Abstract
A new strategy for magnetically manipulating and isolating adherent cells with extremely high post-collection purity and viability is reported. Micromolded magnetic elements (termed microrafts) were fabricated in an array format and used as culture surfaces and carriers for living, adherent cells. A poly(styrene-co-acrylic acid) polymer containing well dispersed magnetic nanoparticles was developed for creating the microstructures by molding. Nanoparticles of γFe2O3 at concentrations up to 1% wt.∕wt. could be used to fabricate microrafts that were optically transparent, highly magnetic, biocompatible, and minimally fluorescent. To prevent cellular uptake of nanoparticles from the magnetic polymer, a poly(styrene-co-acrylic acid) layer lacking γFe2O3 nanoparticles was placed over the initial magnetic microraft layer to prevent cellular uptake of the γFe2O3 during culture. The microraft surface geometry and physical properties were altered by varying the polymer concentration or layering different polymers during fabrication. Cells plated on the magnetic microrafts were visualized using standard imaging techniques including brightfield, epifluorescence, and confocal microscopy. Magnetic microrafts possessing cells of interest were dislodged from the array and efficiently collected with an external magnet. To demonstrate the feasibility of cell isolation using the magnetic microrafts, a mixed population of wild-type cells and cells stably transfected with a fluorescent protein was plated onto an array. Microrafts possessing single, fluorescent cells were released from the array and magnetically collected. A post-sorting single-cell cloning rate of 92% and a purity of 100% were attained.
INTRODUCTION
The ability to efficiently isolate cells or colonies from a mixed population for further expansion or analysis is a process common to many areas of biomedical research and biotechnology.1 Examples of such endeavors include cloning of stem cells or genetically engineered cells for the development of cell lines and creation of animal models and isolation of tumor cells for genetic analysis.2, 3 Admixing of cells with different characteristics from those of interest can lead to skewed or inaccurate results in such biological studies. In many cases, the cells of interest will be in low abundance among the population. For this reason, it is important to have a technique capable of identifying single cells with the desired characteristic, separating those cells from the unwanted cells and then collecting the cells with high purity for further expansion or analysis. Commonly used techniques for performing these types of cell isolation procedures include limiting dilution, colony picking, and fluorescence-activated cell sorting (FACS).4, 5, 6, 7 A number of new technologies for single-cell isolation have been developed in recent years but have yet to be widely adopted including laser micro-dissection or laser ablation,8, 9 optical tweezers,10 dielectrophoresis,11 and microarray technologies.12, 13
The use of magnetism as an external physical force for isolating cells is particularly attractive due to its simplicity, effectiveness, and ease of manipulation.14 Magnetic cell separation (MACS®) developed by Miltenyi Biotec and the related techniques such as magnetic columns, flow channels, arrays, and tweezers rely on magnetic particles bound to the surface of the cells or taken up by the cells to provide magnetic domains encompassing the cell for selective manipulation by an external magnet.15, 16, 17, 18, 19, 20, 21 Magnetic microdevices or microstructures have been fabricated as microtools for precise positioning of cells22 or as mobile structures termed “microtransporters,” “microcarriers,” or “microplates” for manipulation of cells.23, 24, 25 These microstructures, either fabricated from magnetic materials or doped with magnetic nanoparticles, have not yet been shown to be useful for isolating individual cells from a mixed population. Recently, an array of magnetic microstructures was developed in combination with our previous microarray technology for cell sorting by embedding magnetic nanoparticles within the micropallet array elements.26, 27 The transparent microstructures served as sites for attaching adherent cells. After screening the entire array, the cells of interest could be selectively detached from the array using a pulsed laser and collected against gravity with an external magnet to produce very pure populations of collected cells.26
While the micropallet array is an efficient approach for cell sorting, the platform is expensive and complicated as it requires a photolithographically defined array created in a cleanroom environment and a laser integrated into a high quality microscope. An inexpensive and robust platform, termed a “microraft array,” was recently developed by our group for the efficient isolation of viable, single cells or colonies from a mixed population.13 A simple dip-coating process was used to fabricate an array composed of a large number of micron-scale elements (the microrafts) on a polydimethylsiloxane (PDMS) template. Within the array, the microrafts serve as releasable culture sites for individual cells or colonies. After identification of target cells or colonies, microrafts possessing cells of interest can be released with a needle inserted through the PDMS template. Following release, the microraft is allowed to drop from the inverted array onto a collection vessel, such as a Petri dish via gravity. This method has been successful in sorting cells with extremely high collection efficiency (100%) and post-sorting single-cell proliferation capability (95%); however, loosely adherent cells on the array can become detached during the release and collection procedure reducing the purity of isolated cells. Impurity of the isolated cells is undesirable for many applications, such as the creation of stably transfected cell lines. Re-sorting can be generally used to improve purity but results in cell loss and requires additional time and effort. To overcome this problem, magnetism was evaluated as a means to collect the released microrafts and their adherent cells or colonies to achieve high purity of the collected cells. In the current article, the microraft array platform was enhanced by doping the microraft material with magnetic nanoparticles. The dispersion of nanoparticles inside the polymer matrix of the microrafts and the resultant optical properties were examined. The fabrication of magnetic microraft arrays via the dip-coating process was tested. An array of two-layer microrafts composed of a magnetic base and a non-magnetic surface was fabricated to provide an optimal, nanoparticle-free culture surface. Imaging of cells by brightfield, fluorescence, and confocal microscopy was demonstrated. Finally, isolation and magnetic manipulation of single, viable cells from the array was demonstrated, and the purity of isolated cells was determined.
MATERIALS AND METHODS
Materials
The following materials were obtained from the Aldrich Chemical Company (St. Louis, MO): iron(II) chloride tetrahydrate (99%), iron(III) chloride anhydrous (98%), iron(III) nitrate nonahydrate (99+%), 28% ammonium hydroxide solution, oleic acid (90%), toluene (reagent grade), triarylsulfonium hexafluorophosphate salts (mixed, 50% in propylene carbonate), 99+% pure γ-butyralactone (GBL), 1-methoxy-2-propanol (1002F developer, 98.5%), glutaraldehyde, rhodamine B, 2,2′-azobisisobutyionitrile (AIBN, 98%), styrene (≥99%), and acrylic acid (99.5%). EPON resin SU-8 and EPON resin 1002F (phenol, 4,4′-(1-methylethylidene)bis-, polymer with 2,2′-[(1-methylethylidene)bis(4,1-phenyleneoxymethylene]bis-[oxirane]) were obtained from Miller-Stephenson (Sylmar, CA). Dulbecco’s modified eagle medium (DMEM), fetal bovine serum (FBS), 1 × phosphate buffered saline (PBS) pH 7.4, 0.05% trypsin with EDTA solution, penicillin∕streptomycin, CellTracker™ Red CMTPX, CellMask™ Orange plasma membrane stain, and Hoechst dye No. 33342 were obtained from Invitrogen (Carlsbad, CA). Draq-5 DNA dye was from Biostatus (Leicestershire, UK). Poly(dimethylsiloxane) (PDMS, Sylgard 184 silicone elastomer kit) was purchased from Dow Corning (Midland, MI). Collagen I from rat tail tendon and Falcon™ Petri dishes were purchased from BD Biosciences (San Jose, CA). Polycarbonate plates (12″ × 12″ × 0.25″) were purchased from McMaster-Carr (Los Angeles, CA). Wild-type HeLa cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). All other chemicals were procured from Fisher Scientific (Pittsburgh, PA).
Magnetic polystyrene development
Nanoparticles of Fe3O4 were synthesized by the co-precipitation of iron salts in deionized water through the addition of ammonium hydroxide.28 The nanoparticles were magnetically decanted, and the fluid was replaced with fresh deionized water and iron nitrate. Mixing for 1 h at 80 °C in the presence of iron nitrate oxidized the nanoparticles to γFe2O3.29 Magnetically decanting the nanoparticles and replacing the liquid with deionized water produced a magnetic ferrofluid. The nanoparticles were extracted with oleic acid to produce hydrophobic γFe2O3 nanoparticles. The magnetic phase was magnetically decanted, and excess oleic acid was removed by three washes in ethanol. The oleic acid-coated γFe2O3 nanoparticles were then dissolved in toluene (5 g of γFe2O3∕1L toluene). Poly(styrene-co-acrylic acid) (PS-AA) was prepared by copolymerization of styrene and acrylic acid in GBL, as described previously.13 Briefly 95 g styrene, 5 g acrylic acid, 0.1 g 2,2′-azobisisobutyronitrile (AIBN) and 100 g GBL were mixed in a flask and heated in a 60 °C water bath for 72 h to complete copolymerization. A 1:5 v∕v mixture of PS-AA in toluene was slowly added to the γFe2O3 ferrofluid. The toluene was then evaporated (Büchi R200 rotovapor, Flawil, Switzerland) until a thick gel remained. GBL was added to this magnetic polystyrene gel until the desired viscosity for efficient dip coating was achieved.
Fabrication of magnetic microrafts
Releasable magnetic microstructures were molded within PDMS microwells using a previously described protocol (see Supplemental materials).30 For arrays composed of single-layer microrafts, PS-AA, 1002F or SU-8 containing 1% γFe2O3 by weight was applied over the PDMS mold. Trapped air bubbles within the microwells were removed though degassing under vacuum (Oerlikon Leyboid pump). The PDMS mold or template was then attached to a rotary DC motor and lowered into a solution of the magnetic polymer. Slowly raising the PDMS mold produced a convex solution of polymer isolated in each microwell as the template dewetted. Placing the PDMS mold in a 95 °C oven for 2 h evaporated the bulk of the GBL resulting in concave microstructures within the microwells. Further, evaporation of the GBL was achieved by a 1 h bake at 120 °C in a vacuum oven (−30 in. Hg). A magnetic microraft developed with PS-AA containing 1% γFe2O3 dissolved in 75% GBL had a final γFe2O3 concentration of 4% by weight following evaporation of the GBL. For simplicity, the initial concentration of γFe2O3 in the PS-AA was used to define the magnetic loading throughout this report. Multi-layer microrafts were constructed through repeated dip coating and drying of the array in various polymers dissolved in GBL.
Following fabrication of the microraft arrays, the PDMS template was attached to a polycarbonate cassette, with the array facing toward the inside of the cassette. Slight stretching of the PDMS template during attachment to the cassette reduced sagging. While still attached to the cassette, a second polycarbonate structure to create a square inner chamber surrounding the array (25.4 mm × 25.4 mm × 10 mm height—Fig. S4) was glued to the top of the mold using PDMS with a 70 °C bake for 1 h.30
Release and collection of magnetic microrafts
Microrafts were released with the array in one of two orientations—inverted or upright. Microrafts on an inverted array were released by means of a microneedle (anodized steel, 150 μm base diameter and 17.5 μm tip diameter [Fine Science Tools, Foster City, CA]) positioned above the array and inserted through the PDMS template to dislodge the microraft which then settled on the collection dish, as previously reported.13 Release was followed by purification with an external magnet (see Supplemental materials for inverted-release purification).30 Microrafts were also released from an upright array with the microneedle positioned below the array and above the objective of an inverted microscope (Fig. 6a). The microneedle was attached to a “U” brace on an XYZ micromanipulator. The visual field was kept clear of equipment except the microneedle by incorporating a 90° bend in the microneedle. Individual microrafts were released by raising the needle to puncture the PDMS template and dislodge the selected microraft. Following release, the microneedle was lowered to its original position so that the array could be translated with the microscope stage in preparation for the next release. An external magnet positioned above the collection substrate enabled immediate collection following microraft release (Fig. 5). The magnet was kept over the collection plate to retain microrafts in the collection chamber against gravity as the array and collection plates were separated.
Figure 6.
Single cell sorting with magnetic microrafts. (A) Scheme for the magnetic collection of microrafts. (B)–(H) Brightfield and fluorescence images of a HeLa cell expressing a fluorescent protein identified, isolated and expanded into a clonal colony. (B)–(E) A single HeLa cell possessing a fluorescent nucleus is identified on an array composed of two-layer microrafts (100 μm). (F)–(I) The cell seen in “(B)–(E)” immediately following magnetic-assisted collection (F), (G) and after 7 days of incubation (H), (I).
Figure 5.
A series of time-resolved images demonstrating the release and magnetic collection of microrafts. In the displayed images, the neodymium magnet shown at the bottom of the image is 5 mm above the array and out of the focal plane. The microraft array composed of PS-AA containing 0.1% γFe2O3 (A) is deflected out of the focal plane by the microneedle during release of an individual microraft (B). The position of the microraft 1, 2, 3, and 4.3 s following release, panels (C)–(F), respectively, was monitored to assess the movement of a loose magnetic microstructure in a magnetic field. Microrafts are observed to move upward and thus out of focus as they are attracted to the magnet (enhanced online) .
Cell culture on magnetic microrafts
To expedite the attachment of cells to the microraft surface, the array was oxidized in a plasma cleaner (Harrick Plasma, Ithaca, NY) for 1 min. The microraft array and cassette holder were sterilized with 75% ethanol and allowed to dry in a tissue culture hood. Arrays were rinsed ×3 with sterile deionized H2O, and then 1 mL collagen in deionized H2O (100 μg mL−1) was added to the array and incubated for 1 h including a 20 min degassing by vacuum to remove trapped air bubbles within the wells. Alternatively, plasma treatment and collagen coating can be omitted, but it took an extended period of time (>6 h) for cells to attach to the microraft surface. The arrays were rinsed ×3 with deionized H2O followed by the addition of DMEM supplemented with FBS (10%), L-glutamine (584 mg L−1), penicillin (100 units mL−1), and streptomycin (100 μg mL−1). A suspension of 15 000 cells was then added to the microraft array and allowed to settle and adhere to the microrafts over 2 h in a 37 °C incubator with a 5% CO2 atmosphere. Cells used in these studies included wild-type HeLa cells, a human ovarian carcinoma cell line, HeLa cells stably transfected with enhanced green fluorescent protein (eGFP) fused to the nuclear H1-histone protein (a kind gift of Eva Lee, UC Irvine), and C2C12 cells, a murine myoblast cell line.
Prior to cell selection, the arrays were washed ×2 with DMEM and then the chamber surrounding the array was filled with DMEM. A sterile polystyrene Petri dish was then mated to the microraft cassette to create a sealed chamber filled with cell culture media. Following the release procedure, the Petri dish containing the isolated microrafts∕cells was removed from the cassette, immediately filled with 3 mL media, and was returned to a tissue culture incubator for continued culture of the cells.
Imaging of cells on magnetic microrafts
HeLa cells grown on microrafts and the expanded colonies were imaged by both brightfield and fluorescence microscopy using a cooled charge-coupled device (CCD) camera (Photometrics CoolSNAP HQ2, Tucson, AZ) mounted to an inverted epifluorescence microscope (NIKON TE200-U, Melville, NY). Additionally, fluorescence microscopy was used to visualize HeLa cells co-labeled with Hoechst 33342 DNA dye and the cytoplasmic stain CellTracker™ Red CMTPX. Fluorescently labeled C2C12 cells were imaged by differential interference contrast (DIC) and confocal microscopy with an inverted laser scanning microscope (Zeiss 510, Thornwood, NY). After transient transfection with eGFP (see Supplemental materials), C2C12 cells were plated on microraft arrays and stained with CellMask™ orange plasma membrane stain and Draq5 DNA dye following manufacturer protocols.30 Fluorescence images were provided in pseudocolors representative of the fluorophore’s excitation maximum wavelength.
RESULTS
Characterization of transparent magnetic polystyrene
A nanocomposite of uniformly distributed magnetic nanoparticles in a polystyrene:acrylic acid (PS-AA) co-polymer was developed to provide a magnetic and biocompatible material that could be molded into microstructures for cell culture and cell isolation. A ferrofluid containing superparamagnetic γFe2O3 nanoparticles and PS-AA in GBL was prepared as described above. Evaporation of the toluene left a composite of γFe2O3 nanoparticles up to 1 wt. % uniformly dispersed throughout a PS-AA matrix. This nanocomposite was then dissolved in GBL to provide a stable viscous media. The uniformity of the nanoparticle distribution in microrafts was confirmed by imaging films of the polymer under brightfield and with TEM. Films (100 μm thick) of the nanocomposite were transparent and slightly yellow when viewed using brightfield microscopy. TEM demonstrated well separated γFe2O3 nanoparticles (9 ± 4 nm, n = 97) throughout the polymer with no aggregates above 30 nm (Figs. 1a, 1b).
Figure 1.
Magnetic PS-AA characterization. (A) TEM image of PS-AA containing 1% γFe2O3 nanoparticles. (B) The region in the box in (A) is shown at increased magnification. (C) Transmittance curves of films of PS-AA with various concentrations of embedded γFe2O3 nanoparticles.
Brightfield and fluorescence imaging are commonly employed for the detection of cells or other biological specimens. The compatibility of the polystyrene nanocomposite for these uses was assessed by measuring the background absorbance and fluorescence of 50-μm thick films with various concentrations of γFe2O3 spin-coated onto glass slides. Increases in the concentration of γFe2O3 from 0.01 to 1% showed corresponding increases in absorbance at shorter wavelengths. A nanocomposite containing 1% γFe2O3 reached 80% transmittance at a wavelength of 521 nm, whereas 0.1% γFe2O3 reached 80% transmittance at 425 nm (Fig. 1c). The fluorescence of the magnetic films was comparable to that of native PS-AA (data not shown).
Substrates for cell culture should provide good cellular adhesion and support long-term cell growth. Since AA possesses carboxylic acid groups, the surface of PS-AA will present a negative surface charge which should promote cell attachment without the need for surface oxidation or an extracellular matrix coating.13, 31 Fourier transform infrared spectroscopy (FTIR) in the attenuated total reflectance (ATR) mode was used to assess the presence of carboxylic acid groups in the PS-AA copolymer. The absorption peak at 1704 cm−1, characteristic of the carboxylic acid C=O stretch, was observed in films of both PS-AA and magnetic PS-AA, but not native polystyrene, demonstrating the retention of the polymer’s negative charge with and without magnetic nanoparticle incorporation (Fig. S1).30 HeLa cells plated on 1% γFe2O3 PS-AA showed adhesion 6 h after cell addition and well-formed colonies were present after 8 days in culture. These results demonstrated that PS-AA with 1% γFe2O3 was an excellent substrate for cell growth.
Single-layer magnetic rafts
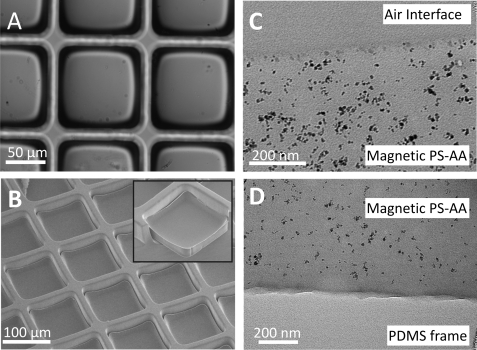
Soft lithography has been used to develop a variety of microdevices for biomedical applications. Previously, microrafts on a PDMS substrate were developed to array and then isolate cells. In that work, a dip-coating process was used to fabricate microstuctures from biocompatible polymers (SU-8, Epon 1002F epoxy resin, Epon 1009F epoxy resin, polystyrene or PS-AA) within an array of PDMS wells. The wells acted as a template to create the molded structures.13 In the current work, magnetic microrafts were created by dip-coating various polymers (SU-8, 1002F, and PS-AA) containing 0.01-1 wt. % uniformly distributed γFe2O3 nanoparticles dissolved in 70 wt. % GBL on a PDMS template consisting of an array of 100 μm × 100 μm microwells isolated by walls 40 μm tall and 20 μm thick. The doped polymers showed successful dewetting on the PDMS as was required to construct the individual microrafts (Figs. 2a and S2).30 Microrafts composed of PS-AA containing 1% γFe2O3 were isolated within the PDMS wells and possessed a slightly concave upper surface as monitored by SEM (Fig. 2b).
Figure 2.
Fabrication of magnetic microrafts. (A) Brightfield and (B) SEM images of PS-AA microrafts containing 1% γFe2O3. Inset shows a side view of a raft with PDMS partially removed. (C) TEM images of microraft-air interface and (D) PDMS-microraft interface.
The transparency of the magnetic polymers was retained during microraft fabrication (Fig. 2a). It has previously been shown that magnetic nanoparticles can accumulate at the air interface of a polymer during photolithographic processing of magnetic photoresists.26 Horizontal slices through the magnetic microrafts were imaged by TEM to determine whether a similar process might occur during raft fabrication. All microrafts composed of 1% γFe2O3 in 1002F showed evenly distributed nanoparticles throughout the polymer with the exception of a 20 nm layer of nanoparticles accumulated at the surface and base of the microrafts (Fig. S2).30 These results confirmed the previous finding that nanoparticles are enriched at the surfaces of the 1002F nanocomposite.26 In contrast, microrafts developed with 1% γFe2O3 in PS-AA possessed uniformly distributed nanoparticles throughout the polymer without noticeable accumulation of nanoparticles at the microraft surface or base (Figs. 2c, 2d). It is likely that γFe2O3 nanoparticles were trapped within the viscous PS-AA matrix during GBL evaporation, whereas the particles in the 1002F monomer were mobile until the resist was exposed to UV light. Since the magnetic PS-AA more closely mimics the oxidized polystyrene surfaces for conventional tissue culture relative to the 1002F surface, the fabrication of microrafts with magnetic PS-AA was the focus of the remainder of this work.
Two-layer magnetic rafts
The application of layers of materials onto the surface of microdevices permits tailoring of surface properties for specific device functions. For example, a layer of native 1002F polymer applied over a magnetic 1002F surface was previously shown to provide a protecting layer to prevent nanoparticle uptake by cells.26 Two-layer microrafts were constructed using sequential dip coating of the PDMS mold. Microrafts were initially formed by dip coating the mold into PS-AA containing 1% γFe2O3. A layer of PS-AA was then overlaid onto the magnetic microrafts using a second dip coating step (Fig. 3a). Following evaporation of solvent, a uniform layer of PS-AA was coated on the magnetic microraft (Fig. 3b). The polymer remained isolated within the PDMS wells and the microrafts retained smooth side walls as confirmed by SEM (Fig. 3c). The central thickness of the 1% γFe2O3-PS-AA and PS-AA layers were 10 and 8 μm, respectively as measured by TEM (Fig. 3d). While the viscosities of the solutions used for the first and second layers were identical, the PS-AA layer was thinner since the effective depth of the well was decreased during the second dip coating step. The thickness of the microraft layers could be adjusted by controlling the concentration of polymer dissolved in GBL during dip coating. For example, addition of PS-AA dissolved in 80 wt. % GBL resulted in a second layer thickness of 3 μm (data not shown).
Figure 3.
Two-layer magnetic raft fabrication. (A) Scheme of two-layer microraft fabrication. (B) Brightfield and (C) SEM images of a 2-layer microraft composed of a 1% γFe2O3 in PS-AA as the base with a PS-AA top layer. Inset shows a side view of a 2-layer microraft with PDMS partially removed. (D) TEM image of a cross section of a 2-layer microraft composed of a 10 μm magnetic PS-AA layer covered with an 8 μm thick layer of PS-AA.
Cell culture on magnetic rafts
Effective devices for culturing and isolating individual cells and cell colonies must be capable of providing both good cellular adhesion and supporting long-term growth on the substrate. PS-AA has previously been shown to be a biologically compatible substrate.13 This substrate can also be coated with extracellular matrices, such as fibronectin and collagen, to further improve cell adherence and growth. HeLa cells plated on magnetic PS-AA microrafts coated with collagen adhered to and spread across the surface of the microrafts within 2 h of plating as observed by brightfield microscopy and SEM (Figs. 4a, 4b). However, plasma treatment or the addition of an extracellular matrix (ECM) also modified the surface of the PDMS walls which reduced their barrier function in keeping the cells localized to individual microrafts. Thus, HeLa cells cultured on arrays treated by oxidation or ECM adsorption were observed to spread across the PDMS wall to adjacent microrafts after three days in culture. On the other hand, native PS-AA and magnetic PS-AA allow cellular adhesion within 6 h of plating without surface modification (Fig. 4c). Colonies of HeLa cells grown on these surfaces remained isolated on the microraft surface and within the confines of the PDMS walls for up to six days.
Figure 4.
Imaging cells on magnetic microrafts. Brightfield (A) and SEM (B) images of HeLa cells adhered to 2-layer microrafts (100 μm) coated with collagen. DIC (C) and confocal fluorescence (D)–(F) images of a C2C12 cell loaded with fluorescent dyes. Individual fluorescent channels show the fluorophores introduced to the cell by transfection with an eGFP expressing plasmid (emission at 517 nm) (D), staining with CellMask™ orange plasma membrane dye (emission 567 nm) (E) and DNA staining (Draq-5 emission at 697 nm) (F).
Many biological assays rely on fluorescent markers to identify the cells of interest. The ability to perform fluorescence imaging on two-layer magnetic rafts was demonstrated by examining cells loaded with fluorescence dyes using both epifluorescence and confocal microscopy. Cells plated on two-layer magnetic microrafts were stained with a nuclear dye (Hoechst 33342, excitation∕emission 350∕461 nm) and a cytoplasmic dye (CellTracker Red, excitation∕emission 570∕602 nm). Imaging by brightfield and fluorescence microscopy demonstrated the visualization of cellular detail on two-layer microrafts (Fig. S3).30 The ability to perform fluorescence confocal imaging of cells on two-layer microrafts was demonstrated using C2C12 cells transfected with a fluorescent protein and co-labeled with nuclear and membrane dyes. C2C12 cells transiently transfected with eGFP (excitation∕emission 492∕517 nm) were plated on unmodified two-layer microrafts then stained with CellMask™ orange plasma membrane dye (excitation∕emission 554∕567 nm) and a DNA dye (Draq-5, excitation∕emission 646∕697 nm). Confocal images showed clear compartmentalization of the dyes without distortion despite imaging through the microrafts (Figs. 4d, 4e, 4f).
Release and collection of magnetic microrafts
The utility of magnetic microrafts relies upon the ability to selectively release and manipulate them with an external magnet. Using a magnetic collection approach can also provide a method for purifying collected cells from non-target cells that may be shed from the array during the collection procedure. Single-layer magnetic microrafts were released in inverted and upright orientations. The efficiency of collection of released magnetic microstructures under varying magnetic field strengths and different concentrations of γFe2O3 was examined (Supplemental materials Table 1).30 Using the upright approach as an example, the microrafts were released and immediately collected onto a glass surface by an external magnet when the magnetic force experienced by the microrafts was sufficient to overcome gravitational force, as shown in Fig. 5. In triplicate experiments, 20 microrafts were released and then magnetically collected in this manner. Microrafts containing 1% γFe2O3 were collected with 100% efficiency (n = 60) at magnet displacements up to 20 mm, corresponding to a magnetic field of 22 mT at the glass surface. Increasing the distance between the glass surface and the collection plate to 24 mm (18 mT) reduced the collection efficiency to 28% ± 17%. Decreasing the concentration of γFe2O3 to 0.1% required reducing the distance between the collection plate and glass slide to 6 mm (166 mT) in order to achieve a collection efficiency of 100% ± 0%. Microrafts containing 0.01% γFe2O3 were not successfully collected when magnet separations down to 1 mm (449 mT) were attempted. Two-layer microrafts composed of 1% magnetic PS-AA bottoms and PS-AA tops produced collection probabilities of 100% at distances up to 16 mm (35 mT) and 73% ± 12% at 20 mm (22 mT).
Cell sorting and purification with magnetic microrafts
Direct collection of cells on microrafts whether or not a magnet is employed has been shown to be efficient, but purity may be limited due to non-target cells being shed from the array during the release procedure. To assess the viability and purity of single cells isolated from the array by magnetically enhanced collection, cell isolation experiments were performed using a heterogeneous population of cells plated on the array (Fig. 6a). A minority population of HeLa cells stably expressing a nuclear eGFP was admixed with wild-type HeLa cells at a 1:3 ratio. To maximize the number of microrafts containing only a single cell, 15 000 cells were plated on an array of 44 000 two-layer microrafts (PS-AA top∕1% magnetic PS-AA bottom) coated with collagen (Figs. 6b, 6c, 6d, 6e). In three independent experiments, 60 microrafts containing a single cell possessing a fluorescent nucleus were released. Immediately after the collection procedure, all released microraft retained their attached cell (Figs. 6f, 6g). After 7 days, 55 of the single cells (92% ± 5%) had expanded into a colony in which all cells possessed fluorescent nuclei with no non-fluorescent cells admixed (Figs. 6h, 6i). Selective isolation of cells attached to magnetically collected microrafts was confirmed by releasing and magnetically collecting 20 microrafts without adherent cells from the microraft array plated with cells. Following 7 days culture, no cell colonies were observed on the collection plate. A cell collection efficiency of 100% with 100% purity and a single-cell cloning efficiency of 92% was attained demonstrating the feasibility of creating highly purified clonal populations of cells from a heterogeneous population.
CONCLUSIONS
Magnetic microstructures were developed to enhance the manipulation and purity of cells isolated from a cell-based microarray. Nanoparticles composed of γFe2O3 were uniformly dispersed in a polystyrene-based polymer to provide biocompatible, transparent, magnetic microrafts. Through the use of multiple dip-coatings, microrafts composed of multiple layers could be easily fabricated. In this manner, microrafts were created with layers composed of differing properties. For example, application of a polymer layer lacking nanoparticles over the magnetic layer overcame potential cell uptake of γFe2O3 from the culture surface. Viable cells cultured on the arrays of single- or two-layer magnetic microrafts could be viewed by brightfield, fluorescence and confocal imaging for identification and selection. Upon release, selected cells were magnetically collected efficiently and with high viability to achieve single-cell cloning rates of 92%. The magnetic properties of the microrafts enabled the attached cells to be readily separated from any contaminating cells shed from the array during the identification and release procedures. The magnetically enhanced retrieval process enabled 100% purity of collected cells to be achieved. These results demonstrated the utility of using magnet microrafts for obtaining highly pure and viable cells for cloning applications.
ACKNOWLEDGMENTS
This research was supported by grants from the National Institutes of Health (EB007612 and EB012549). We thank Dr. Joe Kornegay for supplying the CMV driven eGFP expression plasmid and David Detwiler for his assistance with C2C12 cell transfection. Dr. Michael Chua, director of the Hooker Microscopy Facility, is acknowledged for his assistance with DIC and confocal imaging. Dr. Mark Walters, director of the Shared Materials Instrumentation Facilities at Duke University, is recognized for his acquisition of ATR-FTIR spectra. N.L.A, C.E.S, and Y.W. disclose a financial interest in Cell Microsystems, Inc.
References
- Patel D., Separating Cells (Springer-Verlag, New York, 2001). [Google Scholar]
- Mori K., Kashiwagi A., and Yomo T., Eukaryot J.. Microbiol. 58(1), 37 (2010). 10.1111/j.1550-7408.2010.00517.x [DOI] [PubMed] [Google Scholar]
- Nagrath S., Sequist L. V., Maheswaran S., Bell D. W., Irimia D., Ulkus L., Smith M. R., Kwak E. L., Digumarthy S., Muzikansky A., Ryan P., Balis U. J., Tompkins R. G., Haber D. A., and Toner M., Nature 450(7173), 1235 (2007). 10.1038/nature06385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freshney R. I., Culture of Animal Cells (Wiley-Liss, New York, 2000). [Google Scholar]
- Shapiro H. M., Practical Flow Cytometry, 4th ed. (Wiley-Liss, New York, 2003). [Google Scholar]
- Givan A. L., Flow Cytometry First Principles, 2nd ed. (Wiley-Liss, New York, 2001). [Google Scholar]
- Kirkland D. J., Br. J. Cancer 34, 134 (1976). 10.1038/bjc.1976.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutze K., Posl H., and Lahr G., Cell. Mol. Biol. 44(5), 735 (1998). [PubMed] [Google Scholar]
- Kim J. S., Hur D., Hwang J. K., Chung C., and Chang J. K., Bioanalysis 2(10), 1755 (2010). 10.4155/bio.10.119 [DOI] [PubMed] [Google Scholar]
- Kovac J. R. and Voldman J., Anal. Chem. 79, 9321 (2007). 10.1021/ac071366y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascoyne P. R. C., Wang X. B., Huang Y., and Becker F. F., IEEE Trans. Ind. Appl. 33(3), 670 (1997). 10.1109/28.585856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. L., Young G., Aoto P. C., Pai J. H., Bachman M., Li G. P., Sims C. E., and Allbritton N. L., Cytometry A 71A(10), 866 (2007). 10.1002/cyto.a.v71a:10 [DOI] [PubMed] [Google Scholar]
- Wang Y. L., Phillips C., Xu W., Pai J. H., Dhopeshwarkar R., Sims C. E., and Allbritton N., Lab Chip 10(21), 2917 (2010). 10.1039/c0lc00186d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemshead J. T. and Ugelstad J., Mol. Cell. Biochem. 67, 11 (1985). 10.1007/BF00220980 [DOI] [PubMed] [Google Scholar]
- Miltenyi S., Muller W., Weichel W., and Radbruch A., Cytometry 11(2), 231 (1990). 10.1002/cyto.v11:2 [DOI] [PubMed] [Google Scholar]
- Liu W., Dechev N., Foulds I. G., Burke R., Parameswaran A., and Park E. J., Lab Chip 9(16), 2381 (2009). 10.1039/b821044f [DOI] [PubMed] [Google Scholar]
- Adams J. D., Thevoz P., Bruus H., and Soh H. T., Appl. Phys. Lett. 95, 254103 (2009). 10.1063/1.3275577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pregibon D. C., Toner M., and Doyle P. S., Langmuir 22(11), 5122–8 (2006). 10.1021/la0534625 [DOI] [PubMed] [Google Scholar]
- Ino K., Okochi M., Konishi N., Nakatochi M., Imai R., Shikida M., Ito A., and Honda H., Lab Chip 8(1), 134 (2008). 10.1039/b712330b [DOI] [PubMed] [Google Scholar]
- Lee H., Liu Y., Ham D., and Westervelt R. M., Lab Chip 7(3), 331 (2007). 10.1039/b700373k [DOI] [PubMed] [Google Scholar]
- Xia N., Hunt T. P., Mayers B. T., Alsberg E., Whitesides G. M., Westervelt R. M., and Ingber D. E., Biomed. Microdevices 8(4), 299 (2006). 10.1007/s10544-006-0033-0 [DOI] [PubMed] [Google Scholar]
- Hagiwara M., Kawahara T., Yamanishi Y., and Arai F., Appl. Phys. Lett. 97, 0137013 (2010). 10.1063/1.3459040.1 [DOI] [Google Scholar]
- Sakar M. S., Steager E. B., Kim D. H., Kim M. J., Pappas G. J., and Kumar V., Appl. Phys. Lett. 96, 043705 (2010). 10.1063/1.3293457 [DOI] [Google Scholar]
- Kim L. N., Choi S. E., Kim J., Kim H., and Kwon S., Lab Chip 11(1), 48 (2011). 10.1039/c0lc00369g [DOI] [PubMed] [Google Scholar]
- Ishihara H. and Takeuchi S., in IEEE MEMS 2010 Conference, Hong Kong, China, 24-28 January 2010, p. 959.
- Gach P. C., Sims C. E., and Allbritton N. L., Biomaterials 31(33), 8810 (2010). 10.1016/j.biomaterials.2010.07.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn N. M., Chang R., Westerhof T., Li G. P., Bachman M., and Nelson E. I., Langmuir 26(22), 17703 (2010). 10.1021/la101960v [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massart R., IEEE Trans Magn. 17, 1247 (1981). 10.1109/TMAG.1981.1061188 [DOI] [Google Scholar]
- Bee A., Massart R., and Neveu S., J. Magn. Magn. Mater. 149, 6 (1995). 10.1016/0304-8853(95)00317-7 [DOI] [Google Scholar]
- See supplementary material at http://dx.doi.org/10.1063/1.3608133E-BIOMGB-5-003192 for PDMS template fabrication, measurement of magnetic polystyrene absorption, ART-FTIR procedure, SEM and TEM sample preparation, cell transfection, ATR-FTIR of magnetic PS-AA, microraft fabrication, fluorescence cell imaging, microraft array image, magnetic purification of collected microrafts, and magnetic purification of cells on microrafts.
- Jung H., Kwak B., Yang H. S., Tae G., Kim J. S., and Shin K., Colloid Surf. A 313, 562 (2008). 10.1016/j.colsurfa.2007.05.070 [DOI] [Google Scholar]