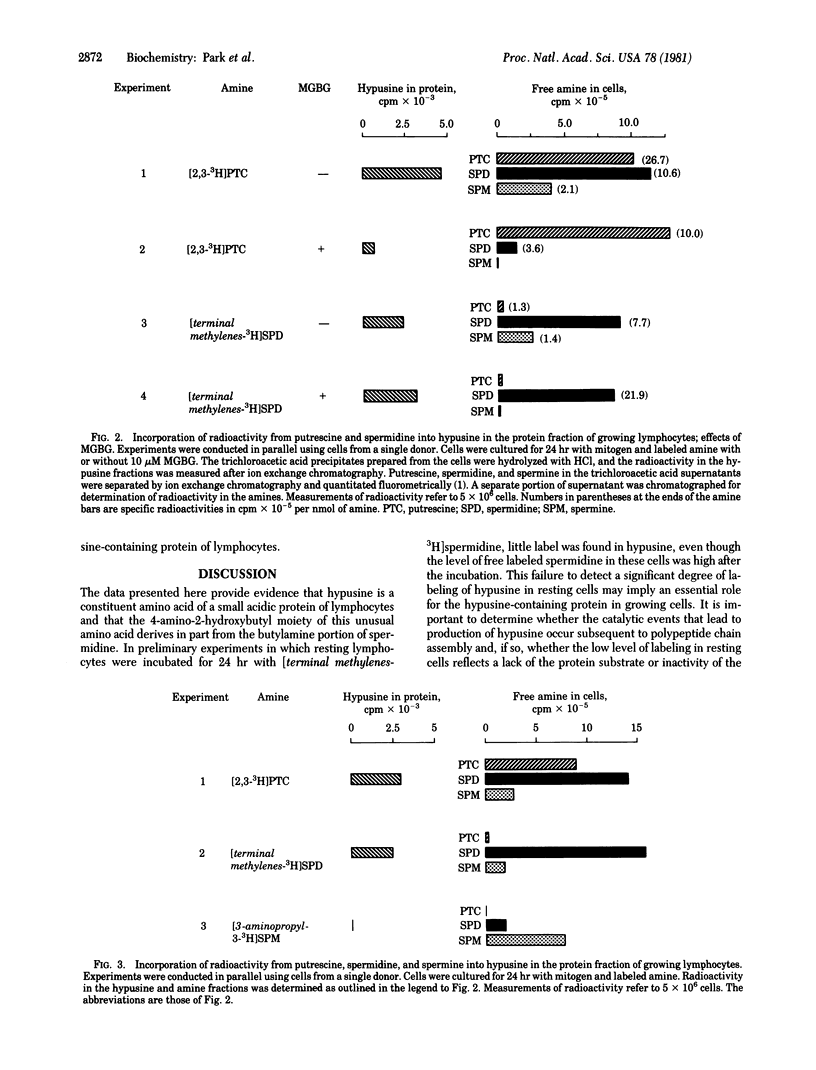
When normal human peripheral lymphocytes are treated with mitogen and grown in the presence of [3H]putrescine or [terminal methylenes-3H]spermidine, label is incorporated predominantly into one cellular protein. The radioactive constituent of this protein was identified as the unusual amino acid hypusine [N epsilon-(4-amino-2-hydroxybutyl)lysine]. This was accomplished by isolation of the component from proteolytic digests or acid hydrolysates and comparison with authentic hypusine by chromatography, conversion to the 2,4-dinitrophenyl derivative, and oxidative degradation. The observed relationships among intracellular levels of labeled putrescine, polyamines, and protein bound hypusine after growth of cells with the various labeled amines and with or without an inhibitor of polyamine biosynthesis supply evidence that spermidine is the immediate amine precursor of hypusine and that the 4-amino-2-hydroxybutyl portion of hypusine derives from the butylamine moiety of spermidine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cooper H. L. Purification of lymphocytes from peripheral blood. Methods Enzymol. 1974;32:633–636. doi: 10.1016/0076-6879(74)32065-4. [DOI] [PubMed] [Google Scholar]
- Corti A., Dave C., Williams-Ashman H. G., Mihich E., Schenone A. Specific inhibition of the enzymic decarboxylation of S-adenosylmethionine by methylglyoxal bis(guanylhydrazone) and related substances. Biochem J. 1974 May;139(2):351–357. doi: 10.1042/bj1390351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durban E., Nochumson S., Kim S., Paik W. K., Chan S. K. Cytochrome c-specific protein-lysine methyltransferase from Neurospora crassa. Purification, characterization, and substrate requirements. J Biol Chem. 1978 Mar 10;253(5):1427–1435. [PubMed] [Google Scholar]
- Folk J. E., Finlayson J. S. The epsilon-(gamma-glutamyl)lysine crosslink and the catalytic role of transglutaminases. Adv Protein Chem. 1977;31:1–133. doi: 10.1016/s0065-3233(08)60217-x. [DOI] [PubMed] [Google Scholar]
- Folk J. E., Park M. H., Chung S. I., Schrode J., Lester E. P., Cooper H. L. Polyamines as physiological substrates for transglutaminases. J Biol Chem. 1980 Apr 25;255(8):3695–3700. [PubMed] [Google Scholar]
- Hölttä E. Oxidation of spermidine and spermine in rat liver: purification and properties of polyamine oxidase. Biochemistry. 1977 Jan 11;16(1):91–100. doi: 10.1021/bi00620a015. [DOI] [PubMed] [Google Scholar]
- Imaoka N., Nakajima T. Hypusine, N6-(4-amino-2-hydroxybutyl)-2,6-diaminohexanoic acid, in tissue proteins of mammals. Biochim Biophys Acta. 1973 Aug 17;320(1):97–103. doi: 10.1016/0304-4165(73)90170-0. [DOI] [PubMed] [Google Scholar]
- LUCAS F., SHAW J. T., SMITH S. G. AMINO ACID ANALYSIS WITH FLUORODINITROBENZENE. Anal Biochem. 1963 Oct;6:335–351. doi: 10.1016/0003-2697(63)90158-1. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Fillingame R. H. Regulation of amino acid decarboxylation. Annu Rev Biochem. 1974;43(0):303–325. doi: 10.1146/annurev.bi.43.070174.001511. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Matsubayashi T., Kakimoto Y., Sano I. Distribution of hypusine, N 6 -(4-amino-2-hydroxybutyl)-2,6-diaminohexanoic acid, in mammalian organs. Biochim Biophys Acta. 1971 Oct;252(1):92–97. doi: 10.1016/0304-4165(71)90095-x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Paik W. K., Kim S. Solubilization and partial purification of protein methylase 3 from calf thymus nuclei. J Biol Chem. 1970 Nov 25;245(22):6010–6015. [PubMed] [Google Scholar]
- Sekeris C. E., Sekeri K. E., Gallwitz D. The methylation of the histones of rat liver nuclei in vitro. Hoppe Seylers Z Physiol Chem. 1967 Dec;348(12):1660–1666. doi: 10.1515/bchm2.1967.348.1.1660. [DOI] [PubMed] [Google Scholar]
- Shiba T., Mizote H., Kaneko T., Nakajima T., Kakimoto Y. Hypusine, a new amino acid occurring in bovine brain. Isolation and structural determination. Biochim Biophys Acta. 1971 Sep 21;244(3):523–531. doi: 10.1016/0304-4165(71)90069-9. [DOI] [PubMed] [Google Scholar]
- Sitaramayya A., Wright L. S., Siegel F. L. Enzymatic methylation of calmodulin in rat brain cytosol. J Biol Chem. 1980 Sep 25;255(18):8894–8900. [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Biosynthesis and metabolism of 1,4-diaminobutane, spermidine, spermine, and related amines. Adv Enzymol Relat Areas Mol Biol. 1972;36:203–268. doi: 10.1002/9780470122815.ch7. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Jänne J., Coppoc G. L., Geroch M. E., Schenone A. New aspects of polyamine biosynthesis in eukaryotic organisms. Adv Enzyme Regul. 1972;10:225–245. doi: 10.1016/0065-2571(72)90016-7. [DOI] [PubMed] [Google Scholar]