Abstract
Study Objectives
Abnormal remodeling of the extracellular matrix (ECM) has been implicated in the pathogenesis of bronchopulmonary dysplasia. However, the contribution of lung parenchymal cells to ECM remodeling after mechanical injury is not well defined. The objective of these studies was to investigate in vitro the release of MMP-2 and -9 and their respective inhibitors TIMP-2 and -1, and to explore potential regulation by IL-10.
Design
Mouse fetal epithelial cells and fibroblasts isolated on E18–19 of gestation were exposed to 20% cyclic stretch to simulate lung injury. MMP-2 and MMP-9 activity were investigated by zymography and ELISA. TIMP-1 and TIMP-2 abundance were analyzed by Western blot.
Results
We found that mechanical stretch increased MMP-2 and decreased TIMP-2 in fibroblasts, indicating that excessive stretch promotes MMP-2 activation, expressed as the MMP-2/TIMP-2 ratio. Incubation with IL-10 did not change MMP-2 activity. In contrast, mechanical stretch of epithelial cells decreased MMP-9 activity and the MMP-9/TIMP-1 ratio by 60–70%. When IL-10 was added, mechanical stretch increased the MMP-9/TIMP-1 ratio by 50%.
Conclusions
We conclude that mechanical stretch differentially affects MMP-2/9 and their inhibitors in fetal lung cells. IL-10 modulates MMP-9 activity through a combination of effects on MMP-9 and TIMP-1 levels.
Keywords: Mechanical injury, Bronchopulmonary dysplasia, TIMP-1/2, Matrix metalloproteinases, Epithelial cells, Fibroblasts
Introduction
Many infants born prematurely require mechanical ventilation for survival. However, excessive stretch of the lungs by mechanical ventilation plays a key role in the development of bronchopulmonary dysplasia (BPD), the most common and serious chronic lung disease in premature infants [1, 2]. Inflammatory cells such as neutrophils and macrophages recruited to the lungs during mechanical injury exacerbate the inflammatory response by releasing proinflammatory cytokines and chemokines. Moreover, release of cytokines by lung parenchymal cells, such as epithelial cells and fibroblasts, may also contribute to the pathogenesis of lung injury secondary to mechanical ventilation [3–6].
In addition to release of cytokines, abnormal remodeling of the extracellular matrix (ECM) significantly contributes to the development of BPD [7]. Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases known for their ability to cleave many components of the ECM. They play crucial roles in lung morphogenesis, growth, and repair after injury [8]. Specifically, MMP-2 and MMP-9 have been associated with inflammatory lung injury [9] and increased levels of these MMPs have been observed in response to hyperoxia as well as in COPD and emphysema [10–12]. MMP-2 (72-kDa gelatinase or gelatinase A) is secreted mainly by noninflammatory cells (fibroblasts, endothelial and epithelial cells), whereas MMP-9 (92-kDa gelatinase or gelatinase B) is secreted mainly by inflammatory cells (neutrophils, monocyte–macrophages) [8]. However, under various forms of stimulation, MMP-9 has been shown to be released by resident cells such as alveolar epithelial cells and fibroblasts [13]. MMP-2 and MMP-9 are present in inactive pro forms which are then secreted into the extracellular environment where they are activated. The activities of MMP-2 and MMP-9 can be regulated through cytokines as well as through their natural inhibitors, tissue inhibitor of MMPs (TIMP)-1 and -2, respectively, that bind to active MMPs in a 1:1 stoichiometric ratio [9]. After inflammation and lung injury, remodeling of the ECM is an important process necessary for successful healing and repair of lung tissues. It is thought that the pathologic tissue degradation and remodeling that occurs in diseases such as asthma, acute respiratory distress syndrome (RDS), pulmonary fibrosis, and emphysema is due to an imbalance between lung tissue-degrading proteases and their inhibitors [14]. A balance between the MMPs and TIMPs is necessary for normal matrix turnover [15]. Furthermore, studies in newborn infants and baboons have also implicated abnormal ECM remodeling in the pathogenesis of BPD [16, 17].
While it is known that MMPs are present at sites of inflammation, it is unclear whether their upregulation contributes to inflammation or is a mechanism to facilitate fast ECM remodeling and wound repair [10]. Additionally, the response of parenchymal lung cells on release of MMPs and TIMPs by mechanical stretch is unknown. Therefore, the objective of this study was to investigate in vitro the response of epithelial cells and fibroblasts to injury induced by mechanical stretch. We hypothesized that activation of MMP-2/9 is affected by stretch. We further speculated that the anti-inflammatory cytokine IL-10 modulates MMPs’ activity.
Materials and Methods
Cell Isolation and Stretch Protocol
Animal experiments were performed in compliance with the Lifespan Institutional Animal Care and Use Committee, Providence, RI. Fetal mouse lungs were obtained from timed-pregnant C57BL6 mice at embryonic days (E) 18–19 (saccular stage of lung development), and fibroblasts and epithelial cells were isolated as previously described [18]. Briefly, after collagenase or dispase digestion, cell suspensions were sequentially filtered through 105-, 30-, and 15-µm nylon meshes using screen cups (Sigma). Clumped nonfiltered cells from the 30- and 15-µm nylon meshes were collected after several washes with DMEM to facilitate the filtration of nonepithelial cells. Further epithelial cell purification was achieved by incubating the cells in 75-cm2 flasks for 30 min. Nonadherent cells were collected and cultured overnight in 75-cm2 flasks containing serum-free DMEM. For fibroblast isolation, the filtrate from the 15-µm nylon mesh was plated onto 75-cm2 flasks and incubated at 37°C for 30–60 min to allow fibroblasts to adhere, then maintained overnight in serum-free DMEM. After overnight culture, cells were harvested with 0.25% (wt/vol) trypsin in 0.4 mM EDTA and plated (around 50% confluency) on Bioflex multiwell plates (Flexcell International, Hillsborough, NC) precoated with fibronectin (1.5 µg/cm2). Monolayers were maintained in culture for 1–2 days until they were approximately 80% confluent and then were mounted in a Flexcell FX-4000 Strain Unit. Before stretch, cells were washed with DMEM media and fresh serum-free DMEM was added to each well. For samples incubated with IL-10, a final concentration of 300 ng/ml of mouse recombinant IL-10 (R&D Systems, 417-ML-025/CF) was added for 30 min before the experiments. The dose of IL-10 was chosen based on previous studies from our laboratory demonstrating that this concentration decreases the release of proinflammatory cytokines and chemokines in fetal epithelial cells [4] and fibroblasts [19] exposed to mechanical stretch. Samples without preadministration of IL-10 underwent the same incubation period in order to keep samples consistent. An equibiaxial cyclical strain regimen of 20% was applied at intervals of 40 cycles/min for 48 h. This regimen, which roughly corresponds to a lung inflation of 80% of total lung capacity in adult rats [20], was chosen to mimic lung cell injury. Cells were grown on nonstretched membranes in parallel and were treated in an identical manner to serve as controls.
Gelatin Zymography Analysis of MMP-2 and MMP-9
Supernatants from each sample were loaded onto a 10% Zymogram (Gelatin) gel (Invitrogen, Carlsbad, CA) and processed according to the manufacturer’s recommendations. The amount of supernatant loaded was normalized to the corresponding cell lysate concentration for each sample and diluted with Novex Tris–Glycine SDS sample buffer (Invitrogen). Gels were stained with SimplyBlue Safestain (Invitrogen) and MMP activity was visualized as clear bands against a dark blue background. Molecular weights for pro and active MMP-2 and -9 were confirmed using recombinant MMPs as positive controls.
Active MMP-2
Supernatants from stretched and nonstretched samples were collected and concentrated to the same volume per sample using a 30-kDa MWCO Centricon® (Amicon®). Corresponding monolayers were lysed with RIPA buffer containing protease inhibitors. Lysates were centrifuged and total protein content was determined by the bicinchoninic acid method. Because there are no commercially available ELISA kits for the detection of active MMP-2 from mouse cells, we have modified the use of the Amersham MMP-2 Biotrak Activity Assay System (GE Healthcare Bio-Sciences Corp., Piscataway, NJ), which is manufactured to detect active human MMP-2. Therefore, in order to detect active mouse MMP-2 levels in supernatants, the plates precoated with anti-human MMP-2 antibody were discarded and substituted with a 96-well Costar flat-bottom clear assay plate coated at room temperature overnight with 20 µg/ml of anti-mouse active MMP-2 antibody (R&D Systems), diluted in coating buffer (0.05 M carbonate/bicarbonate). In the morning, wells were washed three times with 300 µl of 0.05% Tween-20 in PBS and the plate was blocked at room temperature with 1% BSA in PBS for a minimum of 1 h. Wells were then washed again with 0.05% Tween-20 in PBS, making the plates ready for sample addition. The assay was then conducted following the manufacturer’s protocol specific for detection of lower endogenous MMP-2 levels. Recombinant mouse/rat MMP-2 (R&D Systems), diluted in MMP-2 assay buffer [50 mM Tris, 10 mM CaCl2, 150 mM NaCl, 0.05% (w/v) Brij-35, pH 7.5], was used to make a standard curve of absorbance, with concentrations ranging from 0.19 to 12 ng/ml. p-Aminophenylmercuric acetate (APMA) was used to activate MMP-2 only in wells containing standards in order to ensure that endogenous levels of active MMP-2 were detected in samples. Concentrations of active MMP-2 in supernatants were extrapolated from the standard curve and normalized to cell lysate concentrations.
Active MMP-9
The Fluorokine® E Enzyme Activity Assay for detection of Human Active MMP-9 (R&D Systems) was modified in the same manner as the Biotrak Activity Assay kit in order to detect the amount of active MMP-9 present in the supernatants. A 96-well Costar flat-bottom black assay plate was coated at room temperature overnight with 20 µg/ml anti-mouse MMP-9 antibody (R&D Systems, AF909), diluted in coating buffer. The next day the plates were washed, blocked, and washed again in the same manner as for MMP-2 detection and the Fluorokine® E Enzyme Activity Assay was then conducted according to the manufacturer’s protocol. Recombinant mouse MMP-9 (Anaspec, 72069), diluted with calibrator diluent from the kit, was used as a standard for the assay in concentrations ranging from 0.25 to 8 ng/ml. APMA was added only to wells containing standards in order to ensure that endogenous levels of active MMP-9 were detected in samples. Concentrations of MMP-9 in supernatants were extrapolated from a standard curve of fluorescence and the resulting values were normalized to the corresponding cell lysate concentrations.
Western Blot Analysis of TIMP-1 and TIMP-2
Equal amounts of protein lysate samples were fractionated by NU-PAGE-Bis–Tris (4–12%) gel electrophoresis (Novex, San Diego, CA) and transferred to polyvinylidene difluoride membranes. Blots were hybridized with polyclonal antibody against the 22-kDa TIMP-2 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or the 32-kDa TIMP-1 (R&D Systems). Recombinant mouse TIMP-1 Western Blotting Standard (R&D Systems) was used as a positive control in order to help distinguish bands. Secondary antibody was conjugated with horseradish peroxidase; blot was developed with an enhanced chemiluminescence (ECL) detection assay (Amersham Pharmacia Biotech, Piscataway, NJ). Membranes were then stripped and reprobed with antibodies to GAPDH (to control for protein loading) and processed as described before. The intensity of the bands was analyzed by densitometry.
Gene Expression of MMP-2 and MMP-9
Total RNA was isolated as previously described [21] and purified further using the Turbo DNA-free kit (Ambion, Austin, TX). One microgram of total RNA was reverse-transcribed into cDNA using the iScript™ cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s instructions. Pre-designed TaqMan® MMP-2 (Mm00439498_m1*) and MMP-9 (Mm00442991_m1*) primers were purchased from Assays-on-Demand™ Gene Expression Products (Applied Biosystems). To amplify the cDNA by qRT-PCR, 2 µl of the resulting cDNA was added to a mixture of 10 µl of TaqMan Gene Expression Master Mix (Applied Biosystems) and Assays-on-Demand Gene Expression Assay Mix containing forward and reverse primers and TaqMan-labeled probe (Applied Biosystems). Standard curves were generated for each primer set and housekeeping gene GAPDH. Linear regression revealed efficiencies between 96 and 99%. Therefore, fold expressions of stretched samples relative to controls were calculated using the ΔΔCT method for relative quantification (RQ) as previously described [22]. Samples were normalized to GAPDH. The reactions were performed in a 7500 Fast Real-Time PCR System (Applied Biosystems) with the following parameters: 50°C for 2 min, 95°C for 10 min, and 45 cycles of 95°C for 15 s and 60°C for 1 min. All assays were performed in triplicate.
Statistical Analysis
Results are expressed as mean ± SEM from at least three experiments, using different litters for each experiment. Data were analyzed with ANOVA followed by post-hoc tests, and Instat 3.0 (GraphPad Software, La Jolla, CA) was used for statistical analysis; P < 0.05 was considered statistically significant.
Results
Gelatinase Activity
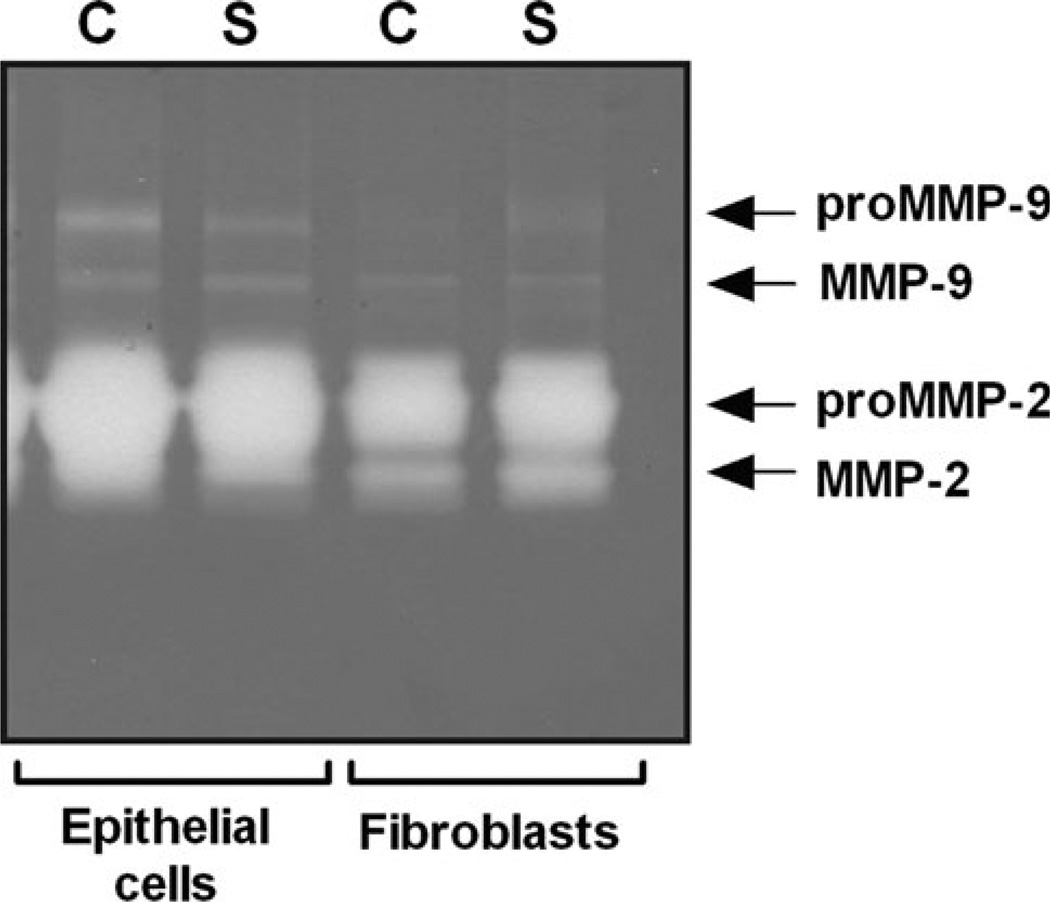
The effect of mechanical stretch on the gelatinolytic activity of MMP-2 and MMP-9 was initially evaluated by gelatin zymography. As seen in Fig. 1, zymography gel pointed to a decrease of pro-MMP-9 levels in epithelial cells after mechanical stretch; however, the signals were too weak to obtain reliable results. In contrast, MMP-2 was clearly present in both cell types. Mechanical stretch did not affect MMP-2 in epithelial cells; however, active MMP-2 (62 kDa) increased in fibroblasts after mechanical stretch. Given the limitations of this technique, we decided to evaluate the effect of stretch and IL-10 on MMP-2 and MMP-9 using a customized ELISA assay as described in “Materials and Methods”.
Fig. 1.
Gelatinase activity in the supernatant of fetal lung cells. Supernatant from epithelial cells and fibroblasts were collected from unstretched (C) and monolayers exposed to 20% cyclic stretch (S). Samples, normalized to the corresponding cell lysate concentrations, were loaded onto a 10% Zymogram (Gelatin) gel and processed according to the manufacturer’s recommendations. MMP activity was visualized as clear bands against a dark blue background. Molecular weights for pro and active MMP-2 and MMP-9 were confirmed using recombinant MMPs as positive controls. This gel is representative from two independent experiments
Mechanical Stretch Releases Active MMP-2 in Mouse Lung Fibroblasts
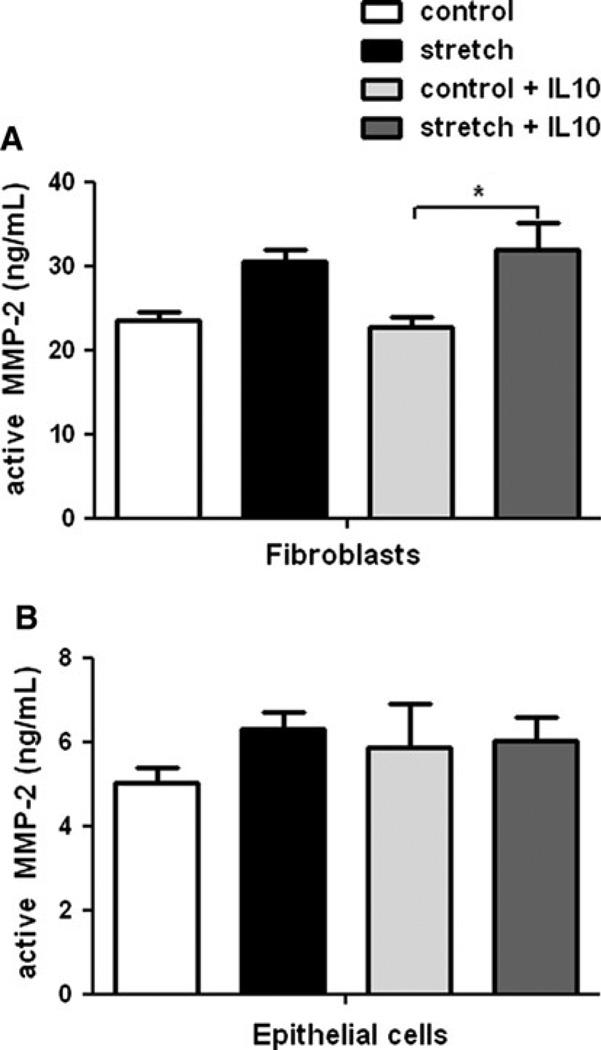
Levels of MMP-2 have been shown to be increased in the lung after hyperoxia [23] and mechanical stretch [24]. Therefore, we sought to investigate the release of MMP-2 in mouse fetal lung cells subjected to mechanical stretch. Culture supernatants were collected, concentrated, and analyzed by a customized ELISA assay as described in Materials and Methods. After 48 h of stretch, active MMP-2 increased by 30% in the supernatant of fibroblasts compared to unstretched samples (23.5 ± 0.9 vs. 30.5 ± 1.5). Similar results were obtained in samples incubated with recombinant IL-10 (22.7 ± 1.35 vs. 32 ± 3.2; n = 5; P < 0.05) (Fig. 2a). In contrast, no changes were observed in fetal epithelial cells exposed to similar experimental conditions (Fig. 2b). The effect of mechanical stretch on MMP-2 activation seems to occur at the post-transcriptional level, given that mechanical stretch did not change MMP-2 mRNA expression (data not shown).
Fig. 2.
Effect of mechanical stretch and IL-10 on MMP-2 release from fibroblasts and epithelial cells. Release of MMP-2 into the supernatant of E18–19 fibroblasts (a) and epithelial cells (b) exposed to 20% mechanical stretch for 48 h in the presence or absence of rIL-10 (300 ng/ml). Unstretched cells served as controls. Supernatants were collected and processed by a customized ELISA, as described in “Material and Methods”. Values are in ng/ml and are mean ± SEM from three to five different experiments. *P < 0.05
Effects of Stretch and IL-10 on TIMP-2 Levels and MMP-2/TIMP-2 Ratio
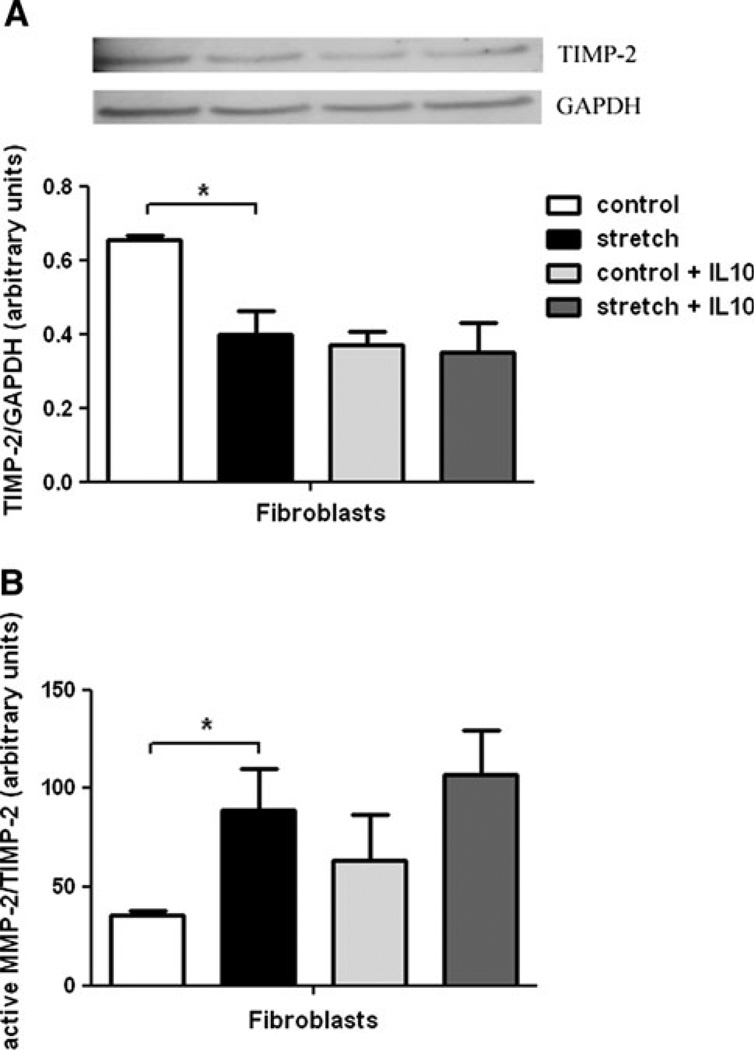
Because MMP-2 activity is also modulated by its natural inhibitor TIMP-2, we also explored the effect of mechanical stretch on TIMP-2 expression. As shown in Fig. 3a, mechanical stretch of fetal fibroblasts decreased TIMP-2 by 35% when compared to unstretched samples (0.66 ± 0.01 vs. 0.40 ± 0.06; n = 5; P < 0.05). In contrast, in stretched samples incubated with IL-10, TIMP-2 did not change when compared to controls. Results were also analyzed as the MMP-2/TIMP-2 ratio, an indicator of MMP-2 activity. Figure 3b shows that the mechanical stretch increases the MMP-2/TIMP-2 ratio by 2.5-fold (35.97 ± 1.85 vs. 88.83 ± 20.95; n = 5; P < 0.05, non-parametric Kruskal–Wallis test). In samples treated with IL-10, mechanical stretch did not change the MMP-2/TIMP-2 ratio when compared to samples without IL-10. Taken together, these data show that mechanical stretch induces MMP-2 activity in fetal fibroblasts and this effect is not altered by the addition of IL-10.
Fig. 3.
Effect of mechanical stretch and IL-10 on TIMP-2 and MMP-2/TIMP-2 ratio. a E18–19 fibroblasts were harvested and subjected to 20% mechanical stretch for 48 h. Unstretched samples were used as controls. Levels of TIMP-2 present in cell lysates before and after stretch in the presence and absence of IL-10 were measured by Western blots. Results were normalized to GAPDH. Upper panels are representative blots. Data in the lower panel are mean ± SEM from five different experiments. *P < 0.05. b Values of MMP-2 release into the supernatant were divided by the corresponding values of TIMP-2 in the cell lysate. *P < 0.05
Mechanical Stretch Decreases Release of MMP-9 in Mouse Epithelial Cells
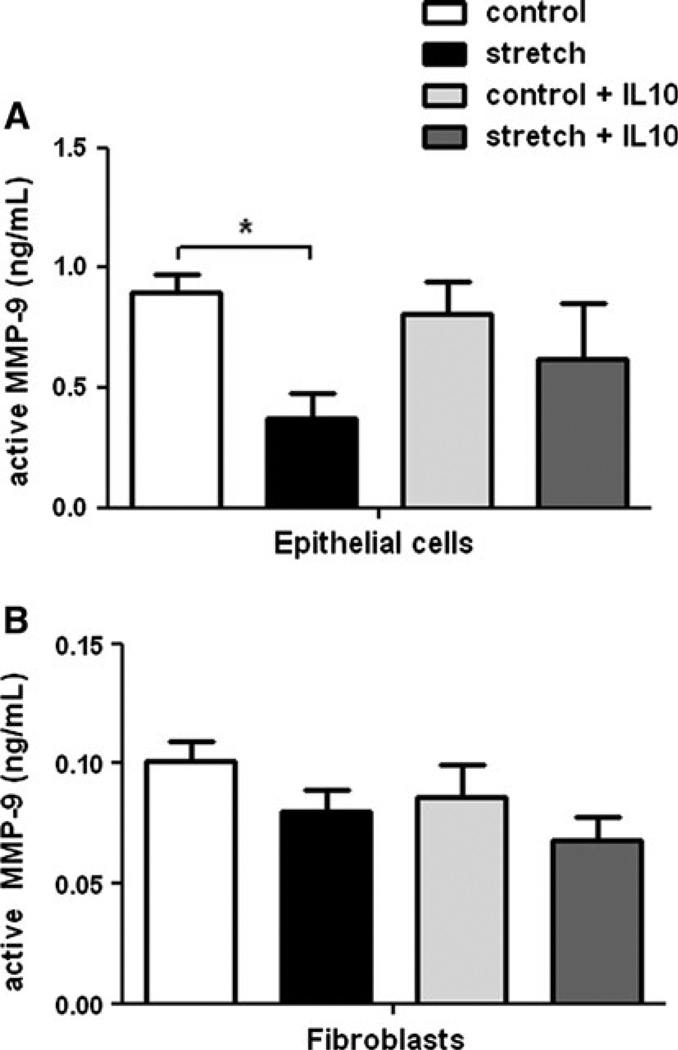
Given the important role played by MMP-9 in lung remodeling [13], we investigated next the effects of mechanical stretch on MMP-9 activation. Figure 4a demonstrates that after 48 h of stretch, the level of active MMP-9 found in the supernatant of epithelial cells decreased by 60% (0.9 ± 0.07 vs. 0.37 ± 0.11; n = 4; P < 0.05). In contrast, in the presence of IL-10, mechanical stretch did not decrease active MMP-9 (0.81 ± 0.14 vs. 0.62 ± 0.23). These data suggest that IL-10 may modulate MMP-9 activity in cells exposed to mechanical stretch. Both mechanical stretch and the addition of IL-10 did not affect the activity of MMP-9 in fibroblasts (Fig. 4b). Similar to MMP-2, mechanical stretch did not affect MMP-9 gene expression (data not shown).
Fig. 4.
Effect of mechanical stretch and IL-10 on MMP-9 release in epithelial cells and fibroblasts. Release of MMP-9 into the supernatant of E18–19 epithelial cells (a) and fibroblasts (b) exposed to 20% cyclic stretch for 48 h in the presence or absence of rIL-10 (300 ng/ml). Unstretched cells served as controls. Supernatants were collected and processed by ELISA, as described in “Material and Methods”. Values are in ng/ml and are mean ± SEM from three to five different experiments. *P < 0.05
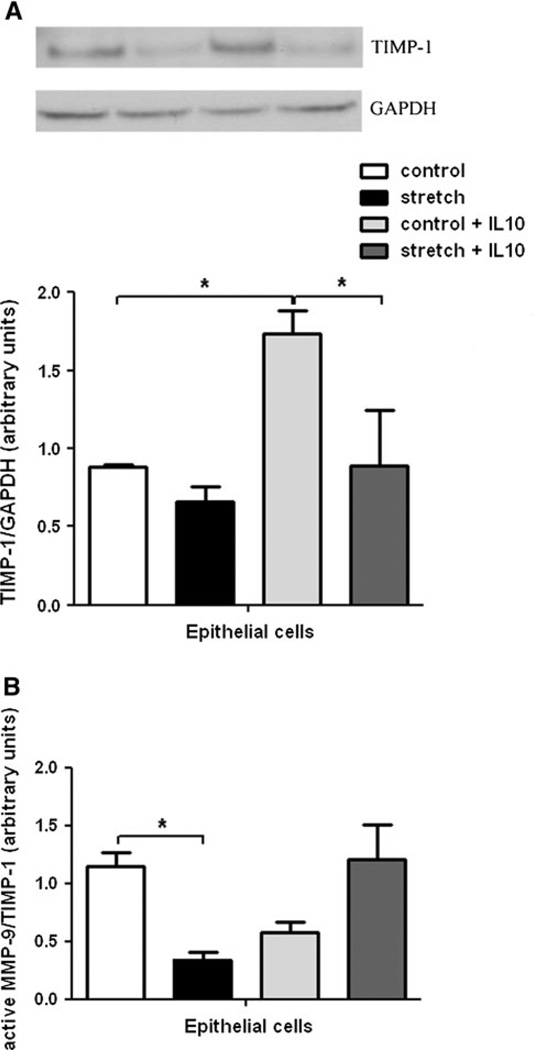
Effects of Mechanical Stretch and IL-10 on TIMP-1 and MMP-9/TIMP-1 Ratio
We then evaluated the effect of mechanical stretch on the MMP-9 inhibitor TIMP-1. In samples without IL-10, mechanical stretch did not affect TIMP-1 protein levels (0.83 ± 0.04 vs. 0.61 ± 0.05). When IL-10 was added, TIMP-1 increased by twofold in unstretched samples (0.83 ± 0.04 vs. 1.65 ± 0.23) and decreased by 50% in samples exposed to mechanical stretch (1.65 ± 0.23 vs. 0.85 ± 0.27) (Fig. 5a). We then investigated the MMP-9/TIMP-1 ratio and found that mechanical stretch decreases MMP-9/TIMP-1 by 70% (1.15 ± 0.11 vs. 0.34 ± 0.05; n = 4; P < 0.05). In samples incubated with IL-10, this ratio increased by 50% when compared to controls (0.57 ± 0.09 vs. 1.2 ± 0.30) (Fig. 5b). Altogether, our data indicate that mechanical stretch of fetal epithelial cells decreases MMP-9 activity (MMP-9/TIMP-1) and the opposite effect was observed in samples incubated with IL-10.
Fig. 5.
Effect of mechanical stretch and IL-10 on TIMP-1 and MMP-9/TIMP-1 ratio. a E18–19 epithelial cells were harvested and subjected to 20% mechanical stretch for 48 h. Unstretched samples were used as controls. Levels of TIMP-1 present in cell lysates before and after stretch in the presence and absence of IL-10 were measured by Western blots. Results were normalized to GAPDH. Upper panels are representative blots. Data in the lower panel are mean ± SEM from five different experiments. *P < 0.05. b Values of MMP-9 release into the supernatant were divided by the corresponding values of TIMP-1 in the cell lysate. *P < 0.05
Discussion
The main findings of this study are that activation of MMP-2 and MMP-9 is affected by mechanical stretch in fetal lung cells. In addition, we observed that this response is selective for different cell types. Excessive mechanical stretch increases active MMP-2 and decreases active MMP-9 released into supernatant from lung fibroblasts and epithelial cells, respectively. Furthermore, we found that IL-10 modulates MMP-9 activity through a combination of effects on MMP-9 and TIMP-1 levels.
MMP-2 and MMP-9 play key roles in pericellular basement membrane turnover by degrading type IV collagen, a main component of the basement membrane. MMP-2 in particular is important for branching morphogenesis and alveolarization [25, 26]. In addition, MMP-2 has been associated with the development of acute lung injury [27]. Previous in vitro studies have shown that mechanical stretch increases MMP-2 in lung endothelial cells [24], airway and vascular smooth muscle cells [28, 29], and atrial myocytes [30]. It has also been shown that MMP-2 levels are increased after injury induced by hyperoxia [23]. Consistent with these observations, we found an increase of active MMP-2 in the supernatant of fibroblasts exposed to injurious stretch. Because the activity of MMP-2 is regulated via the antiprotease TIMP-2, we also investigated how mechanical stretch affects TIMP-2. We were unable to detect TIMP-2 protein in the supernatant; therefore, we used TIMP-2 protein in the cell lysate instead. These data further support activation of MMP-2 in fibroblasts due to mechanical stretch. Stretch not only releases active MMP-2 but also decreases levels of the MMP-2 inhibitor TIMP-2. Therefore, the activity of MMP-2, expressed as MMP-2/TIMP-2 ratio, was significantly increased after stretch (Fig. 3b). Although past investigations have found that IL-10 can regulate MMP-2 activity directly [31–33] or indirectly via TIMP-2 [34], our studies show that IL-10 does not modulate MMP-2 activity in fetal lung cells. This discrepancy could be attributed to differences in the experimental system, such as stimulus applied or cell response specificity.
The mechanism by which mechanical stretch stimulates MMP-2 is not well understood. It has been shown, for example, that MMP-2 activation can be regulated at the cell surface, requiring preliminary binding to a receptor complex formed by the membrane-bound MT1-MMP and TIMP-2 [9]. Another potential mechanism is via proinflammatory cytokines. Recent data from our laboratory [19] have shown that fibroblasts exposed to similar experimental conditions release several proinflammatory cytokines and chemokines, including IL-1β and TNF-α, among others. Both TNF-α and IL-1β have been shown to increase levels of MMP-2 [35, 36].
Previous investigations have found that low MMP-2 levels at birth are associated with the development of BPD [16, 37]. In contrast, other studies have shown an association of increased MMP-2 levels and inflammation. For example, MMP-2 knockout mice demonstrate less inflammation in pulmonary airways [34, 38, 39]. Furthermore, inhibition of MMP-2 has been shown to protect against injury induced by mechanical ventilation [27]. Our in vitro data support the latter studies and suggest that activation of MMP-2 by mechanical stretch might be injurious to the lung.
Contrary to MMP-2, we observed that mechanical stretch decreased the release of active MMP-9 into the supernatant of epithelial cells. In contrast, neither mechanical stretch nor the addition of IL-10 affected the activity of MMP-9 in fibroblasts. One plausible explanation is that MMP-9 is secreted predominantly by inflammatory cells and unlikely to play a key role in fibroblasts. Our results agree with previous investigations in which isolated epithelial cells showed a decrease of MMP-9 after hyperoxic injury [12]. As discussed in the “Introduction”, MMPs are activated upon release into the extracellular environment and their activity can be regulated through their natural inhibitors, TIMPs. The active form/inhibitor ratio is often used as surrogate for the in vivo enzymatic activity. Therefore, activation of MMP could be due to an increase of the active form, a decrease of the inhibitor, or a combination of both. Our data in Fig. 4a show that stretch decreases active MMP-9. TIMP-1 is an inhibitor of MMP-9 and thus if MMP-9 goes down we might expect that TIMP-1 should go up in response to stretch, but in Fig. 5a TIMP-1 does not change. These data indicate that the effect of stretch on the decrease of MMP-9 activity observed in Fig. 5b is not mediated via TIMP-1. Incubation with IL-10 prevented stretch-mediated decrease of MMP-9 (Fig. 4a), suggesting that IL-10 may modulate the activity of MMP-9 in epithelial cells exposed to stretch. When the MMP-9 inhibitor was investigated, we found that TIMP-1 was decreased by stretch in the presence of IL-10 (Fig. 5a). Although mechanical stretch in the presence of IL-10 did not change active MMP-9 when compared to controls (Fig. 4a), the fact that mechanical stretch plus IL-10 decreased TIMP-1 (Fig. 5a) shifted the balance (expressed as MMP-9/TIMP-1) to MMP-9 activation and that is what is reflected in Fig. 5b.
Another observation derived from these studies is the increase of TIMP-1 protein levels mediated by IL-10 (see Fig. 5a, control vs. control + IL-10). The increase of TIMP-1 by IL-10 has been previously shown in alveolar macrophages from smokers [40], prostate tumor cells [41], and hepatic fibrosis [32]. The mechanism by which IL-10 affects TIMP-1 could be by increasing gene transcription of TIMP-1 [42] or indirectly by inhibiting proinflammatory cytokines. The increase of TIMP-1 by IL-10 in control samples can be partially responsible for the increase of the MMP-9/TIMP-1 ratio after stretch, given that as the denominator (TIMP-1) increases, the ratio (active MMP-9/TIMP-1) goes down and therefore we able to observe major differences when compared to stretch samples (Fig. 5b). However, IL-10 also has a direct effect on MMP-9 itself, independent of TIMP-1, as reflected by the lack of increase of TIMP-1 in cell exposed to stretch plus IL-10 when compared to stretch alone (Fig. 5a). Therefore, the overall effect of IL-10 on MMP-9 activity can then be explained as a combination of its effect on TIMP-1 activity and MMP-9 levels.
Whether an increase of MMP-9 is beneficial or harmful during inflammation is not resolved. Some studies have found an increase of MMP-9 and the MMP-9/TIMP-1 ratio in extremely premature baboons with BPD [17] and after hyperoxic injury [43]. In contrast, other investigations have observed that lack of MMP-9 worsens lung injury [44, 45]. Support for the beneficial role of MMP-9 comes from investigations focused on the MMP-9/TIMP-1 ratio. Lanchou et al. [10] found that when the MMP-9/TIMP-1 ratio favored MMP-9 production, the propensity to develop ARDS was lower and patients experienced a quicker healing time. In another study, levels of TIMP-1 were seen to be higher in smokers than in nonsmokers, indicating more inhibition of MMP-9, which is a possible contributor to the development of emphysema [40]. The recruitment of inflammatory cells such as neutrophils and macrophages to sites of injury and their subsequent release of MMP-9 may be another mechanism whereby the immune system works to protect and repair tissues, releasing MMP-9 to initiate matrix remodeling and assist in wound repair rather than contribute to inflammation [46]. Our data support a beneficial role of MMP-9 in lung injury, given that injurious mechanical stretch decreased MMP-9. Our studies also suggest a potential protective role of IL-10.
In summary our in vitro data show that excessive mechanical stretch of fetal lung parenchymal cells induces a differential effect on MMP-2 and MMP-9 activation. IL-10 may modulate some of these responses, specifically MMP-9 activity, by acting on both MMP-9 and TIMP-1 release to shift the ratio in favor of MMP-9 activity. Given that IL-10 is a potent anti-inflammatory cytokine, we speculate that these results might have clinical relevance in ameliorating lung injury induced by mechanical ventilation. Further studies are required to elucidate the exact mechanisms of this anti-inflammatory activity and are currently being explored.
Acknowledgment
This study was supported by grants from National Institutes of Health (HD052670) and Rhode Island Hospital, Department of Pediatrics.
References
- 1.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 2.Tibboel D, Jobe AH. Update in pediatric lung disease 2009. Am J Respir Crit Care Med. 2010;181(7):661–665. doi: 10.1164/rccm.201001-0117UP. [DOI] [PubMed] [Google Scholar]
- 3.Hammerschmidt S, Kuhn H, Sack U, Schlenska A, Gessner C, Gillissen A, et al. Mechanical stretch alters alveolar type II cell mediator release toward a proinflammatory pattern. Am J Respir Cell Mol Biol. 2005;33(2):203–210. doi: 10.1165/rcmb.2005-0067OC. [DOI] [PubMed] [Google Scholar]
- 4.Lee HS, Wang Y, Maciejewski BS, Esho K, Fulton C, Sharma S, et al. Interleukin-10 protects cultured fetal rat type II epithelial cells from injury induced by mechanical stretch. Am J Physiol Lung Cell Mol Physiol. 2008;294(2):L225–L232. doi: 10.1152/ajplung.00370.2007. [DOI] [PubMed] [Google Scholar]
- 5.Vlahakis NE, Schroeder MA, Limper AH, Hubmayr RD. Stretch induces cytokine release by alveolar epithelial cells in vitro. Am J Physiol. 1999;277(1 Pt 1):L167–L173. doi: 10.1152/ajplung.1999.277.1.L167. [DOI] [PubMed] [Google Scholar]
- 6.Gahler A, Stallmach T, Schwaller J, Fey MF, Tobler A. Interleukin-8 expression by fetal and neonatal pulmonary cells in hyaline membrane disease and amniotic infection. Pediatr Res. 2000;48(3):299–303. doi: 10.1203/00006450-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Albertine KH, Jones GP, Starcher BC, Bohnsack JF, Davis PL, Cho SC, et al. Chronic lung injury in preterm lambs. Disordered respiratory tract development. Am J Respir Crit Care Med. 1999;159(3):945–958. doi: 10.1164/ajrccm.159.3.9804027. [DOI] [PubMed] [Google Scholar]
- 8.Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev. 2007;87(1):69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakrabarti S, Patel KD. Matrix metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Exp Lung Res. 2005;31(6):599–621. doi: 10.1080/019021490944232. [DOI] [PubMed] [Google Scholar]
- 10.Corbel M, Boichot E, Lagente V. Role of gelatinases MMP-2 and MMP-9 in tissue remodeling following acute lung injury. Braz J Med Biol Res. 2000;33(7):749–754. doi: 10.1590/s0100-879x2000000700004. [DOI] [PubMed] [Google Scholar]
- 11.Ohbayashi H. Matrix metalloproteinases in lung diseases. Curr Protein Pept Sci. 2002;3(4):409–421. doi: 10.2174/1389203023380549. [DOI] [PubMed] [Google Scholar]
- 12.Buckley S, Warburton D. Dynamics of metalloproteinase-2 and -9, TGF-beta, and uPA activities during normoxic vs hyperoxic alveolarization. Am J Physiol Lung Cell Mol Physiol. 2002;283(4):L747–L754. doi: 10.1152/ajplung.00415.2001. [DOI] [PubMed] [Google Scholar]
- 13.Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol. 2003;28(1):12–24. doi: 10.1165/rcmb.2002-0166TR. [DOI] [PubMed] [Google Scholar]
- 14.Lagente V, Manoury B, Nenan S, Le Quement C, Martin-Chouly C, Boichot E. Role of matrix metalloproteinases in the development of airway inflammation and remodeling. Braz J Med Biol Res. 2005;38(10):1521–1530. doi: 10.1590/s0100-879x2005001000009. [DOI] [PubMed] [Google Scholar]
- 15.Cederqvist K, Sorsa T, Tervahartiala T, Maisi P, Reunanen K, Lassus P, et al. Matrix metalloproteinases-2, -8, and -9 and TIMP-2 in tracheal aspirates from preterm infants with respiratory distress. Pediatrics. 2001;108(3):686–692. doi: 10.1542/peds.108.3.686. [DOI] [PubMed] [Google Scholar]
- 16.Ekekezie II, Thibeault DW, Simon SD, Norberg M, Merrill JD, Ballard RA, et al. Low levels of tissue inhibitors of metalloproteinases with a high matrix metalloproteinase-9/tissue inhibitor of metalloproteinase-1 ratio are present in tracheal aspirate fluids of infants who develop chronic lung disease. Pediatrics. 2004;113(6):1709–1714. doi: 10.1542/peds.113.6.1709. [DOI] [PubMed] [Google Scholar]
- 17.Tambunting F, Beharry KD, Hartleroad J, Waltzman J, Stavitsky Y, Modanlou HD. Increased lung matrix metalloproteinase-9 levels in extremely premature baboons with bronchopulmonary dysplasia. Pediatr Pulmonol. 2005;39(1):5–14. doi: 10.1002/ppul.20135. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Esteban J, Wang Y, Gruppuso PA, Rubin LP. Mechanical stretch induces fetal type II cell differentiation via an epidermal growth factor receptor-extracellular-regulated protein kinase signaling pathway. Am J Respir Cell Mol Biol. 2004;30(1):76–83. doi: 10.1165/rcmb.2003-0121OC. [DOI] [PubMed] [Google Scholar]
- 19.Hawwa RL, Hokenson MA, Wang Y, Huang Z, Sharma S, Sanchez-Esteban J. IL-10 inhibits inflammatory cytokines released by fetal mouse lung fibroblasts exposed to mechanical stretch. Pediatr Pulmonol. 2011 doi: 10.1002/ppul.21433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tschumperlin DJ, Oswari J, Margulies AS. Deformation-induced injury of alveolar epithelial cells. Effect of frequency, duration, and amplitude. Am J Respir Crit Care Med. 2000;162(2 Pt 1):357–362. doi: 10.1164/ajrccm.162.2.9807003. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Maciejewski BS, Drouillard D, Santos M, Hokenson MA, Hawwa RL, et al. A role for caveolin-1 in mechanotransduction of fetal type II epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2010;298(6):L775–L783. doi: 10.1152/ajplung.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Maciejewski BS, Lee N, Silbert O, McKnight NL, Frangos JA, et al. Strain-induced fetal type II epithelial cell differentiation is mediated via cAMP-PKA-dependent signaling pathway. Am J Physiol Lung Cell Mol Physiol. 2006;291(4):L820–L827. doi: 10.1152/ajplung.00068.2006. [DOI] [PubMed] [Google Scholar]
- 23.Pardo A, Barrios R, Maldonado V, Melendez J, Perez J, Ruiz V, et al. Gelatinases A and B are up-regulated in rat lungs by subacute hyperoxia: pathogenetic implication. Am J Pathol. 1998;153(3):833–844. doi: 10.1016/S0002-9440(10)65625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haseneen NA, Vaday GG, Zucker S, Foda HD. Mechanical stretch induces MMP-2 release and activation in lung endothelium: role of EMMPRIN. Am J Physiol Lung Cell Mol Physiol. 2003;284(3):L541–L547. doi: 10.1152/ajplung.00290.2002. [DOI] [PubMed] [Google Scholar]
- 25.Kheradmand F, Rishi K, Werb Z. Signaling through the EGF receptor controls lung morphogenesis in part by regulating MT1-MMP-mediated activation of gelatinase A/MMP2. J Cell Sci. 2002;115(Pt 4):839–848. doi: 10.1242/jcs.115.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukuda Y, Ishizaki M, Okada Y, Seiki M, Yamanaka N. Matrix metalloproteinases and tissue inhibitor of metalloproteinase-2 in fetal rabbit lung. Am J Physiol Lung Cell Mol Physiol. 2000;279(3):L555–L561. doi: 10.1152/ajplung.2000.279.3.L555. [DOI] [PubMed] [Google Scholar]
- 27.Foda HD, Rollo EE, Drews M, Conner C, Appelt K, Shalinsky DR, et al. Ventilator-induced lung injury upregulates and activates gelatinases and EMMPRIN: attenuation by the synthetic matrix metalloproteinase inhibitor, Prinomastat (AG3340) Am J Respir Cell Mol Biol. 2001;25(6):717–724. doi: 10.1165/ajrcmb.25.6.4558f. [DOI] [PubMed] [Google Scholar]
- 28.Hasaneen NA, Zucker S, Cao J, Chiarelli C, Panettieri RA, Foda HD. Cyclic mechanical strain-induced proliferation and migration of human airway smooth muscle cells: role of EMMPRIN and MMPs. FASEB J. 2005;19(11):1507–1509. doi: 10.1096/fj.04-3350fje. [DOI] [PubMed] [Google Scholar]
- 29.O’Callaghan CJ, Williams B. Mechanical strain-induced extracellular matrix production by human vascular smooth muscle cells: role of TGF-beta(1) Hypertension. 2000;36(3):319–324. doi: 10.1161/01.hyp.36.3.319. [DOI] [PubMed] [Google Scholar]
- 30.Saygili E, Rana OR, Meyer C, Gemein C, Andrzejewski MG, Ludwig A, et al. The angiotensin-calcineurin-NFAT pathway mediates stretch-induced up-regulation of matrix metalloproteinases-2/-9 in atrial myocytes. Basic Res Cardiol. 2009;104(4):435–448. doi: 10.1007/s00395-008-0772-6. [DOI] [PubMed] [Google Scholar]
- 31.John M, Oltmanns U, Fietze I, Witt C, Jung K. Increased production of matrix metalloproteinase-2 in alveolar macrophages and regulation by interleukin-10 in patients with acute pulmonary sarcoidosis. Exp Lung Res. 2002;28(1):55–68. doi: 10.1080/019021402753355535. [DOI] [PubMed] [Google Scholar]
- 32.Zheng WD, Zhang LJ, Shi MN, Chen ZX, Chen YX, Huang YH, et al. Expression of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 in hepatic stellate cells during rat hepatic fibrosis and its intervention by IL-10. World J Gastroenterol. 2005;11(12):1753–1758. doi: 10.3748/wjg.v11.i12.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stearns ME, Kim G, Garcia F, Wang M. Interleukin-10 induced activating transcription factor 3 transcriptional suppression of matrix metalloproteinase-2 gene expression in human prostate CPTX-1532 Cells. Mol Cancer Res. 2004;2(7):403–416. [PubMed] [Google Scholar]
- 34.Chou WY, Lu CN, Lee TH, Wu CL, Hung KS, Concejero AM, et al. Electroporative interleukin-10 gene transfer ameliorates carbon tetrachloride-induced murine liver fibrosis by MMP and TIMP modulation. Acta Pharmacol Sin. 2006;27(4):469–476. doi: 10.1111/j.1745-7254.2006.00304.x. [DOI] [PubMed] [Google Scholar]
- 35.Han YP, Tuan TL, Wu H, Hughes M, Garner WL. TNF-alpha stimulates activation of pro-MMP2 in human skin through NF-(kappa)B mediated induction of MT1-MMP. J Cell Sci. 2001;114(Pt 1):131–139. doi: 10.1242/jcs.114.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roomi MW, Monterrey JC, Kalinovsky T, Niedzwiecki A, Rath M. Modulation of MMP-2 and MMP-9 by cytokines, mitogens and inhibitors in lung cancer and malignant mesothelioma cell lines. Oncol Rep. 2009;22(6):1283–1291. doi: 10.3892/or_00000566. [DOI] [PubMed] [Google Scholar]
- 37.Danan C, Jarreau PH, Franco ML, Dassieu G, Grillon C, Abd Alsamad I, et al. Gelatinase activities in the airways of premature infants and development of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2002;283(5):L1086–L1093. doi: 10.1152/ajplung.00066.2002. [DOI] [PubMed] [Google Scholar]
- 38.Tan RJ, Fattman CL, Niehouse LM, Tobolewski JM, Hanford LE, Li Q, et al. Matrix metalloproteinases promote inflammation and fibrosis in asbestos-induced lung injury in mice. Am J Respir Cell Mol Biol. 2006;35(3):289–297. doi: 10.1165/rcmb.2005-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsusaka H, Ide T, Matsushima S, Ikeuchi M, Kubota T, Sunagawa K, et al. Targeted deletion of matrix metalloproteinase 2 ameliorates myocardial remodeling in mice with chronic pressure overload. Hypertension. 2006;47(4):711–717. doi: 10.1161/01.HYP.0000208840.30778.00. [DOI] [PubMed] [Google Scholar]
- 40.Lim S, Roche N, Oliver BG, Mattos W, Barnes PJ, Chung KF. Balance of matrix metalloprotease-9 and tissue inhibitor of metalloprotease-1 from alveolar macrophages in cigarette smokers. Regulation by interleukin-10. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1355–1360. doi: 10.1164/ajrccm.162.4.9910097. [DOI] [PubMed] [Google Scholar]
- 41.Stearns ME, Rhim J, Wang M. Interleukin 10 (IL-10) inhibition of primary human prostate cell-induced angiogenesis: IL-10 stimulation of tissue inhibitor of metalloproteinase-1 and inhibition of matrix metalloproteinase (MMP)-2/MMP-9 secretion. Clin Cancer Res. 1999;5(1):189–196. [PubMed] [Google Scholar]
- 42.Lacraz S, Nicod LP, Chicheportiche R, Welgus HG, Dayer JM. IL-10 inhibits metalloproteinase and stimulates TIMP-1 production in human mononuclear phagocytes. J Clin Invest. 1995;96(5):2304–2310. doi: 10.1172/JCI118286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chetty A, Cao GJ, Severgnini M, Simon A, Warburton R, Nielsen HC. Role of matrix metalloprotease-9 in hyperoxic injury in developing lung. Am J Physiol Lung Cell Mol Physiol. 2008;295(4):L584–L592. doi: 10.1152/ajplung.00441.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lukkarinen H, Hogmalm A, Lappalainen U, Bry K. Matrix metalloproteinase-9 deficiency worsens lung injury in a model of bronchopulmonary dysplasia. Am J Respir Cell Mol Biol. 2009;41(1):59–68. doi: 10.1165/rcmb.2008-0179OC. [DOI] [PubMed] [Google Scholar]
- 45.Albaiceta GM, Gutierrez-Fernandez A, Parra D, Astudillo A, Garcia-Prieto E, Taboada F, et al. Lack of matrix metalloproteinase-9 worsens ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;294(3):L535–L543. doi: 10.1152/ajplung.00334.2007. [DOI] [PubMed] [Google Scholar]
- 46.Cataldo D, Munaut C, Noel A, Frankenne F, Bartsch P, Foidart JM, et al. MMP-2- and MMP-9-linked gelatinolytic activity in the sputum from patients with asthma and chronic obstructive pulmonary disease. Int Arch Allergy Immunol. 2000;123(3):259–267. doi: 10.1159/000024452. [DOI] [PubMed] [Google Scholar]