Abstract
Pancreatic tumors can be classified by their morphologic features on CT. The subtypes include solid tumors, mixed cystic and solid lesions, unilocular cysts, multilocular cystic lesions, and microcystic lesions. Endoscopic US and MRI can provide detailed information for classifying pancreatic lesions. Each subtype has different kinds of tumors and malignant potential, thus the classification can be useful for a better differential diagnosis and treatment planning. For this purpose, we suggest an appropriate modified classification system by using the imaging features of pancreatic tumors with an emphasis on CT findings and illustrate various findings of typical and atypical manifestations.
Keywords: Pancreatic tumors, Computed tomography, Magnetic resonance imaging (MRI), Endoscopic ultrasound (EUS)
INTRODUCTION
While clinical features such as age and sex are important in differentiating pancreatic tumors, imaging features can be very informative for both the detection and characterization of lesions. For example, most solid pancreatic tumors are malignant or potentially malignant, so surgical resection should be initially considered as a curative treatment. On the other hand, cystic pancreatic lesions may be either benign or have malignant potential. Therefore, accurate diagnosis of pancreatic lesions is critical for management planning. However, accurate characterization and differentiation are still challenging because of their morphologic overlap (1, 2).
Multi-detector computed tomography (MDCT) is usually used as an initial imaging tool for evaluating pancreatic lesions and is an excellent modality for both detection and characterization. Once pancreatic lesions are found on MDCT, magnetic resonance imaging (MRI) and endoscopic ultrasound (EUS) can be used for further characterization (3, 4). MRI has been accepted as a useful imaging modality for evaluating cystic lesions due to its excellent soft tissue contrast. EUS is valuable for precisely demonstrating internal structures such as septa and mural nodules, thanks to its high spatial resolution (5). Classification schemes for pancreatic cystic tumors have been previously proposed based on the morphologic features of the lesions (1, 5). However, we suggest another modified classification system based on the imaging features of pancreatic tumors, with an emphasis on CT findings for better differential diagnosis and treatment planning. The correlation between the pathologic specimens to the imaging features is briefly described along the figures.
Classification of the Pancreatic Tumors: Categorization Based on Gross Morphology and Malignant Potential
In this article, we classified pancreatic tumors into solid tumors, mixed cystic and solid lesions, unilocular cysts, multilocular cystic lesions, and microcystic lesions. This classification is based on the previously suggested classifications for pancreatic cystic tumors (1, 5). However, under the influence of the ovarian tumor classification of the International Ovarian Tumor Analysis (IOTA) group, we thought "solid tumors" should be included as a category in pancreatic tumor classification according to its morphologic features. Therefore we created a category for solid and cystic tumors. Multilocular cystic tumors were divided into either a lobulated, pleomorphic, or smooth shape with septation for better differentiation (Table 1). Because each pancreatic neoplasm has characteristic morphologic features and different malignant potential, this classification system of pancreatic tumors can be helpful in characterizing and managing the lesions (Fig. 1). Quite a number of potentially malignant cystic pancreatic tumors (e.g., mucinous cystic neoplasms [MCNs] or intraductal papillary mucinous neoplasms [IPMNs]), show typical morphologic features, allowing therapeutic planning to be determined based on imaging findings.
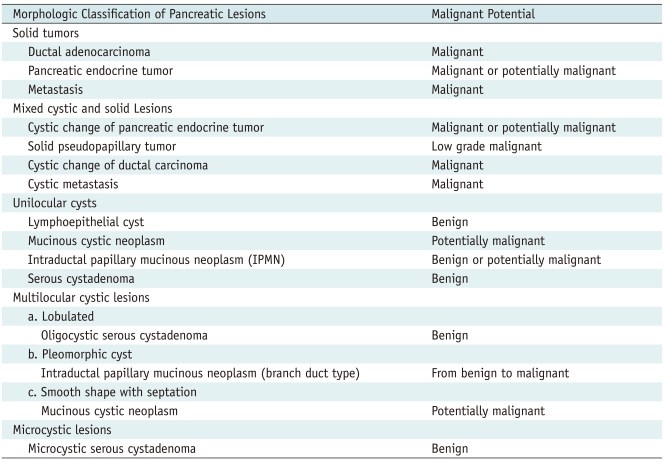
Table 1.
Classification of Pancreatic Tumors Based on Gross Morphology and Malignant Potential
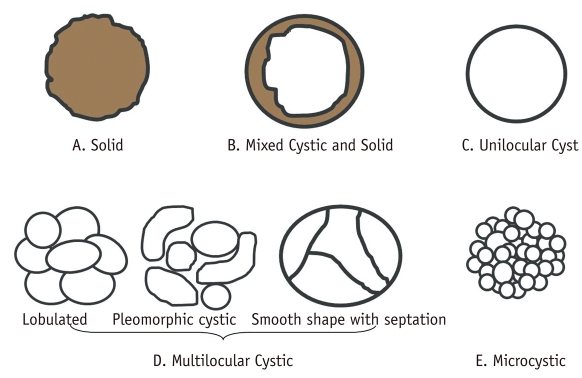
Fig. 1.
Morphologic subtypes of pancreatic lesions.
Because each pancreatic neoplasm has different malignant potential, it is clinically valuable to determine characteristic imaging findings that can enable differentiation. We classified pancreatic tumors into solid tumors (A malignant or potentially malignant), mixed cystic and solid lesions (B malignant or potentially malignant), unilocular cysts (C benign or potentially malignant), multilocular cystic lesions (D benign or potentially malignant), and microcystic lesions (E benign). Multilocular cystic lesions are divided into three categories including lobulated (benign), pleomorphic (from benign to malignant), and smooth in shape with septation (potentially malignant). These figures are made by referring to previously suggested classification schema (5).
Solid Tumors
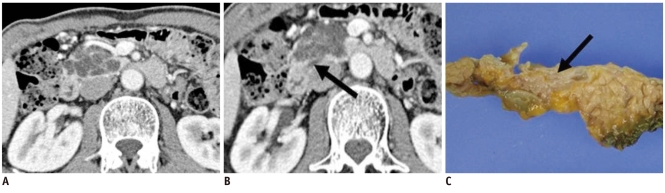
Although there are a wide variety of pancreatic neoplasms, the majority of them are epithelial tumors and 90% of these are ductal adenocarcinomas and their variants (e.g., adenosquamous carcinomas and undifferentiated carcinomas). Ductal adenocarcinoma is highly invasive and often elicits a desmoplastic reaction. Typically, ductal adenocarcinoma is a hypoattenuating solid mass relative to the normally enhancing pancreatic parenchyma in the pancreatic and portal venous phases because of fibroblastic proliferation and decreased vascularity. Tumors in the head and body of the pancreas frequently cause pancreatic or biliary duct obstruction with upstream ductal dilatation and pancreatic parenchymal atrophy. Neoplasms originating outside the exocrine ductal epithelium are rare. They include pancreatic endocrine tumors and metastasis. Pancreatic endocrine tumors are typically hyperattenuating in the arterial and venous phases on contrast-enhanced CT because of their rich capillary network, while most pancreatic metastases are hypodense except for those from renal cell carcinoma and melanoma (Fig. 2). Unlike ductal adenocarcinomas, pancreatic endocrine tumors are well demarcated and rarely result in pancreatic ductal dilatation. In addition, owing to their hypervascularity, the tumors intensely enhance on either contrast enhanced CT or MRI (6).
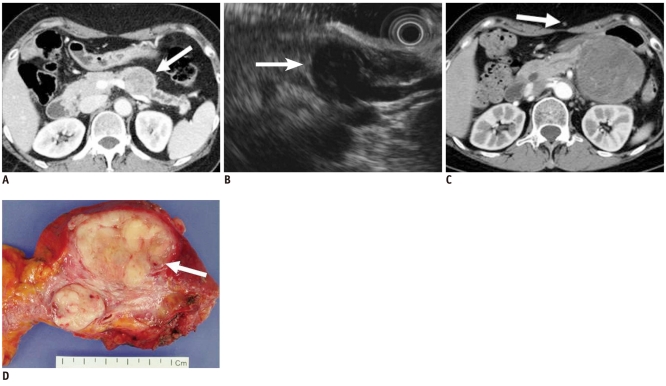
Fig. 2.
58-year-old woman with metastatic leiomyosarcoma manifesting as solid lesion.
A. Contrast-enhanced CT image during venous phase shows well-marginated, low attenuating solid tumor in pancreas (white arrow). B. Endoscopic US image depicts heterogeneously hypoechoic solid tumor (white arrow). Woman had multiple painless metastatic tumors in her stomach, chest, thigh and pancreas, which were too numerous to excise. Consequently, surgery was ruled out, and only chemotherapy was performed. C. Woman began to feel sick and mass increased in size at 1-year follow-up CT. Note metastatic nodule in subcutaneous layer (white arrow). D. Tumor was finally excised and appeared as whitish solid tumor in gross specimen and was confirmed as metastatic leiomyosarcoma (white arrow).
Mixed Cystic and Solid Lesions
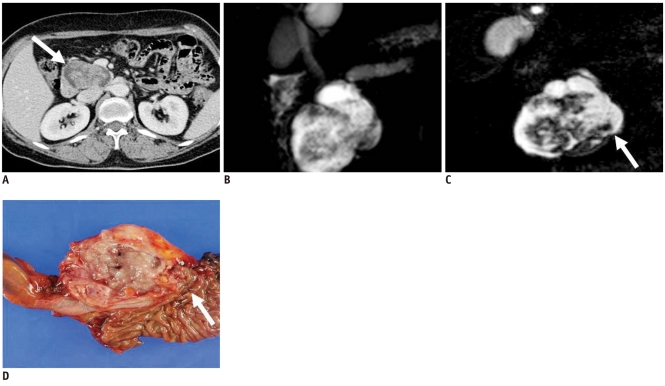
This category includes true cystic tumors as well as solid neoplasm associated with a cystic component or cystic degeneration. Solid tumors associated with a cystic component include cystic pancreatic endocrine tumor (Fig. 3), solid pseudopapillary tumor (SPT), cystic change of ductal carcinoma, and metastasis (5). Usually a cystic pancreatic endocrine tumor is a single unilocular cyst that occupies the majority of the tumor. In rare cases, ductal adenocarcinoma with cystic change could also manifest as a unilocular cystic lesion (7). Cystic change of ductal carcinoma commonly has low-attenuating regions consistent with necrosis, dilated duct or mucin pools. In comparison with cystic pancreatic endocrine tumor, cystic change of ductal carcinoma appears as relatively poor enhancement of solid portion and has irregular outer margin due to a desmoplastic reaction. On the other hand, when central necrosis is present in pancreatic endocrine tumor, enhancement of the non-necrotic portions is typical on contrast enhanced CT or MRI (6). SPT appears as a well-demarcated mass with a fibrous capsule and varying amount of hemorrhage, necrosis, or calcification (8). Because all of these tumors are either malignant or have a high potential for being malignant, surgical resection is the treatment of choice.
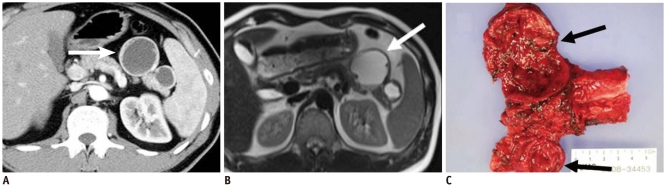
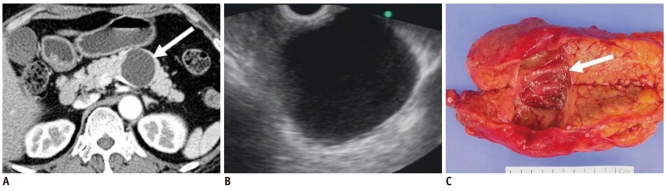
Fig. 3.
40-year-old man with cystic change of islet cell tumor manifesting as mixed cystic and solid lesion.
A. Contrast enhanced CT image during arterial phase shows two unilocular cystic lesions with small solid components in tail of pancreas (white arrow). B. T2-weighted image shows cystic character of lesions (white arrow). C. Surgical specimen shows two predominantly cystic lesions with solid portion and small mural nodules. Microscopic examination revealed cystic change of islet cell tumors (black arrows).
Unilocular Cysts
Unilocular cysts are probably the most difficult to manage because they are frequently small and consist of a broad-spectrum of benign to potentially malignant pathologies, including MCN (Fig. 4), IPMN, and serous cystadenoma. A unilocular cyst in a patient with a clinical history of pancreatitis is almost always a pseudocyst. Other less common unilocular cysts include simple cyst or lymphoepithelial cyst (Fig. 5). Although accurate characterization of unilocular cysts is challenging, CT findings, including the location in the pancreatic head, lobulated contour, absence of wall enhancement, and lack of mural nodule, are specific for the service of serous cystadenoma (9). The presence of peripheral tumoral calcification has a significant association with mucinous cystic neoplasms (10). Moreover, when differentiation is not possible at imaging, symptomatic patients can be further treated with EUS-guided cyst aspiration or surgical resection (5).
Fig. 4.
57-year-old woman with mucinous cystic neoplasm manifesting as unilocular cyst.
A. Transverse contrast enhanced CT image during arterial phase shows unilocular cyst (white arrow) in body of pancreas. B. Endoscopic US image depicts cyst without solid portion or septation. C. Gross specimen reveals unilocular cystic lesions without septation or solid mural nodule (white arrow). Communication with pancreatic duct was not found. Tumor was confirmed as mucinous cystadenoma.
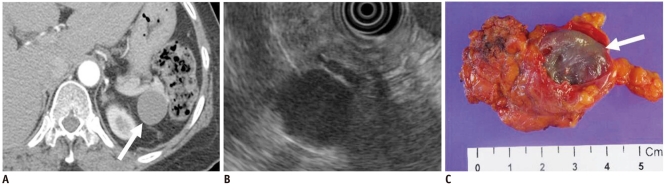
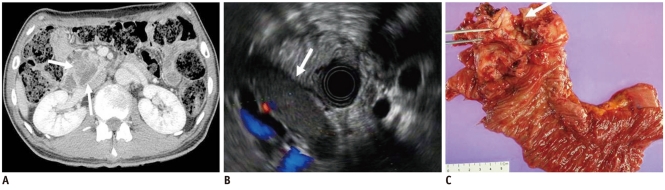
Fig. 5.
47-year-old woman with lymphoepithelial cyst manifesting as unilocular cyst.
A. Transverse contrast enhanced CT image during arterial phase shows unilocular cystic lesion in tail of pancreas (white arrow). B. Endoscopic US image shows homogeneous echoic cystic lesion. C. Surgical specimen shows round tumor with surrounding fibrous capsule (white arrow).
Multilocular Cystic Lesions
Multilocular cystic lesions can be divided into three categories: (a) lobulated; (b) pleomorphic, (c) smooth shape with septation (1, 5). A "lobulated" shape is defined as the shape of a simple closed curve that could not be described as the borders of the same circle, with or without internal septation and corresponding with oligocystic serous cystadenomas. A "pleomorphic" shape is defined as one containing three or more cysts, including more than one oval or tubular cyst, and is typical of branch duct type IPMNs. A "smooth shape with septation" is defined as a simple closed curve with the borders of the same circle, and this is a typical finding of MCNs (Fig. 6).
Fig. 6.
27-year-old woman with mucinous cystic neoplasm manifesting as multilocular cystic lesion (smooth shape with septation).
A. Contrast enhanced CT image during venous phase obtained after intravenous injection of contrast material reveals smooth-shaped cystic lesion in tail of pancreas. B. Axial T2-weighted MR image reveals multiple internal septa in cystic lesion. C. Endoscopic US image also depicts multilocular cystic lesion with smooth shape and multiple inner septa, which was consistent with mucinous cystic neoplasm.
The cystic tumors in this category are also difficult to be characterized because of their overlapping morphology. The differentiation of oligocystic serous cystadenoma from MCN is important because of the malignancy potential of the mucinous tumors. Oligocystic serous cystadenoma appears as a multicystic or lobulated cystic lesion with septation (Fig. 7), while MCN shows a smooth shape, with or without septations. Calcification in serous cystadenomas is typically central within the fibrous stroma, whereas MCN may have a peripheral eggshell calcification (1, 9).
Fig. 7.
37-year-old woman with serous cystadenoma manifesting as multilocular cystic lesion (lobulated).
A. Contrast-enhanced CT image during venous phase shows multilocular cyst in body of pancreas. Lobulated shape of tumor corresponds to polycystic serous cystadenoma rather than mucinous cystadenoma. B. Endoscopic US depicts tumor composed of multiple cysts. Endoscopic US-guided aspiration showed no malignant cell. C. Ill-defined multilocular cystic tumor with inner smooth and trabeculated surface is seen in gross specimen (white arrows) and confirmed as serous cystadenoma composed of variable sized cysts lined by simple cuboidal cells.
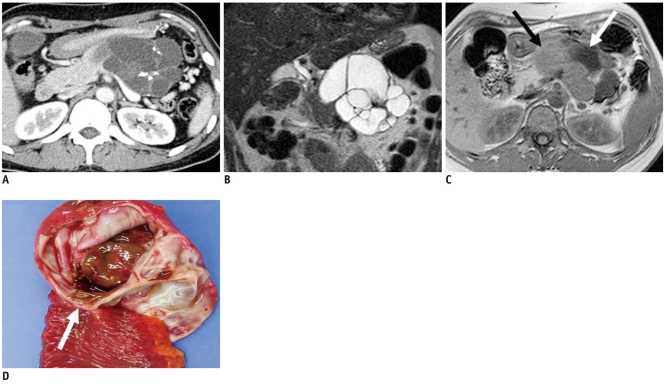
A branch duct type or combined type IPMN can have the morphologic features of pleomorphic cysts. They arise from the epithelial lining of the pancreatic duct and typically produce a copious amount of mucin, which may cause ductal dilatation and obstruction resulting from mucus plugs. Histologically, IPMN represents a spectrum ranging from hyperplasia to carcinoma. Identification of a pleomorphic cyst that communicates with the main pancreatic duct is highly suggestive of a branch duct type or combined type IPMN (Fig. 8). Usually solid tumors, serous cystadenomas, and most mucinous cystic neoplasms do not show ductal communication. For predicting ductal communication and differentiating IPMNs from other lesions, MRI is generally known to show a better diagnostic performance than MDCT (4). However, the lack of communication with the main pancreatic duct does not exclude the possibility of an IPMN. Features predictive of invasive carcinoma in IPMN on CT and other imaging studies include involvement of the main pancreatic duct, marked dilatation of the main pancreatic duct, diffuse or multifocal involvement, the presence of a large mural nodule or solid mass, large size of the mass, and obstruction of the common bile duct (Fig. 9) (11).
Fig. 8.
43-year-old man with branch duct type intraductal papillary mucinous neoplasm manifesting as multilocular cystic lesion (pleomorphic cystic).
A. Contrast enhanced CT image during venous phase shows multilocular pleomorphic cystic lesion in head of pancreas. B. Note dilated downstream pancreatic duct (black arrow). C. Gross specimen showing ill-defined multilocular cystic tumor in head of pancreas (black arrow) which contained mucinous material. Tumor connects with irregularly dilated branches of pancreatic duct, and is confirmed as branch duct type intraductal papillary mucinous neoplasm.
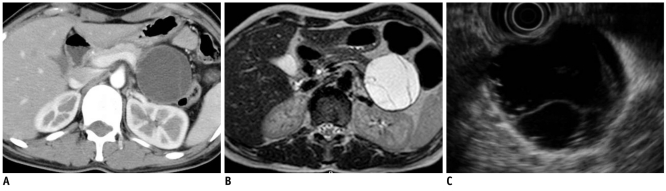
Fig. 9.
52-year-old man with invasive intraductal papillary mucinous neoplasm.
A. Contrast-enhanced CT image during venous phase shows dilatation of main pancreatic duct with adjacent solid mass in head of pancreas (white arrows). B. Endoscopic US image shows cystic lesion with inner solid portion (white arrow). C. Surgical specimen shows tumor involvement of dilated main pancreatic duct (white arrow). Microscopic examination revealing invasive intraductal papillary mucinous neoplasm.
Endoscopic US is helpful in excluding a pseudocyst to determine the specific type of the cystic pancreatic tumors, and identifying a malignant transformation by evaluation of the cyst wall (thickness, focal irregularity, mass, or papillary projections) and intracystic structures (septations, mucous, or debris). EUS features that correlate with malignancy include the presence of focal cyst wall thickening or irregularity, septal thickening, an adjacent solid mass, and the presence of collateral vessels (12).
Microcystic Lesions
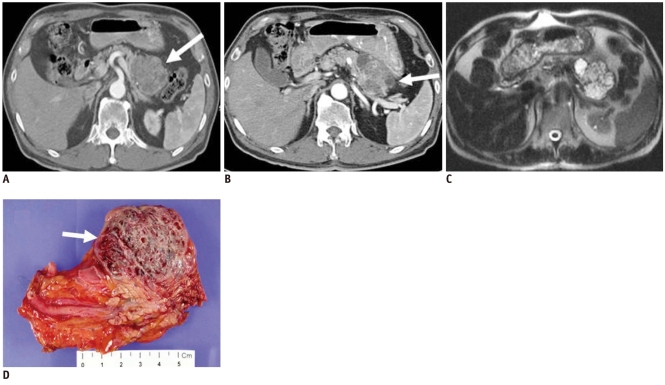
Serous microcystic cystadenoma is the only cystic lesion included in this category. The typical CT appearance of this tumor is similar to that of a sponge or honeycomb with innumerable tiny cystic spaces septated by thin septa. The septa may coalesce into a characteristic central stellate scar that may calcify itself, which is considered to be pathognomonic for serous cystadenoma and found in about 20% of tumors. The small size of the cysts and the innumerable enhancing septa may cause the mass to appear solid on CT. Microcysts may be seen as numerous discrete foci with bright signal intensity on T2-weighted MR images (Fig. 10), and have little free fluid in the locules on EUS (5, 10, 12, 13).
Fig. 10.
66-year-old man with microcystic serous cystadenoma manifesting as microcystic lesion.
A. Contrast-enhanced transverse CT during arterial phase shows hypervascular mass (white arrow) in tail of pancreas. It appears as solid lesion on CT, owing to innumerable cysts and fine septa. B. Note cystic portion in periphery of tumor (white arrow). C. Axial T2-weighted image reveals microcystic mass. D. Gross specimen shows multilocular, well-defined bulging mass containing multiple tiny cystic spaces and sanguineous clear fluid (white arrow), which are confirmed as microcystic serous cystadenoma.
Limitations and Pitfalls in the Differentiation of the Cystic Pancreatic Tumors
Although MCN usually manifests itself as a multilocular cyst with a smooth contour, with or without septation, there is definite overlap between MCN and serous cystadenoma. In our case (Fig. 11), the cystic tumor in the tail of the pancreas has a lobulated contour with internal septation and calcification in the central area, but it was confirmed as MCN (Fig. 11).
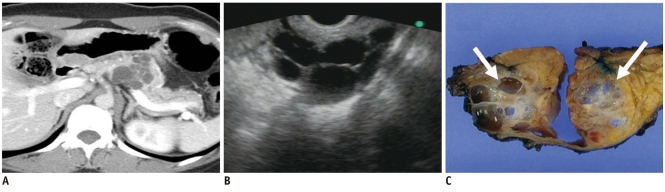
Fig. 11.
37-year-old woman with mucinous cystic neoplasm: pitfall in differentiating multilocular cystic lesions.
A. Contrast-enhanced CT image during venous phase shows multilocular lobulated cyst with internal septation and calcification in tail of pancreas. B. Coronal T2-weighted image reveals multilocular cystic mass. C. T1-weighted gradient-echo image shows multiple locules with different signal intensity (black and white arrows). D. Gross specimen shows multilocular cystic tumor with inner smooth and glistening surface (white arrow) which was confirmed as mucinous cystadenoma at microscopy.
Serous cystadenomas typically have a polycystic, honeycomb, or oligocystic appearance. However, they can also atypically show giant tumors with ductal dilatation, intratumoral hemorrhages, solid variants, unilocular cystic tumors, interval growth, and disseminated forms, so that imaging findings may be unlikely to be diagnostic (14). Even though almost all serous cystic pancreatic neoplasms are benign, serous cystadenocarcinomas rarely are benign, but also have been reported (15).
Identification of a pleomorphic cyst that communicates with the main pancreatic duct is highly suggestive of a branch duct type or combined type IPMN. However, the lack of communication with the main pancreatic duct does not exclude an IPMN. EUS and EUS-guided fine needle aspiration (FNA) may provide additional information about the malignant transformation. A three-dimensional MR pancreatography can be used for differentiation of IPMN from other pancreatic cystic lesions by showing ductal communication (16) (Fig. 12).
Fig. 12.
59-year-old-woman with branch duct type intraductal papillary mucinous neoplasm manifesting as mixed cystic and solid lesion.
A. Contrast-enhanced CT image during venous phase shows cystic mass and internal solid portion in head of pancreas (white arrow). B. Two-dimensional thick slab MR cholangiopancreatography image shows cystic mass in head of pancreas. Communication between cystic mass and pancreatic duct is not clear. C. Source image of 3D-MR cholangiopancreatography shows narrow tumor neck suggesting communication between tumor and main pancreatic duct more clearly than 2D-MR cholangiopancreatography (white arrow). D. Gross specimen shows whitish polypoid friable mass containing translucent mucoid materials. Tumor communicates with main pancreatic duct (white arrow) and was confirmed as branch duct type intraductal papillary mucinous neoplasm.
CONCLUSION
Pancreatic tumors can be classified by morphologic features on CT and their pathologic features are correlated with CT. Each category has a different malignant potential. Therefore, knowledge of the spectrum of morphologic features observed in pancreatic tumors is useful for differential diagnosis and treatment planning. EUS and MRI can provide detailed information for classifying the pancreatic lesions.
References
- 1.Kim SY, Lee JM, Kim SH, Shin KS, Kim YJ, An SK, et al. Macrocystic neoplasms of the pancreas: CT differentiation of serous oligocystic adenoma from mucinous cystadenoma and intraductal papillary mucinous tumor. AJR Am J Roentgenol. 2006;187:1192–1198. doi: 10.2214/AJR.05.0337. [DOI] [PubMed] [Google Scholar]
- 2.Park MS, Kim KW, Lim JS, Lee JH, Kim JH, Kim SY, et al. Unusual cystic neoplasms in the pancreas: radiologic-pathologic correlation. J Comput Assist Tomogr. 2005;29:610–616. doi: 10.1097/01.rct.0000172561.62810.6c. [DOI] [PubMed] [Google Scholar]
- 3.Irie H, Honda H, Aibe H, Kuroiwa T, Yoshimitsu K, Shinozaki K, et al. MR cholangiopancreatographic differentiation of benign and malignant intraductal mucin-producing tumors of the pancreas. AJR Am J Roentgenol. 2000;174:1403–1408. doi: 10.2214/ajr.174.5.1741403. [DOI] [PubMed] [Google Scholar]
- 4.Song SJ, Lee JM, Kim YJ, Kim SH, Lee JY, Han JK, et al. Differentiation of intraductal papillary mucinous neoplasms from other pancreatic cystic masses: comparison of multirow-detector CT and MR imaging using ROC analysis. J Magn Reson Imaging. 2007;26:86–93. doi: 10.1002/jmri.21001. [DOI] [PubMed] [Google Scholar]
- 5.Sahani DV, Kadavigere R, Saokar A, Fernandez-del Castillo C, Brugge WR, Hahn PF. Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. Radiographics. 2005;25:1471–1484. doi: 10.1148/rg.256045161. [DOI] [PubMed] [Google Scholar]
- 6.Mergo PJ, Helmberger TK, Buetow PC, Helmberger RC, Ros PR. Pancreatic neoplasms: MR imaging and pathologic correlation. Radiographics. 1997;17:281–301. doi: 10.1148/radiographics.17.2.9084072. [DOI] [PubMed] [Google Scholar]
- 7.Lee LY, Hsu HL, Chen HM, Hsueh C. Ductal adenocarcinoma of the pancreas with huge cystic degeneration: a lesion to be distinguished from pseudocyst and mucinous cystadenocarcinoma. Int J Surg Pathol. 2003;11:235–239. doi: 10.1177/106689690301100315. [DOI] [PubMed] [Google Scholar]
- 8.Choi JY, Kim MJ, Kim JH, Kim SH, Lim JS, Oh YT, et al. Solid pseudopapillary tumor of the pancreas: typical and atypical manifestations. AJR Am J Roentgenol. 2006;187:W178–W186. doi: 10.2214/AJR.05.0569. [DOI] [PubMed] [Google Scholar]
- 9.Cohen-Scali F, Vilgrain V, Brancatelli G, Hammel P, Vullierme MP, Sauvanet A, et al. Discrimination of unilocular macrocystic serous cystadenoma from pancreatic pseudocyst and mucinous cystadenoma with CT: initial observations. Radiology. 2003;228:727–733. doi: 10.1148/radiol.2283020973. [DOI] [PubMed] [Google Scholar]
- 10.Curry CA, Eng J, Horton KM, Urban B, Siegelman S, Kuszyk BS, et al. CT of primary cystic pancreatic neoplasms: can CT be used for patient triage and treatment? AJR Am J Roentgenol. 2000;175:99–103. doi: 10.2214/ajr.175.1.1750099. [DOI] [PubMed] [Google Scholar]
- 11.Kawamoto S, Horton KM, Lawler LP, Hruban RH, Fishman EK. Intraductal papillary mucinous neoplasm of the pancreas: can benign lesions be differentiated from malignant lesions with multidetector CT? Radiographics. 2005;25:1451–1468. doi: 10.1148/rg.256055036. discussion 1468-1470. [DOI] [PubMed] [Google Scholar]
- 12.Levy MJ, Clain JE. Evaluation and management of cystic pancreatic tumors: emphasis on the role of EUS FNA. Clin Gastroenterol Hepatol. 2004;2:639–653. doi: 10.1016/s1542-3565(04)00235-6. [DOI] [PubMed] [Google Scholar]
- 13.Brugge WR. The use of EUS to diagnose cystic neoplasms of the pancreas. Gastrointest Endosc. 2009;69:S203–S209. doi: 10.1016/j.gie.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 14.Choi JY, Kim MJ, Lee JY, Lim JS, Chung JJ, Kim KW, et al. Typical and atypical manifestations of serous cystadenoma of the pancreas: imaging findings with pathologic correlation. AJR Am J Roentgenol. 2009;193:136–142. doi: 10.2214/AJR.08.1309. [DOI] [PubMed] [Google Scholar]
- 15.Shintaku M, Arimoto A, Sakita N. Serous cystadenocarcinoma of the pancreas. Pathol Int. 2005;55:436–439. doi: 10.1111/j.1440-1827.2005.01850.x. [DOI] [PubMed] [Google Scholar]
- 16.Choi JY, Lee JM, Lee MW, Kim SJ, Choi SY, Kim JY, et al. Magnetic resonance pancreatography: comparison of two- and three-dimensional sequences for assessment of intraductal papillary mucinous neoplasm of the pancreas. Eur Radiol. 2009;19:2163–2170. doi: 10.1007/s00330-009-1391-9. [DOI] [PubMed] [Google Scholar]