Abstract
The metabolism of mRNA--protein complexes present in polyribosomes of mouse L cells was investigated with the aid of ultraviolet light-induced crosslinking of proteins to RNA. It was shown that a set of at least seven proteins which are synthesized and become associated with mRNA under conditions permitting mRNA synthesis also are synthesized and become associated with mRNA when mRNA synthesis is inhibited with a high dose of actinomycin D. This set includes a protein of Mr 78,000 which can be crosslinked to poly(A). These findings imply that mRNA-associated proteins exchange in the cytoplasm with a pool of free proteins. Taken together with results from other laboratories, they suggest a role for mRNA-associated proteins in translation. The labeling kinetics of mRNA-associated proteins were also investigated and found to be consistent with cytoplasmic addition to mRNA and a half-life of more than 2 hr. Additional implications of these findings are discussed.
Full text
PDF
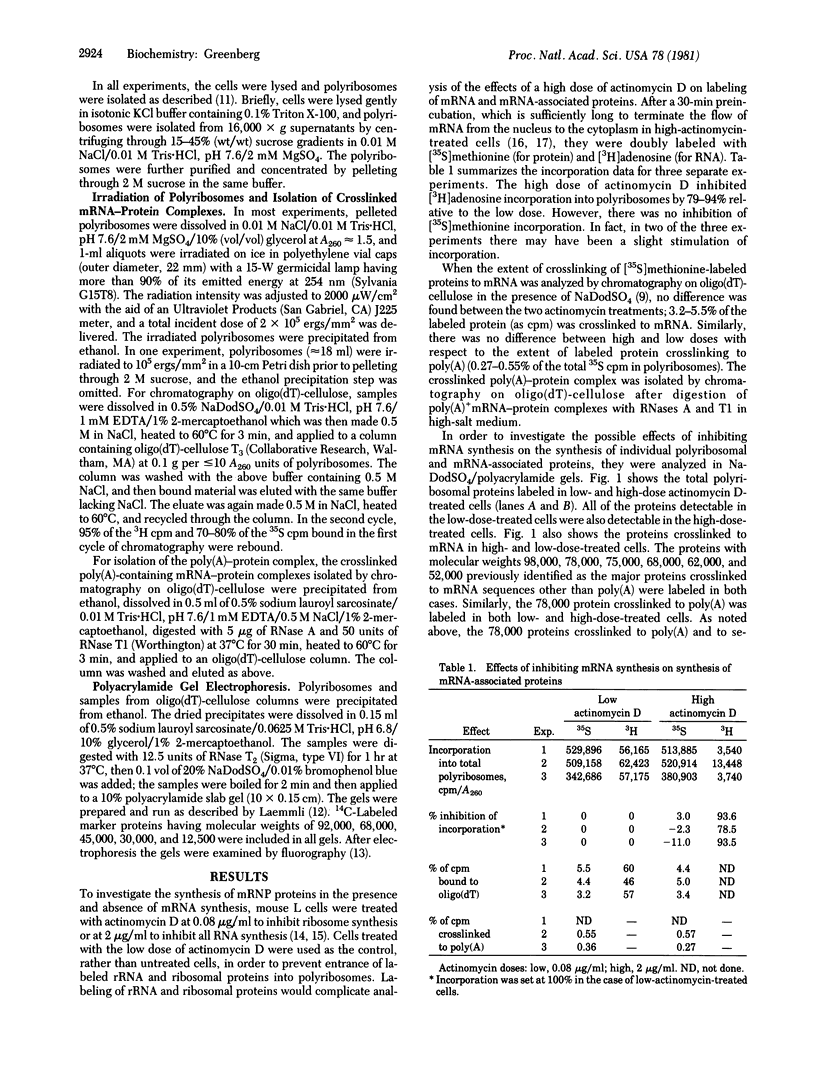
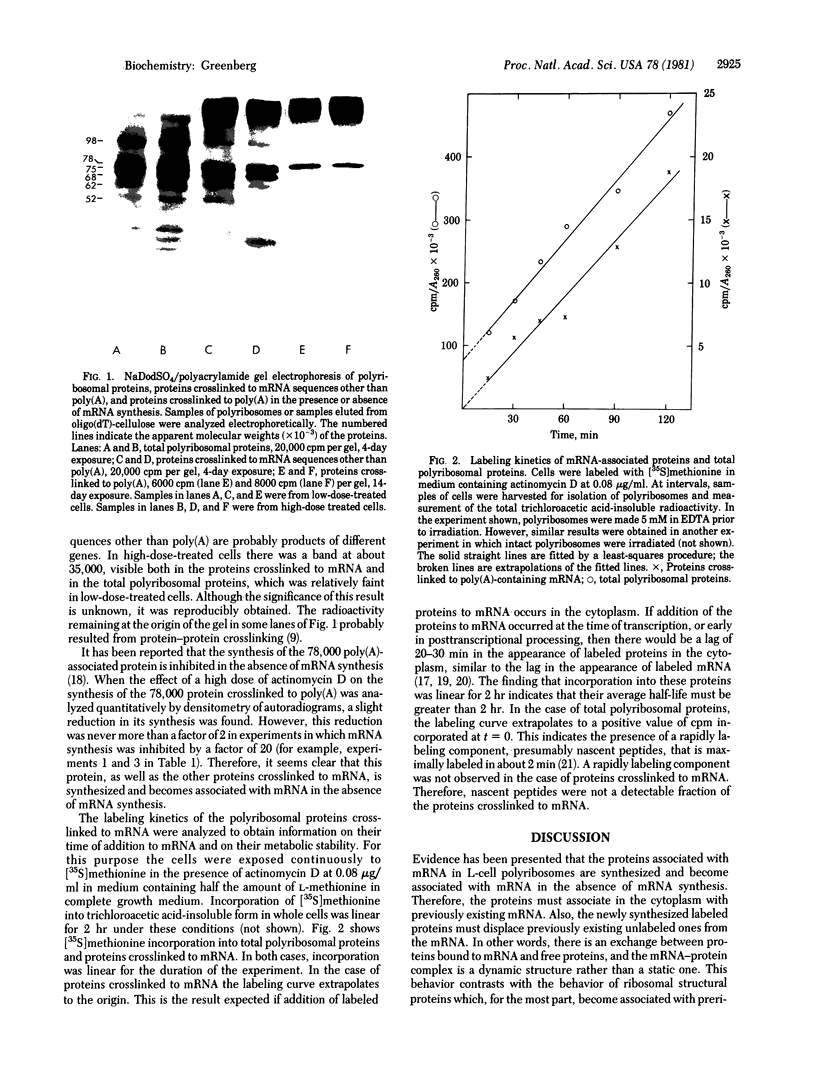

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer B. W., Kornberg R. D. Repeating structure of cytoplasmic poly(A)-ribonucleoprotein. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1890–1892. doi: 10.1073/pnas.77.4.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig N. C. On the regulation of the synthesis of ribosomal proteins in L-cells. J Mol Biol. 1971 Jan 14;55(1):129–134. doi: 10.1016/0022-2836(71)90288-9. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. High stability of messenger RNA in growing cultured cells. Nature. 1972 Nov 10;240(5376):102–104. doi: 10.1038/240102a0. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. Isolation of L-cell messenger RNA which lacks poly(adenylate). Biochemistry. 1976 Aug 10;15(16):3516–3522. doi: 10.1021/bi00661a019. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. Messenger RNA metabolism of animal cells. Possible involvement of untranslated sequences and mRNA-associated proteins. J Cell Biol. 1975 Feb;64(2):269–288. doi: 10.1083/jcb.64.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. R. Proteins crosslinked to messenger RNA by irradiating polyribosomes with ultraviolet light. Nucleic Acids Res. 1980 Dec 11;8(23):5685–5701. doi: 10.1093/nar/8.23.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish V. M., Pederson T. Poly (A)-rich ribonucleoprotein complexes from HeLa cell messenger RNA. J Biol Chem. 1976 Oct 10;251(19):5888–5894. [PubMed] [Google Scholar]
- Kumar A., Pederson T. Comparison of proteins bound to heterogeneous nuclear RNA and messenger RNA in HeLa cells. J Mol Biol. 1975 Aug 15;96(3):353–365. doi: 10.1016/0022-2836(75)90165-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Liautard J. P., Setyono B., Spindler E., Köhler K. Comparison of proteins bound to the different functional classes of messenger RNA. Biochim Biophys Acta. 1976 Apr 2;425(4):373–383. doi: 10.1016/0005-2787(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Mazur G., Schweiger A. Identical properties of an mRNA-bound protein and a cytosol protein with high affinity for polyadenylate. Biochem Biophys Res Commun. 1978 Jan 13;80(1):39–45. doi: 10.1016/0006-291x(78)91101-4. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov L. P., Spirin A. S., Erni B., Staehelin T. RNA-binding proteins of rabbit reticulocytes contain the two elongation factors and some of the initiation factors of translation. FEBS Lett. 1978 Apr 1;88(1):21–26. doi: 10.1016/0014-5793(78)80598-5. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Inhibition of RNA synthesis by actinomycin D: characteristic dose-response of different RNA species. J Cell Physiol. 1970 Oct;76(2):127–139. doi: 10.1002/jcp.1040760202. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA turnover in mouse L cells. J Mol Biol. 1973 Oct 5;79(4):681–696. doi: 10.1016/0022-2836(73)90071-5. [DOI] [PubMed] [Google Scholar]
- Preobrazhensky A. A., Spirin A. S. Informosomes and their protein components: the present state of knowledge. Prog Nucleic Acid Res Mol Biol. 1978;21:1–38. doi: 10.1016/s0079-6603(08)60265-2. [DOI] [PubMed] [Google Scholar]
- Schwartz H., Darnell J. E. The association of protein with the polyadenylic acid of HeLa cell messenger RNA: evidence for a "transport" role of a 75,000 molecular weight polypeptide. J Mol Biol. 1976 Jul 15;104(4):833–851. doi: 10.1016/0022-2836(76)90185-6. [DOI] [PubMed] [Google Scholar]
- Setyono B., Schmid H. P., Köhler K. The role of acidic proteins from cytoplasmic fractions of Krebs II ascites cells for efficient translation. Z Naturforsch C. 1979 Jan-Feb;34(1-2):64–75. doi: 10.1515/znc-1979-1-215. [DOI] [PubMed] [Google Scholar]
- Vlasik T. N., Ovchinnikov L. F., Radjabov K. M., Spirin A. S. Translation factors of the wheat embryo extract are RNA-binding proteins. FEBS Lett. 1978 Apr 1;88(1):18–20. doi: 10.1016/0014-5793(78)80597-3. [DOI] [PubMed] [Google Scholar]
- Warner J. R. The assembly of ribosomes in HeLa cells. J Mol Biol. 1966 Aug;19(2):383–398. doi: 10.1016/s0022-2836(66)80012-8. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Warner J. R. Cytoplasmic synthesis of nuclear proteins. Kinetics of accumulation of radioactive proteins in various cell fractions after brief pulses. J Cell Biol. 1971 Dec;51(3):643–652. doi: 10.1083/jcb.51.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]