Abstract
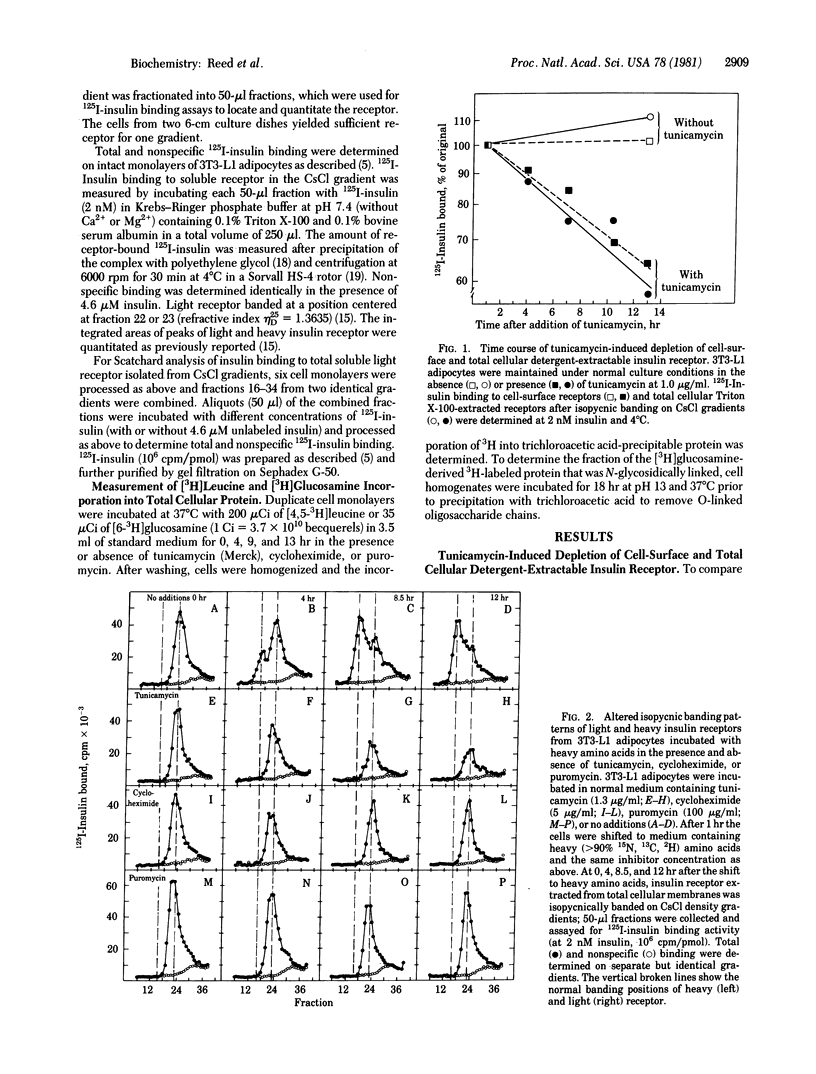
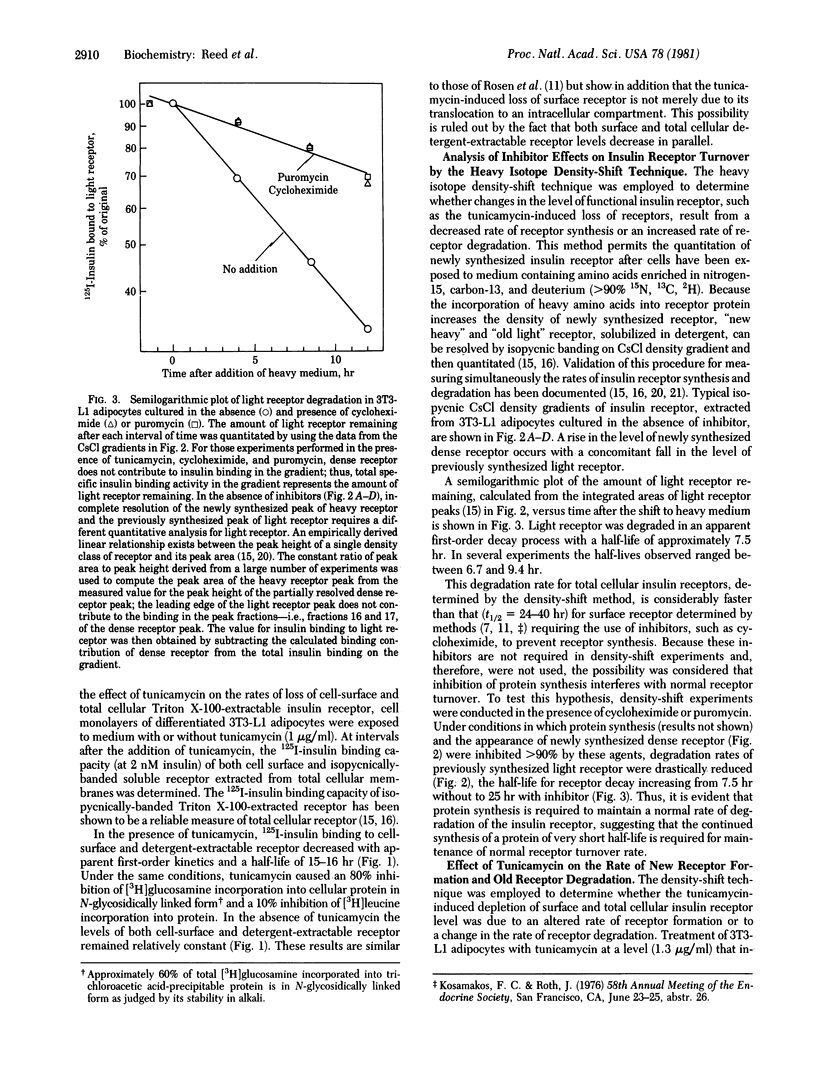
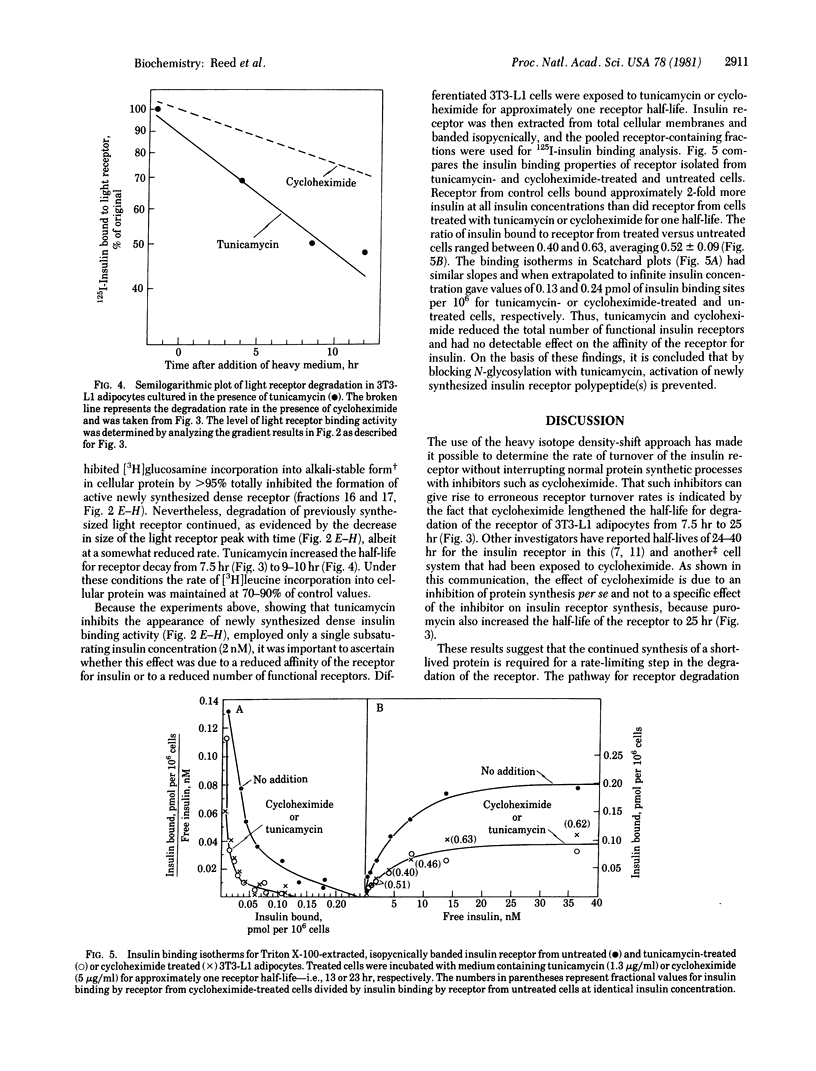
The roles of glycosylation and protein synthesis in the maintenance of insulin receptor levels and turnover rates in 3T3-L1 adipocytes were investigated. The heavy isotope density-shift technique was employed to determine the effects of inhibitors of these processes on the rates of synthesis and degradation of cellular insulin receptors. Inhibitors of protein synthesis--i.e., cycloheximide and puromycin--markedly decreased the rate of degradation of the insulin receptor, the half-life for receptor decay increasing from 7.5 hr without to 25 hr with inhibitor. The continued synthesis of a short-lived protein appears to be necessary for normal insulin receptor turnover. Tunicamycin, a potent inhibitor of core oligosaccharide addition in the formation of N-glycosidically linked glycoproteins, caused the depletion of cell-surface and total cellular detergent-extractable insulin receptors. This inhibitor totally prevented the formation of functional newly synthesized insulin receptor, yet receptor degradation was affected minimally. Thus, glycosylation of the receptor appears to be required for its activation after translation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. S., Goldstein J. L. Receptor-mediated endocytosis: insights from the lipoprotein receptor system. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3330–3337. doi: 10.1073/pnas.76.7.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crettaz M., Jeanrenaud B. Postreceptor alterations in the states of insulin resistance. Metabolism. 1980 May;29(5):467–473. doi: 10.1016/0026-0495(80)90172-9. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Isolation of the insulin receptor of liver and fat-cell membranes (detergent-solubilized-( 125 I)insulin-polyethylene glycol precipitation-sephadex). Proc Natl Acad Sci U S A. 1972 Feb;69(2):318–322. doi: 10.1073/pnas.69.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Membrane receptors. Annu Rev Biochem. 1974;43(0):169–214. doi: 10.1146/annurev.bi.43.070174.001125. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Freychet P. O., Orci L. Internalization of polypeptide hormones: mechanism, intracellular localization and significance. Diabetologia. 1980 Apr;18(4):263–274. doi: 10.1007/BF00251003. [DOI] [PubMed] [Google Scholar]
- Harrison L. C., Martin F. I., Melick R. A. Correlation between insulin receptor binding in isolated fat cells and insulin sensitivity in obese human subjects. J Clin Invest. 1976 Dec;58(6):1435–1441. doi: 10.1172/JCI108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S. S., Kolodny G. M. Insulin receptors in 3T3 fibroblasts. Relationship to growth phase, transformation and differentiation into new cell types. Exp Cell Res. 1977 Jul;107(2):293–299. doi: 10.1016/0014-4827(77)90352-4. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Hazum E., Cuatrecasas P. The subunit structure of rat liver insulin receptor. Antibodies directed against the insulin-binding subunit. J Biol Chem. 1980 Jul 25;255(14):6937–6940. [PubMed] [Google Scholar]
- Kahn C. R. Role of insulin receptors in insulin-resistant states. Metabolism. 1980 May;29(5):455–466. doi: 10.1016/0026-0495(80)90171-7. [DOI] [PubMed] [Google Scholar]
- Karlsson F. A., Grunfeld C., Kahn C. R., Roth J. Regulation of insulin receptors and insulin responsiveness in 3T3-L1 fatty fibroblasts. Endocrinology. 1979 May;104(5):1383–1392. doi: 10.1210/endo-104-5-1383. [DOI] [PubMed] [Google Scholar]
- Kosmakos F. C., Roth J. Insulin-induced loss of the insulin receptor in IM-9 lymphocytes. A biological process mediated through the insulin receptor. J Biol Chem. 1980 Oct 25;255(20):9860–9869. [PubMed] [Google Scholar]
- Krupp M. N., Livingston J. N. Insulin binding to solubilized material from fat cell membranes: evidence for two binding species. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2593–2597. doi: 10.1073/pnas.75.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp M., Lane M. D. On the mechanism of ligand-induced down-regulation of insulin receptor level in the liver cell. J Biol Chem. 1981 Feb 25;256(4):1689–1694. [PubMed] [Google Scholar]
- Mackall J. C., Student A. K., Polakis S. E., Lane M. D. Induction of lipogenesis during differentiation in a "preadipocyte" cell line. J Biol Chem. 1976 Oct 25;251(20):6462–6464. [PubMed] [Google Scholar]
- Maturo J. M., 3rd, Hollenberg M. D. Insulin receptors in transformed fibroblasts and in adipocytes: a comparative study. Can J Biochem. 1979 Jun;57(6):497–506. doi: 10.1139/o79-063. [DOI] [PubMed] [Google Scholar]
- Reed B. C., Kaufmann S. H., Mackall J. C., Student A. K., Lane M. D. Alterations in insulin binding accompanying differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4876–4880. doi: 10.1073/pnas.74.11.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed B. C., Lane M. D. Expression of insulin receptors during preadipocyte differentiation. Adv Enzyme Regul. 1980;18:97–117. doi: 10.1016/0065-2571(80)90011-4. [DOI] [PubMed] [Google Scholar]
- Reed B. C., Lane M. D. Insulin receptor synthesis and turnover in differentiating 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):285–289. doi: 10.1073/pnas.77.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed B. C., Ronnett G. V., Clements P. R., Lane M. D. Regulation of insulin receptor metabolism. Differentiation-induced alteration of receptor synthesis and degradation. J Biol Chem. 1981 Apr 25;256(8):3917–3925. [PubMed] [Google Scholar]
- Rosen O. M., Chia G. H., Fung C., Rubin C. S. Tunicamycin-mediated depletion of insulin receptors in 3T3-L1 adipocytes. J Cell Physiol. 1979 Apr;99(1):37–42. doi: 10.1002/jcp.1040990106. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Hirsch A., Fung C., Rosen O. M. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J Biol Chem. 1978 Oct 25;253(20):7570–7578. [PubMed] [Google Scholar]
- Struck D. K., Lennarz W. J. Evidence for the participation of saccharide-lipids in the synthesis of the oligosaccharide chain of ovalbumin. J Biol Chem. 1977 Feb 10;252(3):1007–1013. [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Weintraub B. D., Stannard B. S., Linnekin D., Marshall M. Relationship of glycosylation to de novo thyroid-stimulating hormone biosynthesis and secretion by mouse pituitary tumor cells. J Biol Chem. 1980 Jun 25;255(12):5715–5723. [PubMed] [Google Scholar]


