Abstract
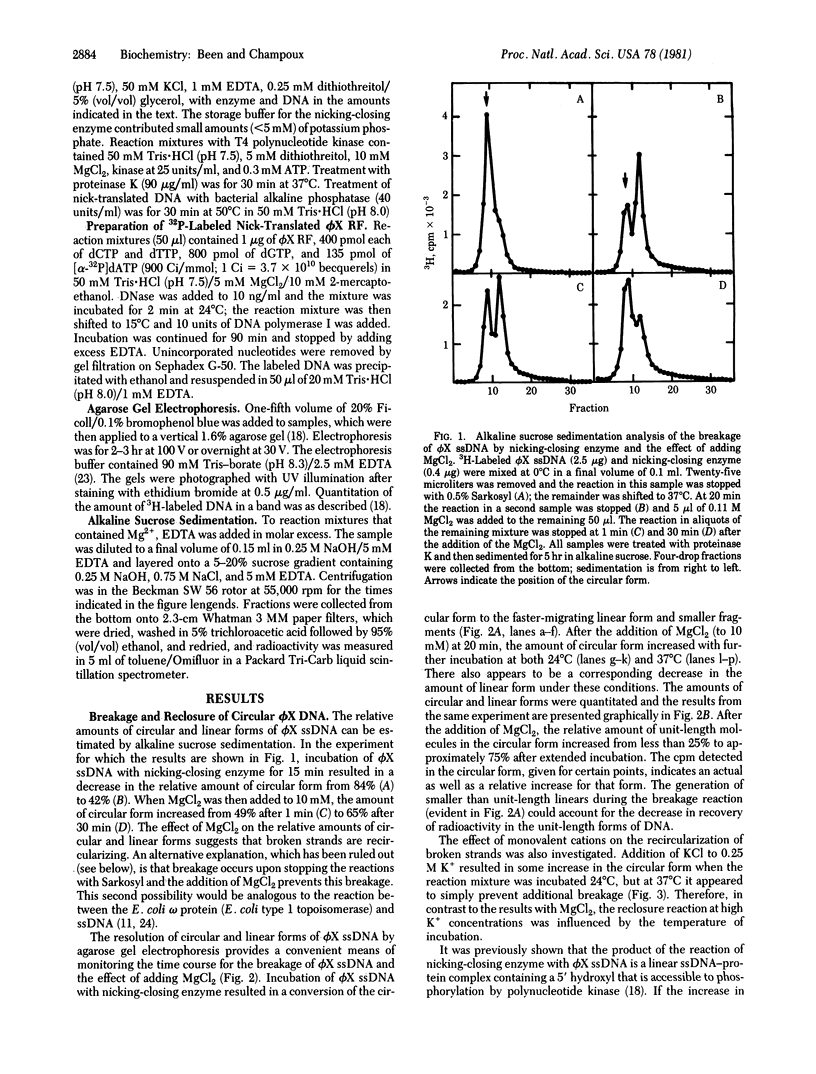
Circular single strands of bacteriophage phi X174 DNA are broken by rat liver DNA nicking-closing enzyme (type 1 topoisomerase) in low salt (50 mM KCl) at 37 degrees C, generating linear strands containing covalently bound enzyme [Been, M. D. & Champoux, J. J. (1980) Nucleic Acids Res. 8, 6129-6142]. The linear strands can be recircularized in the presence of 10 mM MgCl2 at 24 degrees C and 37 degrees C or 250 mM KCl at 24 degrees C. Recircularization is blocked when the hydroxyl group at the 5' terminus is phosphorylated. The linears generated by the nicking-closing enzyme can also be joined to other DNA fragments containing 5' hydroxyls, but not 5' phosphates. The linkage formed in both the intrastrand and interstrand reactions is stable to alkali. Reclosure of broken single strands is presumed to be analogous to the closure step that occurs durng nicking and closing cycles on duplex DNA.
Full text
PDF
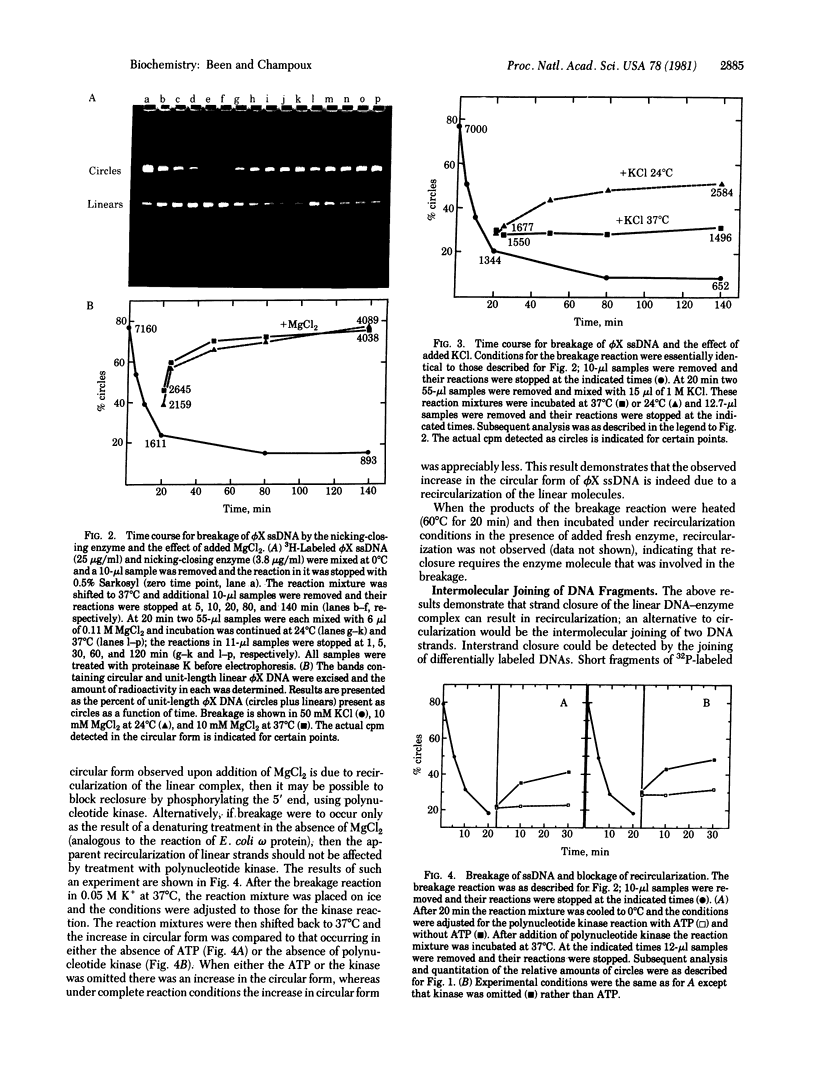

Images in this article
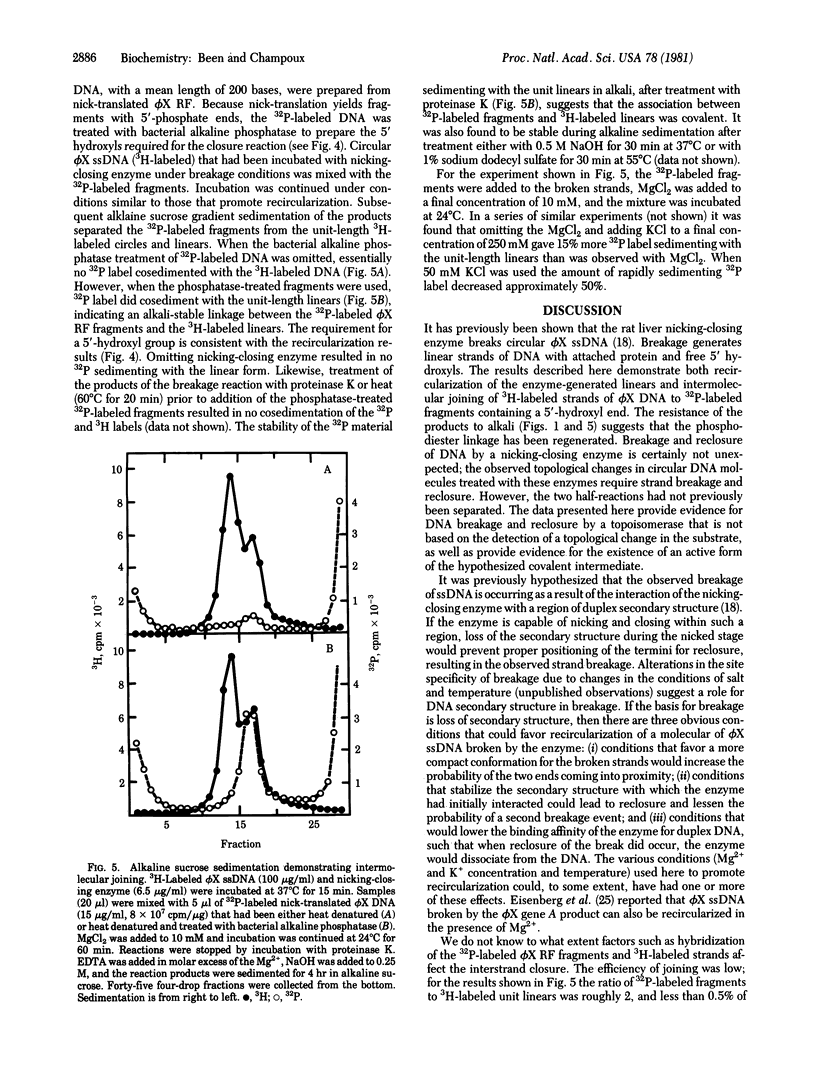
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Been M. D., Champoux J. J. Breakage of single-stranded DNA by rat liver nicking-closing enzyme with the formation of a DNA-enzyme complex. Nucleic Acids Res. 1980 Dec 20;8(24):6129–6142. doi: 10.1093/nar/8.24.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux J. J., Dulbecco R. An activity from mammalian cells that untwists superhelical DNA--a possible swivel for DNA replication (polyoma-ethidium bromide-mouse-embryo cells-dye binding assay). Proc Natl Acad Sci U S A. 1972 Jan;69(1):143–146. doi: 10.1073/pnas.69.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux J. J. Evidence for an intermediate with a single-strand break in the reaction catalyzed by the DNA untwisting enzyme. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3488–3491. doi: 10.1073/pnas.73.10.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux J. J., McConaughy B. L. Priming of superhelical SV40 DNA by Escherichia coli RNA polymerase for in vitro DNA synthesis. Biochemistry. 1975 Jan 28;14(2):307–316. doi: 10.1021/bi00673a017. [DOI] [PubMed] [Google Scholar]
- Champoux J. J., McConaughy B. L. Purification and characterization of the DNA untwisting enzyme from rat liver. Biochemistry. 1976 Oct 19;15(21):4638–4642. doi: 10.1021/bi00666a014. [DOI] [PubMed] [Google Scholar]
- Champoux J. J. Mechanism of the reaction catalyzed by the DNA untwisting enzyme: attachment of the enzyme to 3'-terminus of the nicked DNA. J Mol Biol. 1978 Jan 25;118(3):441–446. doi: 10.1016/0022-2836(78)90238-3. [DOI] [PubMed] [Google Scholar]
- Champoux J. J. Proteins that affect DNA conformation. Annu Rev Biochem. 1978;47:449–479. doi: 10.1146/annurev.bi.47.070178.002313. [DOI] [PubMed] [Google Scholar]
- Champoux J. J. Renaturation of complementary single-stranded DNA circles: complete rewinding facilitated by the DNA untwisting enzyme. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5328–5332. doi: 10.1073/pnas.74.12.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux J. J. Strand breakage by the DNA untwisting enzyme results in covalent attachment of the enzyme to DNA. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3800–3804. doi: 10.1073/pnas.74.9.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depew R. E., Liu L. F., Wang J. C. Interaction between DNA and Escherichia coli protein omega. Formation of a complex between single-stranded DNA and omega protein. J Biol Chem. 1978 Jan 25;253(2):511–518. [PubMed] [Google Scholar]
- Eisenberg S., Griffith J., Kornberg A. phiX174 cistron A protein is a multifunctional enzyme in DNA replication. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3198–3202. doi: 10.1073/pnas.74.8.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson G. N., Vapnek D. A simple method of preparing large amounts of phiX174 RF 1 supercoiled DNA. Biochim Biophys Acta. 1973 Apr 11;299(4):516–520. doi: 10.1016/0005-2787(73)90223-2. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y., Nash H. A. Nicking-closing activity associated with bacteriophage lambda int gene product. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3760–3764. doi: 10.1073/pnas.76.8.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard K., Wang J. C. Escherichia coli DNA topoisomerase I catalyzed linking of single-stranded rings of complementary base sequences. Nucleic Acids Res. 1978 Oct;5(10):3811–3820. doi: 10.1093/nar/5.10.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer K. N., Cozzarelli N. R. Formation and resolution of DNA catenanes by DNA gyrase. Cell. 1980 May;20(1):245–254. doi: 10.1016/0092-8674(80)90252-4. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Loeb L. A. On the fidelity of DNA replication. Effect of divalent metal ion activators and deoxyrionucleoside triphosphate pools on in vitro mutagenesis. J Biol Chem. 1979 Jul 10;254(13):5718–5725. [PubMed] [Google Scholar]
- Liu L. F., Depew R. E., Wang J. C. Knotted single-stranded DNA rings: a novel topological isomer of circular single-stranded DNA formed by treatment with Escherichia coli omega protein. J Mol Biol. 1976 Sep 15;106(2):439–452. doi: 10.1016/0022-2836(76)90095-4. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Liu C. C., Alberts B. M. Type II DNA topoisomerases: enzymes that can unknot a topologically knotted DNA molecule via a reversible double-strand break. Cell. 1980 Mar;19(3):697–707. doi: 10.1016/s0092-8674(80)80046-8. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Interaction between DNA and Escherichia coli DNA topoisomerase I. Formation of complexes between the protein and superhelical and nonsuperhelical duplex DNAs. J Biol Chem. 1979 Nov 10;254(21):11082–11088. [PubMed] [Google Scholar]
- Mizuuchi K., Fisher L. M., O'Dea M. H., Gellert M. DNA gyrase action involves the introduction of transient double-strand breaks into DNA. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1847–1851. doi: 10.1073/pnas.77.4.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuuchi K., Gellert M., Nash H. A. Involement of supertwisted DNA in integrative recombination of bacteriophage lambda. J Mol Biol. 1978 May 25;121(3):375–392. doi: 10.1016/0022-2836(78)90370-4. [DOI] [PubMed] [Google Scholar]
- Prell B., Vosberg H. P. Analysis of covalent complexes formed between calf thymus DNA topoisomerase and single-stranded DNA. Eur J Biochem. 1980 Jul;108(2):389–398. doi: 10.1111/j.1432-1033.1980.tb04734.x. [DOI] [PubMed] [Google Scholar]
- Sugino A., Higgins N. P., Cozzarelli N. R. DNA gyrase subunit stoichiometry and the covalent attachment of subunit A to DNA during DNA cleavage. Nucleic Acids Res. 1980 Sep 11;8(17):3865–3874. doi: 10.1093/nar/8.17.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J. Physical and topological properties of circular DNA. J Gen Physiol. 1966 Jul;49(6):103–125. doi: 10.1085/jgp.49.6.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. Interaction between DNA and an Escherichia coli protein omega. J Mol Biol. 1971 Feb 14;55(3):523–533. doi: 10.1016/0022-2836(71)90334-2. [DOI] [PubMed] [Google Scholar]




