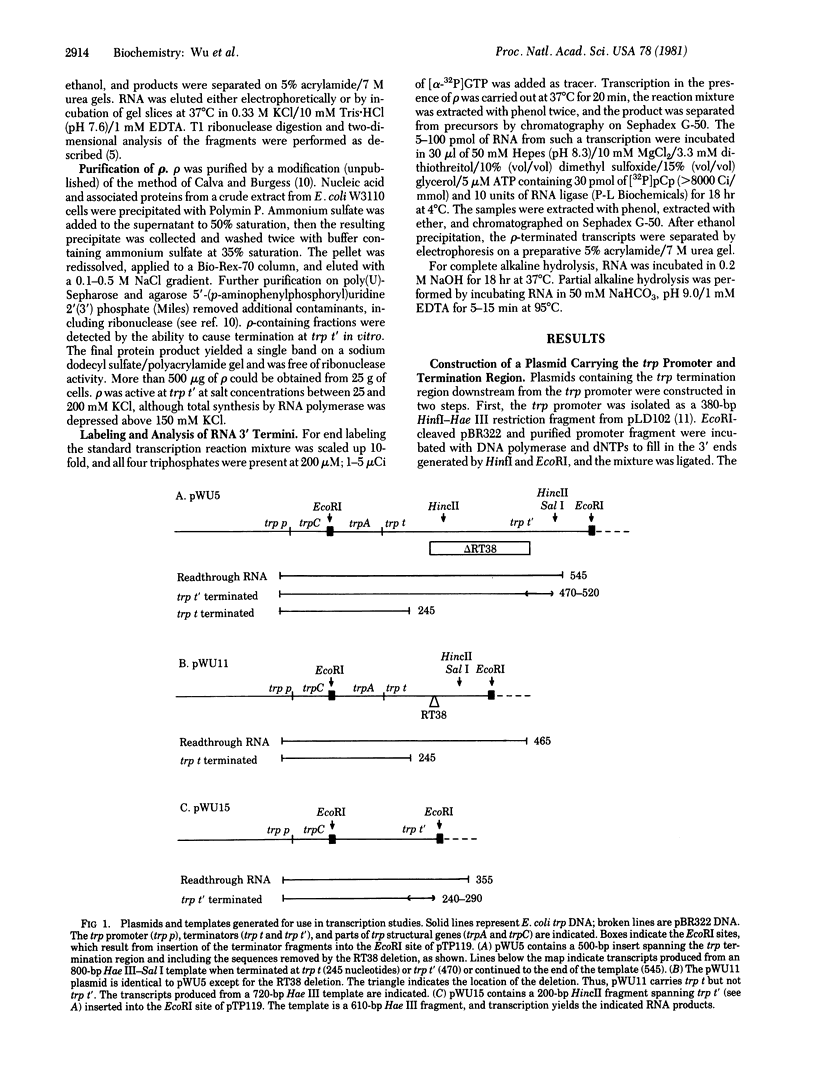
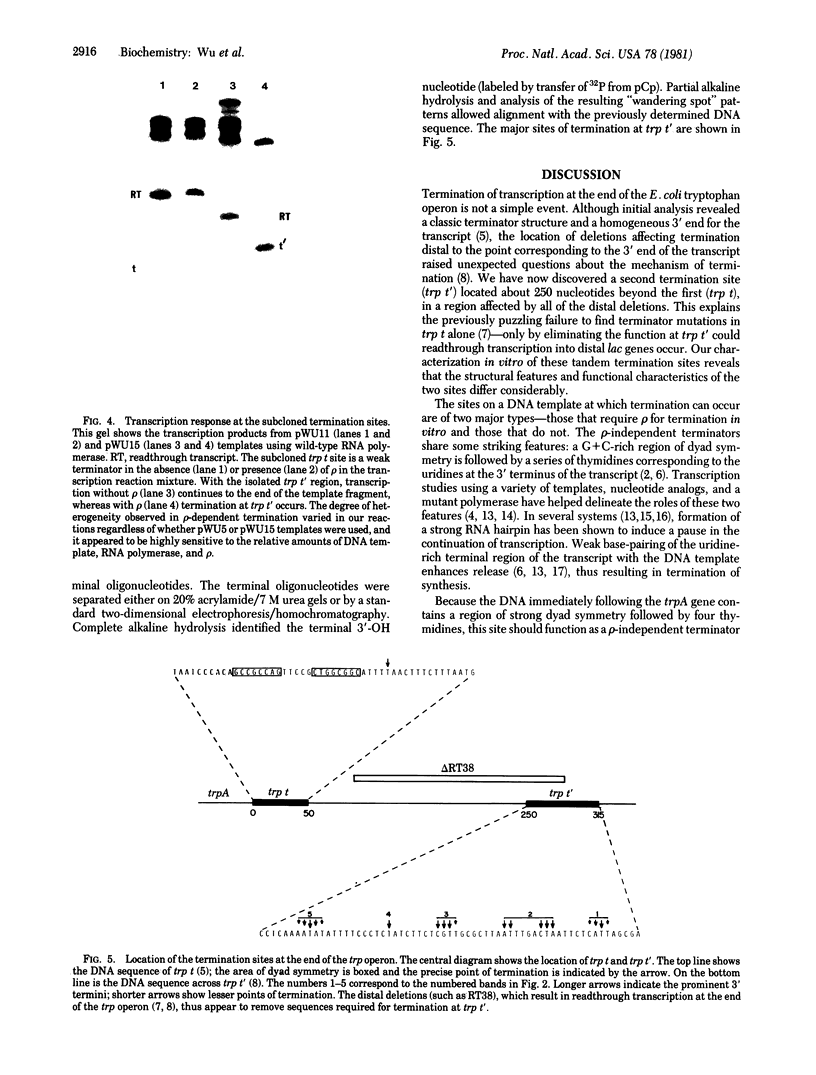
Abstract
In vivo, transcription of tryptophan (trp) operon mRNA appears to terminate at a site (trp t) 36 nucleotides after the last structural gene, and efficient function at this site requires the protein factor rho. However, distal nucleotide sequences also seem to play a role in modulating termination at trp t. We report here our in vitro studies of DNA fragments carrying portions of the trp termination region. Transcription of these DNA fragments in a purified system demonstrates that RNA polymerase actually recognizes two different termination sites. Termination at the previously characterized site, trp t, is only 25% efficient, and it is unaffected by the presence of rho factor in vitro. However, addition of rho to the transcription reaction mixture reveals that termination also occurs within a region that we have designated trp t', located about 250 bases past trp t. These two sites behave independently in vitro, whether in the tandem configuration or cloned separately, and their structural features and functional characteristics are quite different. This contrasts with the observation that termination of transcription at the end of the trp operon in vivo appears to require a rho-mediated interaction between trp t and trp t'. The possible involvement of other factors and the significance of multiple termination sites is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Calva E., Burgess R. R. Characterization of a rho-dependent termination site within the cro gene of bacteriophage lambda. J Biol Chem. 1980 Nov 25;255(22):11017–11022. [PubMed] [Google Scholar]
- Calva E., Rosenvold E. C., Szybalski W., Burgess R. R. Analysis of the in vitro synthesis of 5'-gamma-32P-labeled transcripts from coliphage lambda by gel electrophoresis, RNA-DNA hybridization, and RNase T1 digestion. J Biol Chem. 1980 Nov 25;255(22):11011–11016. [PubMed] [Google Scholar]
- Christie G. E., Platt T. A functional hybrid ribosome binding site in tryptophan operon messenger RNA of Escherichia coli. J Mol Biol. 1980 Nov 5;143(3):335–341. doi: 10.1016/0022-2836(80)90195-3. [DOI] [PubMed] [Google Scholar]
- Farnham P. J., Platt T. A model for transcription termination suggested by studies on the trp attenuator in vitro using base analogs. Cell. 1980 Jul;20(3):739–748. doi: 10.1016/0092-8674(80)90320-7. [DOI] [PubMed] [Google Scholar]
- Farnham P. J., Platt T. Rho-independent termination: dyad symmetry in DNA causes RNA polymerase to pause during transcription in vitro. Nucleic Acids Res. 1981 Feb 11;9(3):563–577. doi: 10.1093/nar/9.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J., Li J. Interaction of the sigma factor and the nusA gene protein of E. coli with RNA polymerase in the initiation-termination cycle of transcription. Cell. 1981 May;24(2):421–428. doi: 10.1016/0092-8674(81)90332-9. [DOI] [PubMed] [Google Scholar]
- Greenblatt J. Regulation of transcription termination by the N gene protein of bacteriophage lambda. Cell. 1981 Apr;24(1):8–9. doi: 10.1016/0092-8674(81)90495-5. [DOI] [PubMed] [Google Scholar]
- Guarente L. P., Beckwith J. Mutant RNA polymerase of Escherichia coli terminates transcription in strains making defective rho factor. Proc Natl Acad Sci U S A. 1978 Jan;75(1):294–297. doi: 10.1073/pnas.75.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. P., Beckwth J., Wu A. M., Platt T. A mutation distal to the messenger RNA endpoint reduces transcription termination in the tryptophan operon in Escherichia coli. J Mol Biol. 1979 Sep 5;133(1):189–197. doi: 10.1016/0022-2836(79)90258-4. [DOI] [PubMed] [Google Scholar]
- Küpper H., Sekiya T., Rosenberg M., Egan J., Landy A. A rho-dependent termination site in the gene coding for tyrosine tRNA su3 of Escherichia coli. Nature. 1978 Mar 30;272(5652):423–428. doi: 10.1038/272423a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F. H., Tinoco I., Jr DNA-RNA hybrid duplexes containing oligo(dA:rU) sequences are exceptionally unstable and may facilitate termination of transcription. Nucleic Acids Res. 1980 May 24;8(10):2295–2299. doi: 10.1093/nar/8.10.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. P., Blumenberg M., Yanofsky C. Comparison of the nucleoside sequence of trpA and sequences immediately beyond the trp operon of Klebsiella aerogenes. Salmonella typhimurium and Escherichia coli. Nucleic Acids Res. 1981 Apr 10;9(7):1743–1755. doi: 10.1093/nar/9.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T. Termination of transcription and its regulation in the tryptophan operon of E. coli. Cell. 1981 Apr;24(1):10–23. doi: 10.1016/0092-8674(81)90496-7. [DOI] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D., Shimatake H., Brady C., Wulff D. L. The relationship between function and DNA sequence in an intercistronic regulatory region in phage lambda. Nature. 1978 Mar 30;272(5652):414–423. doi: 10.1038/272414a0. [DOI] [PubMed] [Google Scholar]
- Wu A. M., Chapman A. B., Platt T., Guarente L. P., Beckwith J. Deletions of distal sequence after termination of transcription at the end of the tryptophan operon in E. coli. Cell. 1980 Apr;19(4):829–836. doi: 10.1016/0092-8674(80)90073-2. [DOI] [PubMed] [Google Scholar]
- Wu A. M., Platt T. Transcription termination: nucleotide sequence at 3' end of tryptophan operon in Escherichia coli. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5442–5446. doi: 10.1073/pnas.75.11.5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]






