Abstract
Summary: Mycobacterium haemophilum is a slowly growing acid-fast bacillus (AFB) belonging to the group of nontuberculous mycobacteria (NTM) frequently found in environmental habitats, which can colonize and occasionally infect humans and animals. Several findings suggest that water reservoirs are a likely source of M. haemophilum infections. M. haemophilum causes mainly ulcerating skin infections and arthritis in persons who are severely immunocompromised. Disseminated and pulmonary infections occasionally occur. The second at-risk group is otherwise healthy children, who typically develop cervical and perihilar lymphadenitis. A full diagnostic regimen for the optimal detection of M. haemophilum includes acid-fast staining, culturing at two temperatures with iron-supplemented media, and molecular detection. The most preferable molecular assay is a real-time PCR targeting an M. haemophilum-specific internal transcribed spacer (ITS), but another approach is the application of a generic PCR for a mycobacterium-specific fragment with subsequent sequencing to identify M. haemophilum. No standard treatment guidelines are available, but published literature agrees that immunocompromised patients should be treated with multiple antibiotics, tailored to the disease presentation and underlying degree of immune suppression. The outcome of M. haemophilum cervicofacial lymphadenitis in immunocompetent patients favors surgical intervention rather than antibiotic treatment.
INTRODUCTION
Mycobacterium haemophilum is an acid-fast bacillus (AFB) belonging to the group of nontuberculous mycobacteria (NTM) frequently found in environmental habitats, which can colonize and occasionally infect humans and animals (98). M. haemophilum can cause localized or disseminated disease in immunocompromised hosts and is a rare cause of disease in immunologically competent individuals. In 1996, Saubolle et al. (123) presented an overview of 64 cases reported in the literature. Since that time, another 154 cases have been reported. The purpose of this review is to present an update of the clinical picture, diagnostic approach, and therapeutic options for M. haemophilum infections.
GENERAL DESCRIPTION AND TAXONOMY
M. haemophilum, or the “blood-loving” mycobacterium, is a slowly growing AFB that differs from all other identified Mycobacterium species in preferring a lower growth temperature and having a unique culture requirement for iron supplementation. Thus, the classification of mycobacteria into several Runyon groups based on growth characteristics and pigment production may not be applicable to M. haemophilum. Many infections with M. haemophilum likely remain unrecognized, although suspicion should arise when AFB are visualized in smears and when cultures fail to yield an etiologic agent.
M. haemophilum was first described in 1978 as a pathogen causing skin infections most frequently in immunocompromised patients, which may explain its preferred growth temperature of 30°C (130). In 1981, Dawson and colleagues described a case of submandibular lymphadenitis due to M. haemophilum in an otherwise healthy child (31), and M. haemophilum has since been recognized as an emerging pathogen in a variety of syndromes. The microorganism is now also known to cause cutaneous and subcutaneous infections, septic arthritis, osteomyelitis, and pneumonitis in immunocompromised patients. Cervicofacial lymphadenitis is the most common manifestation in immunocompetent children. Reports of such cases originate from all continents. However, although our understanding of M. haemophilum infections in humans has increased considerably in recent years, the natural habitat and how an infection is acquired remain unknown.
M. haemophilum most resembles Mycobacterium marinum and M. ulcerans in regard to its role in skin infections. The relatedness can also be observed for genomic traits, as all three species have a relatively low GC content compared to those of most other Mycobacterium species.
Some interesting similarities also exist between M. haemophilum and Mycobacterium leprae. First, the fatty acid docosanoic acid is found in abundant quantities in both species. Second, M. haemophilum has also been shown to possess a specific phenolic glycolipid antigen that closely resembles the corresponding lipid in M. leprae (10). Third, M. leprae has major membrane protein I (35 kDa), which is absent in members of the M. tuberculosis complex, but homologous sequences have been detected in M. avium, M. haemophilum, and M. smegmatis (159).
Taxonomic relationships between mycobacteria can be investigated by comparing the sequences of gene targets used to differentiate species, such as ribosomal gene fragments (i.e., the 16S rRNA gene and internal transcribed spacer [ITS]) and housekeeping genes (i.e., hsp65 and rpoB). The taxonomic relationship between M. haemophilum and other Mycobacterium species is not completely clear because different panels of mycobacterial species were included in previous studies, and different gene fragments were used in alignments: the 16S rRNA, rpoB, hsp65, and sod genes. A phylogenetic analysis of 500-bp 5′ 16S rRNA gene sequences in the RIDOM database indicated that M. leprae, M. malmoense, and M. bohemicum are the species genetically most closely related to M. haemophilum (62). Another tree constructed from the 16S rRNA gene sequences from 80 species indicated that M. leprae and the M. avium complex are closely related (50). A study using an unrooted phylogenetic analysis of 16S rRNA gene sequences (1,325 bp) from 18 species showed that M. bohemicum and M. szulgai are the most closely related species (58), and M. leprae has a relatively large genetic distance from M. haemophilum. Last, using a multigene approach, including the sod, 16S rRNA, hsp65, and rpoB genes, Devulder and colleagues showed that M. haemophilum has no immediate neighboring species, although M. leprae was not included in this analysis (34). At the National Institute for Public Health and the Environment (RIVM), about 700 nucleotides of the 3′ end of the rpoB gene were sequenced. With the sequence data obtained, a dendrogram (Fig. 1) was created by using BioNumerics software (Applied Maths, Kortrijk, Belgium). Based on rpoB similarities, the closest relationship was observed for M. leprae (93.5%). Other mycobacterial species were at larger genetic distances, with M. gordonae (92%), M. malmoense (91.7%), M. avium (91%), and M. szulgai being most closely related (R. de Zwaan, RIVM, unpublished data).
Fig. 1.
Dendrogram made by using the rpoB gene sequences of 29 mycobacterial species. M. leprae was most closely associated (93.5%).
CLINICAL PRESENTATION
In contrast to infections caused by M. tuberculosis, M. haemophilum is not a reportable infection, and the number of cases may be higher than what is represented by published case reports. A second reason for the underestimation of the actual number of M. haemophilum infections is the difficulties in diagnosing the disease. Based on the available literature, two groups appear to be at risk for M. haemophilum infection (123). The main group consists of severely immunocompromised patients, in whom M. haemophilum occurs as an opportunistic infection (1, 2). M. haemophilum is being increasingly recognized in persons who are severely immunocompromised by HIV infection; after renal, bone marrow, or cardiac transplantation; or after treatment for lymphoma or rheumatoid arthritis. The second at-risk group is otherwise healthy children, who typically develop cervical and perihilar lymphadenitis similar to that caused by infection with the Mycobacterium avium complex (3, 90, 164).
IMMUNOCOMPROMISED PATIENTS
Cutaneous Manifestations
M. haemophilum causes mainly skin lesions in immunocompromised patients (42, 150). Cutaneous infections with potentially pathogenic mycobacterial species are important for the differential diagnosis of skin lesions in these patients (36, 61, 93, 106). M. haemophilum infections have been reported, especially in patients with lymphoma or HIV and in organ transplant recipients (19, 66, 81, 84, 112, 153). The clinical spectrum of cutaneous infections caused by M. haemophilum appears to be broad (19, 30), varying from localized disease to systemic disease with cutaneous dissemination (49). Multiple skin lesions tend to occur and can present as erythematous papules, plaques, nodules, necrotic abscesses, or chronic ulcers. Cutaneous lesions are found most frequently on the extremities, particularly over joints, and less commonly on the trunk and face. Purpuric and annular lesions have also been described (47). Skin lesions typically evolve from papules to asymptomatic pustules and eventually to very painful deep-seated ulcers. The erythematous or violaceous papules and/or nodules are usually painless at first, but they can develop into potentially very painful abscesses or ulcers. Patients with cutaneous and articular manifestations have a more favorable prognosis than those with pulmonary involvement (126). An overview of the skin infections reported since the review by Saubolle et al. (123) is presented in Table 1. Thirty-three new cases have been reported, with a median age of the patients of 48 years (range, 14 months to 67 years). The sex distribution was equal. Most of the reports were from the United States (10 cases), followed by Germany (4 cases), Australia (4 cases), and Singapore (4 cases). The majority of the patients had a history of solid-organ transplant or AIDS.
Table 1.
Reported cutaneous manifestations in immunocompromised patients, 1996 to 2011a
| Reference | Age of patient (yr)/sex | Underlying disease(s) | Country | Treatment | Outcome | Duration of treatment |
|---|---|---|---|---|---|---|
| 8 | 48/M | IgA deficiency | Germany | CLR, RB, E | Resolved | 6 mo |
| 22 | 59/M | Renal transplant | Brazil | CLR, CI | Resolved | 1 yr |
| 114 | 52/M | Renal transplant | United States | CI, CLR | Resolved | 1 yr |
| 119 | 62/M | Heart transplant | Israel | CI, CLR, R | Regression within 3 wk | 1 mo |
| 72 | 65/F | CLL | United States | R,CLR, CI | Resolved | 6 mo |
| 72 | 17/F | SLE, MDS | United States | R, CLR, G | Resolved | NA |
| 28 | 45/F | Renal transplant | Venezuela | CLR | Resolved | 6 mo |
| 28 | 14 mo/F | Unknown immunodeficiency, CD4+ <16% | Venezuela | CLR, TMS, R, I | Resolved | 6 mo |
| 125 | 67/F | RA | Germany | (i) CLR, CI; (ii) RB, E, CLR; (iii) CLR monotherapy after 8 wk | Resolved | >6 mo, NA |
| 94 | 38/F | Autoimmune cirrhosis | United States | CI, CLR | Resolved | 6 mo |
| 94 | 47/F | Myasthenia gravis, corticosteroids | United States | D, RB, AZI | Improvement | 8 mo |
| 20 | 37/M | AIDS | Spain | I, R, E, AK, CLR, CI, Min | Resolved | 5 mo |
| 21 | 16/M | Renal transplant | United States | E, R, CLR, CI, AK | Partial resolution | 14mo |
| 149 | 59/M | Polymyalgia rheumatica | Germany | I, E, R | Died | |
| 53 | 27/F | AIDS | United States | RB, CLR, CI | Improvement | 1 mo |
| 138 | 59/F | SLE | Singapore | (i) CLR, CI, R, I, E; (ii) RB, CLR | Resolved; recurrence and resolution after 2nd course | 13 mo |
| 138 | 64/F | Cutaneous vasculitis | Singapore | CLR, CI | Resolution | 18 mo |
| 138 | 42/F | Sjogren's syndrome, Crohn's disease | Singapore | CLR, D | NA | 18 mo |
| 98 | 51/M | AIDS | Italy | RB, E, CLR; later RB, AZI, L | Resolution | 5 mo |
| 85 | 44/M | Renal transplant | Taiwan | CI, R, CLR | Resolution | 1 yr |
| 49 | 59/M | Diabetes | United States | CI, RB, CLR | Resolution | NA |
| 139 | 25/F | SLE | Singapore | CLR, E, I, R | Resolution | 6 mo |
| 108 | 30/F | AIDS | Germany | Only antiretroviral therapy | Resolution | 14 mo |
| 38 | 51/M | AIDS | Japan | CLR, E | Resolution | 8 mo |
| 126 | 29/F | AIDS | United States | R, CI, CLR, D | Died | 11 mo |
| 47 | 62/M | AIDS | Switzerland | AZI, RB | Resolution | 6 mo |
| 51 | 51/M | AIDS | United States | CLR, TMS, CI | Resolution | NA |
| 97 | 56/M | Lung transplant | Australia | I, CLR, P, D, CI | Resolution | 42 mo |
| 97 | 49/F | Lung transplant | Australia | CLR, E, R | Resolution | 17 mo |
| 97 | 53/F | Lung transplant | Australia | CLR, R, CI, D | Resolution | 31 mo |
| 97 | 39/M | Lung transplant | Australia | CLR, R, CI, D | Resolution | 18 mo |
| 80 | 30/F | AIDS | The Netherlands | (i) D, CI, R, CLR; (ii) Min, RB, E; (iii) I, CI, CY | Resolution | 6 mo |
| 6 | 35/M | AIDS | Germany | R, E, I, CLR | Resolution | 7 wk, relapse and retreatment with same regimen |
I, isoniazid; R, rifampin; RB, rifabutin; E, ethambutol; CY, cycloserine; CI, ciprofloxacin; AK, amikacin; AZI, azithromycin; CLR, clarithromycin; TMS, trimethoprim-sulfamethoxazole; P, pyrazinamide; D, doxycycline; Min, minocycline; G, gatifloxacin; L, levofloxacin; RA, rheumatoid arthritis; MDS, myelodysplastic syndrome; SLE, systemic lupus erythematosus; CLL, chronic lymphocytic leukemia; NA, data not available; M, male; F, female.
Cutaneous lesions have been rarely reported for children (21, 28), but the manifestations of the skin lesions are similar to those of immunosuppressed adults.
Pyomyositis
Mycobacterial infection of the skeletal muscle is very rare; in particular, large muscles are involved, and the condition usually presents as localized muscle involvement through direct extension from a proximal focus of infection. Only four cases of pyomyositis caused by M. haemophilum have been reported (70, 82, 124, 127).
In a recent report by Lee et al. (82), a 23-year-old immunosuppressed female patient with multiple, tender, erythematous, and palpable fluctuant abscesses on the left leg due to an M. haemophilum infection was described. In another case, the patient had been on long-term steroid treatment for polymyositis and presented with ulcerations over both thighs and the left arm after a year of steroid therapy (127). A 24-year-old female renal transplant recipient was described as having tender, erythematous, and palpable fluctuant swelling on the left calf (70). The patient had undergone kidney transplantation 8 years earlier, after which she had been on immunosuppressive treatment with cyclosporine and mycophenolate mofetil.
Disseminated and Pulmonary Infections
Several cases of septicemia and pneumonitis due to M. haemophilum have been documented (Tables 2 and 3).
Table 2.
Reported disseminated infections in immunocompromised patientsa
| Reference(s) | Age of patient (yr)/sex | Country | Underlying disease(s) | Initial presentation(s) | Culture source(s) | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| 136 | 64/M | Germany | AIDS | Skin, pulmonary | Blood, sputum | RB, E, CLR + TMS | Cure |
| 11 | 6/F | The Netherlands | ALL | Skin, pulmonary, joints | Skin, bone marrow | E, R, CLR, AK, drainage of abscesses | Cure |
| 39 | 46/F | United States | Cardiac transplant | Skin, pulmonary, joints | Skin, synovial fluid | Imi, CI, CLR, D | Cure |
| 121 | 67/M | Brazil | Renal transplant | NA | Blood | NA | NA |
| 126 | 32/M | United States | BMT, MDS | Pulmonary infiltrate | Blood | None | Died |
| 19 | 51/F | United States | Multiple myeloma, RA | Skin disease | Skin, blood | R, E, CLR, CI | Cure |
| 123 | 33/M | United States | AIDS | Synovial involvement | Synovial fluid, blood | E, CLR, CI, AK | Relapse, died of AIDS |
| 75, 76, 135, 156 | 30/F | United States | BMT, APML | Subcutaneous nodules | Skin, blood | CI, CLR, D, I, R, E | Cure |
| 75, 76, 79 | 36/M | United States | AIDS | Skin lesions, septic arthritis | Synovial fluid, blood | PAS, R | Cure |
| 75, 135, 163 | 37/M | United States | AIDS | Skin lesions, septic arthritis | Skin, blood | AK, CI, I, CL, D, E, I, R | Cure |
| 75, 117 | 34/M | United States | AIDS | Subcutaneous nodules | Skin, blood | E, I, R | Persisted |
| 23 | NA | United States | NA | NA | Blood | NA | NA |
I, isoniazid; Imi, imipenem; R, rifampin; RB, rifabutin; E, ethambutol; CI, ciprofloxacin; AK, amikacin; CLR, clarithromycin; TMS, trimethoprim-sulfamethoxazole; D, doxycycline; PAS, p-aminosalicylic acid; RA, rheumatoid arthritis; BMT, allogeneic bone marrow transplantation; MDS, myelodysplastic syndrome; APML, acute promyelocytic leukemia; CML, chronic myelogenous leukemia; ALL, acute lymphocytic leukemia; NA, data not available.
Table 3.
Reported pulmonary manifestations in immunocompromised patientsa
| Reference(s) | Age of patient (yr)/sex | Country | Underlying disease(s) | Initial presentation(s) | Culture source(s) | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| 137 | 72/F | The Netherlands | RA/OSAS | Pneumonia | Sputum | R, AZI | Resolution |
| 126 | 32/M | United States | BMT, MDS | Pulmonary infiltrate | Blood | None | Death |
| 157 | 62/F | United States | NA | Pulmonary nodule | Lung biopsy specimen | R, E | Cure |
| 123 | 30/F | United States | Renal transplant, subsequent AIDS | Skin lesions, septic arthritis, subsequent pulmonary involvement | NA | E, I, R, subsequent Min | Initial resolution, subsequent death |
| 135 | 51/M | United States | AIDS | Bronchitis | Sputum, bone | CI, D, E, I, P, R | Resolution |
| 76 | 42/M | United States | BMT, MDS | Pulmonary infiltrate | BAL fluid, sputum | R, E, CI, CLR, AK, P | Death |
| 76 | 37/M | United States | AIDS | Skin lesions, pneumonia | Skin, sputum | AK, CI, D, E, I, R | Responded and then relapsed and died |
| 27, 75, 76, 135 | 35/M | United States | AIDS | Skin, pulmonary infiltrate | Skin, sputum | R, E, CI, AK, D, Ery | Death |
| 27, 75, 76, 135, 156 | 27/M | United States | BMT, AA | Pulmonary nodules | Sputum, BAL fluid, lung biopsy specimen | R, E, AK, P, I, S | Death |
I, isoniazid; R, rifampin; RB, rifabutin; E, ethambutol; CI, ciprofloxacin; AK, amikacin; AZI, azithromycin; CLR, clarithromycin; P, pyrazinamide; D, doxycycline; Min, minocycline; Ery, erythromycin; S, streptomycin; RA, rheumatoid arthritis; BMT, allogeneic bone marrow transplantation; MDS, myelodysplastic syndrome; AA, aplastic anemia; CML, chronic myelogenous leukemia; BAL, bronchoalveolar lavage; OSAS, obstructive sleep apnea syndrome; NA, data not available.
The patients with disseminated disease in Table 2 include 11 adults aged 30 to 67 years and 1 6-year-old child. Nine patients were from the United States, one was from Germany, and one was from Brazil. Five patients had AIDS, one had received a renal transplant, one had received a cardiac transplant, two had received a bone marrow transplant, and one was undergoing treatment for multiple myeloma. Only one case of a pediatric disseminated infection has been described (11). A 6-year-old child from The Netherlands with a history of B cell precursor acute lymphoblastic leukemia presented with fever and painful suppurative skin lesions on the knees, elbows, and face. The patient later developed arthritis and osteomyelitis of the right knee in addition to several subcutaneous abscesses, and she remained febrile.
Nine patients have been reported to have M. haemophilum pulmonary infections. Six patients were male, and three were female, with a median age of 38 years (range, 27 to 72 years) (Table 3). Eight reports were from the United States, and the most recent report was from The Netherlands. Despite several multidrug regimens (Table 3), a resolution of the infection was observed for less than half of the patients.
Ophthalmologic Manifestations
Two reports in the literature described primary ophthalmologic infections due to M. haemophilum (102, 104). Millar et al. (102) described a 55-year-old man with a history of acute myeloid leukemia with chronic bilateral conjunctivitis and dry eyes for a period of 6 months. Skin lesions were also noted on the patient's face and arms. The clinical condition improved with moxifloxacin and clarithromycin with the addition of valacyclovir and clindamycin 1 week later. Modi et al. (104) presented a unilateral chronic granulomatous iridocyclitis in a 66-year-old man with previous cardiac transplantation and cyclosporine and mycophenolate mofetil treatment. Antibiotic therapy did not prevent the progression of intraocular inflammation, and the patient developed a corneal ulcer that perforated. Enucleation was performed 1 year after the initial presentation. However, the skin lesions regressed with antibiotic therapy.
Osteomyelitis
A less common manifestation of M. haemophilum in immunocompromised patients is septic arthritis or osteomyelitis with or without cutaneous lesions. Osteomyelitis caused by M. haemophilum resembles that caused by other microorganisms (107, 163). On radiographs, bony resorption with clear margins, cortical destruction, and adjacent soft tissue swelling are apparent. Magnetic resonance imaging (MRI) can reveal well-circumscribed medullary lesions with cortical disruption and a large soft tissue component (83). Table 4 provides an overview of the cases of skeletal M. haemophilum infections described in the literature. Twenty-six cases have been reported, with a median age of the patients of 45.5 years (range, 20 to 77 years). The underlying illnesses most frequently included AIDS (15 cases) and organ transplantation (7 cases).
Table 4.
Reported septic arthritis/osteomyelitis in immunocompromised patientsa
| Reference(s) | Age of patient (yr)/sex | Underlying disease | Country | Area(s) of septic arthritisb | OMb | Other site(s) | Therapy | Outcome |
|---|---|---|---|---|---|---|---|---|
| 101 | 58/M | Lymphoma | Australia | Foot | I, R, E | Partial response | ||
| 101 | 55/F | Renal transplant | Australia | Ankle | I, R, E | Died | ||
| 26 | NA | Cardiac transplant | South Africa | Limbs | NA | NA | Died | |
| 96 | 32/M | AIDS | United States | Ankles, L wrist | Blood | R, I, P, E, ET | No improvement | |
| 117 | 34/M | AIDS | United States | R finger | Soft tissue abscess, BAL fluid | I, R, E | No improvement | |
| 54 | 48/M | Renal transplant | France | L middle finger, L knee | Skin | Surgery; Min, Ery for 2 mo; I, R, E, Min for 6 mo | Cure | |
| 79 | 36/M | AIDS | United States | R knee | Skin, blood | R, I, P, E | Improvement, stable at 19-mo follow-up | |
| 57 | 21/F | AIDS | United States | L ankle, tibia | Skin | R, Min | Resolution | |
| 33 | 44/M | AIDS | United States | Bilateral tibia and fibula | Skin, sputum | I, R, E, CI, CL, AK | Initial improvement, relapse in 6 wk | |
| 163 | 31/F | AIDS | United States | L knee | R 3rd finger, R calcaneus | Skin | R, I, E, AK, CI, CL, P | Resolution |
| 76, 135, 163 | 37/M | AIDS | United States | L ankle | Skin | R, I, P, E for 14 mo | Resolution | |
| 118 | NS | AIDS | France | Bilat knees | Finger, toes, tibia, elbow, T9-10 vertebrae | Skin, lungs | I, R, E | No improvement |
| 133 | 39/M | AIDS | Australia | L foot | CL, AK, D, R | Improvement in 10 wk | ||
| 132 | 41/M | AIDS | United States | R elbow | R olecranon | Skin | R, I, P | Resolved 9 mo later |
| 64 | 49/M | AIDS | United States | Knees, ankles | R ankle and tibia | Skin, blood, lymph nodes | R, I, P, E, A, CI, CL | No improvement |
| 65 | 46/M | AIDS | United States | Foot | CI, RB, CY, AZI | Improved after treatment | ||
| 123 | 30/F | Renal transplant/AIDS | United States | Hand | Hand | Skin, pulmonary | I, R, E, Min | Resolution of lesion, died |
| 123 | 33/M | AIDS | United States | Knee | Skin | E, CLR, CI, AK | Died of AIDS complications | |
| 123 | 77/M | T cell lymphoma | United States | Hand | Curettage | Relapse after 1 yr, died of lymphoma complications | ||
| 123 | 66/F | RA (corticosteroids) | United States | Hip | D, R, subsequent excision + D, R | Cure | ||
| 123 | 56/M | AIDS | United States | Ankle | NA | Died 2 mo after initial presentation | ||
| 123 | 45/F | Renal transplant | United States | Finger | Skin | CI, RB | Cure | |
| 112 | 20/M | Cardiac transplant | United States | Olecranon | CLR, R | Cure | ||
| 121 | 30/M | AIDS | Brazil | Elbow | NA | NA | NA | |
| 126 | 47/F | AA/BMT | United States | NS | CI, CLR, D, R (>6 mo) | Improved | ||
| 39 | 46/F | Cardiac transplant | United States | Wrist, knees, ankles | Skin, pneumonia | Imi, CI, CLR, D, 2 mo | Resolution | |
| 56 | 53/F | AIDS | Germany | Tibia | E, R, CLR | Cure | ||
| 37 | 56/F | Polycythemia vera | Canada | R wrist, R ankle | Skin | CI, RB, CLR | Cure |
I, isoniazid; Imi, imipenem; R, rifampin; RB, rifabutin; E, ethambutol; ET, ethionamide; CY, cycloserine; CL, clofazimine; CI, ciprofloxacin; AK, amikacin; AZI, azithromycin; CLR, clarithromycin; P, pyrazinamide; D, doxycycline; Min, minocycline; Ery, erythromycin; RA, rheumatoid arthritis; AA, aplastic anemia; BMT, bone marrow transplant; OM, osteomyelitis; NA, data not available.
L, left; R, right.
Uncommon Clinical Presentations
Central catheter infections.
Ward et al. (152) described an M. haemophilum infection of the central venous catheter tunnel in two young (26 and 29 years old) immunosuppressed patients with hematological malignancy undergoing high-dose chemotherapy supported by bone marrow transplantation. The M. haemophilum infections occurred at the site of the tunneled catheter after the line had been removed. For one patient, therapy consisted of amikacin, clarithromycin, ciprofloxacin, and meropenem for 3 weeks, after which amikacin and meropenem were ceased and ethambutol was started. For the other patient, repeated surgical excisions were combined with clarithromycin, amikacin, and meropenem treatment. The drugs were later changed to rifampin, ciprofloxacin, and clarithromycin. For both patients, the wound eventually healed.
Epididymal abscess.
Keller et al. (73) described an epididymal abscess due to M. haemophilum infection in a renal transplant patient. A right orchidectomy was performed, combined with clarithromycin, rifabutin, and ethambutol treatment. The symptoms resolved over 5 months of follow-up.
Mixed infections.
Dual infections with M. haemophilum and other NTM species are extremely rare but also difficult to diagnose. Since the first case report by Branger et al. (12), describing a mixed infection with M. haemophilum and M. xenopi, two new cases have been reported. Bekou and colleagues (8) reported a skin infection with multifocal nodules of variable sizes arranged in a sporotrichoid-like manner on the extremities and back of a 48-year-old male patient with an IgA deficiency. Both M. haemophilum and M. kansasii were cultured. Treatment with clarithromycin, rifabutin, and ethambutol for 6 months led to a complete clinical remission of the skin lesions. Phowthongkum et al. (111) described a 40-year-old male patient with AIDS who developed a spindle cell pseudo-brain tumor as a result of M. haemophilum and M. simiae infection. He was treated with isoniazid, rifampin, pyrazinamide, ethambutol, and clarithromycin. One month after hospitalization, he commenced antiretroviral treatment, including zidovudine, lamivudine, and efavirenz. He was discharged home, and was seen for the last time 3 months after the operation.
IMMUNOCOMPETENT PATIENTS
Adult Infections
Cervicofacial infections.
An outbreak of 12 cases of M. haemophilum skin infection with lymphadenitis after permanent makeup on the eyebrows was described recently (52). The ink used by the tattoo artist was found to be contaminated with M. haemophilum. All 12 patients were female, with a median age of 56 years, and none of the patients were immunosuppressed. The patients presented with an inflammatory lesion consisting of a few red papules or pustules or an erythematous plaque on one eyebrow. In all cases, the lesion was associated with ipsilateral lymphadenopathy in the parotid region, affecting one or more lymph nodes (median, 2; range, 1 to 5). Eight patients presented with an abscess, which later developed into a fistula in seven cases, whereas none of the patients reported systemic symptoms.
Minani et al. (103) described a 27-year-old immunocompetent woman with a right buccal abscess and submandibular lymphadenitis. The patient was cured with surgical excisional therapy of the affected lymph nodes and drainage of the buccal abscess. A retrospective overview of another six patients with cervicofacial lymphadenitis (five females and one male, with an age range of 19 to 65 years) seen over a 15-year period in Phoenix, AZ, was also presented (103).
“Other” skin lesions.
Skin lesions in immunocompetent adults due to M. haemophilum infection are rare and the result of injury. Two cases have been reported (99, 128): a 61-year-old male who sustained several lacerations to the forearm when he was thrown against coral while surfing and a 65-year-old female who developed subcutaneous skin nodules after coronary artery bypass surgery.
Pediatric M. haemophilum Infections
Cervicofacial infections.
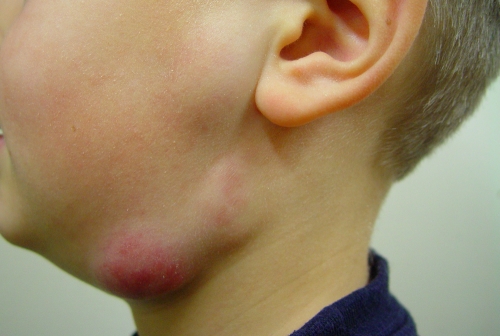
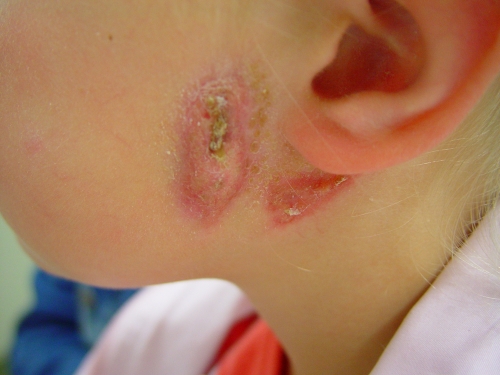
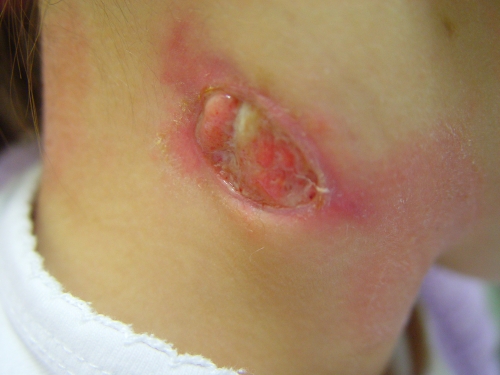
Lymphadenitis is the most common clinical manifestation of NTM infection of children (155). Since the first reported case of cervicofacial lymphadenitis in an immunocompetent child in 1981 (31), seven additional cases of children with head and neck lymphadenitis have been added to the literature (3, 123, 141, 147). M. haemophilum was recently reported to be a major cause of lymphadenitis in immunocompetent children in Israel and The Netherlands (90, 164). These reports showed that M. haemophilum is the second most commonly recognized pathogen in children with cervicofacial NTM lymphadenitis. Patients with M. haemophilum lymphadenitis tended to be older than patients with the more common M. avium lymphadenitis (25, 90). In the study from The Netherlands (90), the M. avium-infected and M. haemophilum-infected patients did not differ with respect to sex, duration of lymph node swelling prior to presentation, or clinical symptoms, but M. haemophilum infections of the head and neck were associated with an infection of multiple lymph nodes (Fig. 2, 3, and 4) and the involvement of extranodal areas, such as the medial canthus, cheek, or ear lobe (60, 90, 92). Children with M. avium or M. haemophilum cervicofacial lymphadenitis seldom exhibited general clinical symptoms (90, 164), although some children experienced a loss of appetite. As a result of a diagnostic delay, most children with M. haemophilum lymphadenitis (80%) presented in a secondary or tertiary center in the stage of lymph node fluctuation with discoloration of the skin.
Fig. 2.
Clinical picture of a child with a cervicofacial Mycobacterium haemophilum lymphadenitis presenting as a fluctuant swelling with red skin discoloration.
Fig. 3.
Clinical picture of Mycobacterium haemophilum lymphadenitis after skin breakdown.
Fig. 4.
Ulcerating open wound as a result of a cervicofacial Mycobacterium haemophilum infection.
Inguinal lymphadenitis.
One case of a 5-year-old girl with a painful, enlarged lymph node in the groin has been reported (89). The portal of entry was most likely a wound on the dorsum of her foot. During antimycobacterial therapy with clarithromycin and rifabutin, the inguinal lymph node started suppurating, and after 12 weeks of treatment, complete necrosis of the lymph node was visible. The surgical excision of the affected inguinal lymph nodes led to complete resolution.
Pulmonary involvement.
Armstrong et al. (3) described a 12-month-old male infant with a 6-week history of daily fever, anorexia, and weight loss. Examination revealed fever, cough, tachypnea, tachycardia, and decreased breath sounds over the right upper lobe of the lung. No immunodeficiencies were detected, and after mediastinal biopsy, antituberculous medication with pyrazinamide, rifampin, isoniazid, and pyridoxine reduced the clinical symptoms. After 6 weeks, the antibiotic therapy was changed to erythromycin, which was prolonged for 15 months, with a final resolution of the disease.
ANIMAL INFECTIONS
M. haemophilum infection is not restricted to a human host. M. haemophilum appears to be pathogenic in fish and has caused clinical manifestations in a snake and a bison similar to those seen in humans (63, 69, 74, 154). A royal python was diagnosed with pulmonary mycobacteriosis caused by both M. marinum and M. haemophilum (63). Normal lung tissue was largely replaced by granulomatous tissue containing necrotic foci, as is often observed for mycobacterial disease in humans. Cultures of tissue biopsy specimens contained numerous AFB representing both species. Another report described an intradural mass compressing the spinal cord in a bison (69). Again, histological examination showed necrotic granulomatous tissue containing a large number of AFB. 16S rRNA gene sequencing analysis of the mycobacterial culture identified M. haemophilum.
M. haemophilum appears to be highly pathogenic in zebrafish, as several outbreaks have been reported (74, 154). At least three unrelated outbreaks, with mortality rates of up to 20%, were caused by this species. All organs seemed to be infected, and massive amounts of bacilli were observed in granulomas and throughout regions of diffuse inflammation.
PATHOGENESIS
M. haemophilum infections are similar to those caused by M. marinum and M. ulcerans; they occur most commonly as necrotic lesions within the regions of the body with the lowest temperatures (19). Histological examination usually reveals a granulomatous reaction with necrotic foci.
M. haemophilum is apparently of low virulence, as most healthy mice and guinea pigs in earlier studies survived for an observation period of 3 months after intramuscular, intravenous, and subcutaneous inoculations of large numbers of bacilli (130, 131). However, some of the mice died after 2 to 4 weeks, with large numbers of AFB in liver, spleen, and kidneys. The intramuscular injection of M. haemophilum into the thighs of frogs did not result in abnormalities when the frogs were kept at room temperature. However, the animals died within 20 days when kept at 30°C, with M. haemophilum infestation in the liver and kidneys. In vitro, M. haemophilum seems to have a preference for growth in cultured human endometrial carcinoma cells (Hec-1-B), compared to human microvascular endothelial cells (HMEC-1) (43, 44). An epithelial cell culture infection model suggested greater intracellular replication at 33°C than at 37°C and showed that the bacilli are associated with cytotoxicity at the lower temperature (43, 44). These observations indicate that M. haemophilum is a facultative intracellular bacterium. Additionally, M. haemophilum exhibits contact-dependent cytolytic activity at 33°C, similar to the effect observed for M. tuberculosis infections. Thus, the pathogenicity of M. haemophilum appears to be temperature dependent, which is consistent with infection and tissue damage in skin and other superficial body sites with a lower temperature.
EPIDEMIOLOGY
Typing of M. haemophilum
Several Mycobacterium species have been examined extensively by molecular typing, but limited information is available on the genetic diversity of M. haemophilum. Three typing studies have been conducted to date, based on pulsed-field gel electrophoresis (PFGE) (162), restriction fragment length polymorphism (RFLP) analysis (77), and amplified fragment length polymorphism (AFLP) analysis (16).
All three methods demonstrated a high degree of clustering among the clinical isolates investigated, and a sufficient degree of discrimination was observed among isolates that were not epidemiologically related. PFGE and RFLP analysis were used to type isolates from the United States, most of which came from the New York City area. In the AFLP study (16), isolates from different continents were tested, including the strains from the United States that were also subjected to RFLP analysis and PFGE.
The general conclusion from these three studies was that a high degree of clustering exists among isolates from the same geographic area and that a high degree of genetic stability is present over time. Clusters of identical DNA fingerprint types were observed within close geographical proximity, but the isolates were not necessarily derived from the same hospitals and not found in geographically distant locations. Genetic conservation was also demonstrated by several clusters of clonal types for extended time periods; one cluster from New York linked isolates over a period of 16 years, and two clusters from Australia remained unchanged for 15 and 18 years, which suggests an extremely low evolutionary rate for this mycobacterium. This bacterium may survive in a highly suitable niche, such as tap water, without any selective pressure.
Although the typing results of the three studies are in accordance and technically reliable, typing results should be analyzed with caution because isolates with (nearly) the same DNA fingerprinting profiles are not necessarily epidemiologically linked. Whole-genome sequencing of multiple strains will facilitate the establishment of a robust and detailed phylogenetic tree that may serve to clarify the epidemiology of M. haemophilum infections in humans and the environment. This method was recently shown to be highly informative when it was applied to an M. tuberculosis outbreak in which two separate lineages were identified to occur simultaneously in one social network (48).
Environmental Findings
Although no clinical isolates have been linked directly to environmental isolates, several findings suggest that water reservoirs are a likely source of M. haemophilum infection. For a cluster of M. haemophilum infections in New York, the hospital drinking water supply was suspected to be the common source, but this was not proven (T. E. Kiehn, Memorial Sloan Kettering Cancer Center, New York, NY, personal communication). The resistance to common disinfectants, temperature tolerance, and ability to form biofilms exhibited by mycobacteria are all preferential characteristics for survival and persistence in water systems and reservoirs (41). One paper describing an M. haemophilum infection in a patient after sustaining a coral injury suggested that seawater or coral is also an environmental source (128).
Several studies have been conducted with the objective of investigating the presence of NTM in water systems (146) However, the specific requirements for the detection of M. haemophilum were often not met in these studies. For example, Covert et al. (27) employed molecular identification after culturing without specific requirements for M. haemophilum. Chang and colleagues (24), using a PCR-RFLP method for the direct detection of mycobacteria in water samples, showed a high prevalence of AFB. However, the reverse primer sequence used in that study did not match the M. haemophilum sequence, and thus, direct detection was compromised. Molecular detection using concentrated water samples containing AFB was unsuccessful overall, and the method was eventually applied to the identification of isolates cultured without the culturing requirements necessary for M. haemophilum. Both studies showed that a variety of mycobacterial species were present in chlorine-treated water supplies and were thorough, but M. haemophilum might have been overlooked.
Only a few studies allowed the detection of M. haemophilum by molecular methods or specific culturing methods (40, 68, 113, 154). Hussein and colleagues (68) did include species detection, but they encountered only other NTM. Three studies detected M. haemophilum. Falkinham et al. (40) found it in three samples, comprising one water sample and two biofilm samples, all from different water distribution systems in the United States. Whipps et al. (154) detected M. haemophilum in biofilms from four zebrafish tank meniscuses and one tank drain, all from a zebrafish research center in which M. haemophilum caused significant mortality among the fish population. Pryor et al. (113) cultured M. haemophilum from a water distribution system (unknown sample type) as one of many other Mycobacterium species.
In one publication, an environmental M. haemophilum isolate not directly associated with water was described. Mycobacterial isolates were cultured from the intestines and surface of hospital cockroaches in Taiwan, and M. haemophilum was found on the surface of one cockroach (109).
DIAGNOSTICS
Skin Testing
No specific antigen test is available for M. haemophilum infections, although in the past, purified protein derivatives (PPDs) of M. avium, M. kansasii, M. scrofulaceum and M. marinum, M. intracellulare, M. gordonae, and M. fortuitum have been used for the diagnosis of NTM infections. Unfortunately, a few years ago the production of NTM-PPD (Statens Serum Institute, Denmark) was terminated, although skin testing appeared to be useful for the diagnosis of NTM infections in children. Because of cross-reactivity between the immune reactions to PPDs of different species, the tuberculin-PPD test often shows false-positive reactions due to previous encounters with NTM (91). The problem with previous NTM encounters is not expected in young children; therefore, a positive tuberculin test can be indicative of NTM disease in this patient group, except for children living in a country where tuberculosis is highly endemic. For the initial diagnosis of NTM lymphadenitis, the tuberculin test has an optimal cutoff value of 5 mm for a positive skin induration (91). Using a 5-mm cutoff, the tuberculin PPD has 71% sensitivity for M. haemophilum and a 98% positive predictive value (PPV). Using a 10-mm cutoff (the induration cutoff considered positive for M. tuberculosis reactivity), 57% of all confirmed M. haemophilum infections yielded positive skin indurations.
Histopathology
Tissues infected with M. haemophilum show, almost without exception, granulomatous infiltrates with necrosis (19, 35). The granulomas comprise variable forms of granulocytes, lymphocytes, monocytes, and multinucleated giant cells. Bacilli can be observed both extracellularly and intracellularly, and they can be abundant or scarce in affected tissue (19, 35, 126). No specific clinical and histological manifestations can be attributed to M. haemophilum. M. haemophilum skin infection often mimics M. marinum infection: it forms erythematous papules or nodules, often overlying or above the joints, and in later stages, it becomes suppurative/ulcerative. However, in contrast to M. marinum infections, the nodules are painful, and sporotrichoid spread is seldom seen in M. haemophilum infections (19). Skin manifestations sporadically include lichenoid dermatitis, panniculitis, vasculitis, or annular plaques.
Histological findings for 16 skin biopsy specimens from 11 immunocompromised patients with culture-proven M. haemophilum infections revealed most commonly (7 of 16 biopsy specimens) a mixed histopathological pattern of suppurative and granulomatous reactions (19). Four biopsy specimens showed well-formed epithelioid granulomas. The authors of that study noted that infections by M. haemophilum can also present with nongranulomatous or paucigranulomatous reactions without necrosis, probably due to the immunocompromised state of the patients.
Microscopy
M. haemophilum is a strongly acid-fast bacterium and can be stained with Ziehl-Neelsen, modified Kinyoun, or auramine dye. The bacilli appear as short, and often curved, rods (1.2 μm to 2.5 μm in length) and can be pleomorphic. No specific growth or morphological differences exist between this and other species. Because M. haemophilum has the tendency to clump, a stain from a cultured isolate can exhibit strings of AFB, as is sometimes attributed exclusively to M. tuberculosis. Cord formation or cording should no longer be attributed exclusively to isolates of M. tuberculosis, as has recently also been demonstrated for nonpathogenic mycobacteria (71).
Culture
Like most of the pathogenic Mycobacterium species, M. haemophilum is slowly growing. Visible growth can take as long as 8 weeks. The normal growth temperature for mycobacteria is 35°C to 37°C. M. haemophilum, however, prefers a lower growth temperature of 30°C to 32°C and requires iron supplements such as hemin or ferric ammonium citrate, which can be added to both liquid and solid media (32, 122). Culturing of mycobacteria is most frequently applied to a system measuring the assimilation of bacteria in broth medium such as the BBL Mycobacteria Growth Indicator Tube (MGIT) containing Middlebrook 7H9 medium. A combination of a liquid culture medium with a solid medium is recommended. Solid egg-based media such as Löwenstein-Jensen (LJ), Coletsos, Stonebrink, Herrold's, or Dubos medium and solid agar-based media such as Middlebrook 7H10 and 7H11 agars are commercially available but must be supplemented with iron or hemin to allow the growth of M. haemophilum, as previously described (4).
Growth enhancers, such as mycobactin and OADC (containing oleic acid, albumin, dextrose, catalase, and NaCl), and antibiotics to inhibit the growth of contaminants are often added: PANTA (containing polymyxin, amphotericin B, nalidixic acid, trimethoprim, and azlocillin) and/or PACT (containing polymyxin B, amphotericin B, carbenicillin, and trimethoprim) (129, 158). The effect of these growth enhancers or antibiotic supplements on M. haemophilum has not been examined. The application of a decontamination protocol prior to culture helps to further decrease contamination with commensals and to release culturable bacilli from tissue (17). Several decontamination protocols are available, but it should be considered that most of them also decrease to some extent the recovery of mycobacteria. In our institute, we follow a NALC (N-acetyl-l-cysteine)-NaOH procedure for those samples that are contaminated and culture positive for rapidly growing bacteria on a standard blood agar medium (13).
Molecular Identification Methods
M. haemophilum can easily be differentiated from other species by sequencing. The representation of the species in the publicly available GenBank databases is sufficient for identification. Complete or partial ITSs and 16S rRNA, rpoB, and hsp65 genes represent 28 of 48 M. haemophilum sequences submitted to the database to date (January 2011). For most other housekeeping genes, only one sequence is available. Although the genetic marker most suitable for species identification is still unclear, all sequence targets in the database enable the identification of M. haemophilum.
A few commercial assays are available for the identification of cultured NTM isolates. Two reverse line probe assays include M. haemophilum: the GenoType Mycobacterium AS (Hain Lifescience GmbH, Nehren, Germany) (115) and the Inno-LiPA-Mycobacteria V2 (Innogenetics, Ghent, Belgium) (143, 144) assays. Other assays do not include the species, such as the AccuProbe assay, a chemiluminescence assay (Gen-Probe Inc./bioMérieux, Marcy l'Etoile, France), and the Speed-Oligo Mycobacteria assay, a hybridization dipstick test (Vircell, Spain). The newest software and database versions of Microseq 500 ID (Microseq ID 16S rDNA Full Gene Library v2.0, Applied Biosystems, Foster City, CA), a sequencing system, include a database with 86 mycobacterial species, including M. haemophilum (Applied Biosystems).
Also, several noncommercial molecular assays have been developed to differentiate between Mycobacterium species and include M. haemophilum. High-performance liquid chromatography (HPLC) has also been successfully applied (140) The new assays either employ species-specific probe hybridization, such as array probes (142, 161), or use restriction patterns to differentiate between species (118).
Newly developed methods that are currently being evaluated for application as tools to identify bacterial isolates might be applicable for the identification of species of Mycobacterium isolates. Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) and Raman spectrometry (18, 95, 120) as well as the new-generation sequencing method pyrosequencing (145) have been described for the differentiation of NTM species. Although M. haemophilum has been included in the NIH database, clinical isolates have not yet been tested (120).
Direct Detection Methods
The direct detection of NTM as a group is still being challenged, as only a few molecular assays have been described and validated for direct application to clinical materials (5, 13, 67, 116, 134).
Only two of these assays have been applied to the detection of M. haemophilum in clinical materials. Conventional PCR and subsequent restriction analysis (PRA) of hsp65 in all Mycobacterium species were applied successfully to biopsy specimens from four patients with M. haemophilum skin infections (28, 151). A 439-bp fragment was amplified and digested into species-specific band patterns by two restriction enzymes. However, the assay includes the handling of PCR products and therefore poses a contamination risk.
The second assay is a real-time PCR assay targeting the ITS between the 16S rRNA and 23S genes of all slowly growing Mycobacterium species (13). Mismatches in the forward primer and genus-specific probe have been encountered in several rapid-growing mycobacteria; therefore, for the detection of this group of species, this assay is less proficient. A species-specific probe subsequently enables the recognition of M. haemophilum. M. haemophilum-specific culture was found to be less sensitive than the real-time PCR assay when applied directly to biopsy specimens from children with cervicofacial lymphadenitis (14). Of 16 patients with evidence of M. haemophilum infection, 9 (56%) were positive by auramine staining, and 9 (56%) were positive by M. haemophilum-specific cultures. Thirteen specimens (81%) were positive by genus-specific detection, 11 of which were also positive by M. haemophilum-specific detection.
This assay was also applied to formalin-fixed/paraffin-embedded biopsy specimens from patients with granulomatous inflammation of the skin, which were stored between 1984 and 2004 (15). Of 30 patient materials tested, 13 (43%) were found to contain mycobacterial DNA. Only 5 of the patients had been previously diagnosed with a mycobacterial disease. M. haemophilum was identified as the most common species (n = 7). In this study, PCR was not compared with conventional techniques.
Another possible approach for direct detection is the application of generic PCR targeting a Mycobacterium-specific fragment that is subsequently sequenced to identify the involved species. This approach was applied in several reported M. haemophilum cases (52, 70, 114). The method can be performed by using a number of gene fragments (see “Molecular Identification Methods” above).
Diagnostic Approach
M. haemophilum infection should be considered for immunocompetent patients with nonpyogenic cervicofacial lymphadenitis. M. haemophilum can induce reactions in the tuberculin PPD skin test similar to those induced by M. tuberculosis and could be misdiagnosed when positive culture results are lacking (3, 59, 91). In general, a 10-mm tuberculin PPD cutoff point is recommended for the identification of latent M. tuberculosis infections, whereas a reaction of 5 to 9 mm is more likely to indicate NTM infection (45, 46). Therefore, although it is not decisive, the tuberculin PPD test can be helpful as a diagnostic tool with an induration cutoff of >5 mm as an indication of NTM infection in children.
M. haemophilum involvement should also be suspected for immunocompromised patients with typical NTM manifestations combined with skin lesions. Specific M. haemophilum detection should be carried out concurrently with standard mycobacterial detection for clinical samples obtained from superficial body sites, such as skin biopsy specimens and superficial lymph node biopsy specimens.
Overall, the failure to isolate a pathogen from clinical specimens with positive acid-fast stains should prompt a targeted search for M. haemophilum using appropriate culture conditions and molecular techniques.
A full diagnostic regimen for the optimal detection of M. haemophilum in biopsy specimens includes acid-fast staining, mycobacterial culturing at two temperatures using media with and without iron additives, and molecular detection. The diagnosis of mycobacterial infection by the direct detection of the pathogen is achieved by use of fine-needle aspiration biopsy (13), excision of the affected tissue, or respiratory specimens. After decontamination using, for example, the NALC-NaOH decontamination protocol, biopsy specimens should be stained with auramine and investigated microscopically, followed by standard mycobacterial culturing at 35°C in liquid MGIT medium and on solid LJ medium. In addition to this generic protocol, M. haemophilum-specific culturing should be performed at 30°C on LJ medium supplemented with iron citrate (preferably combined with a liquid medium using hemin supplementation). Because culture for M. haemophilum is less sensitive than the real-time PCR assay described above (14), molecular diagnosis should also be attempted, preferably using genus-specific detection and M. haemophilum-specific detection. Molecular detection also enables biopsy specimens and other histopathological materials to be examined for the presence of mycobacterial DNA when culturing is not possible due to tissue fixation (15). This approach offers an excellent opportunity to investigate the presence of newly identified Mycobacterium species in stored patient materials.
However, positive PCR results need to be interpreted with caution. The widespread presence of NTM in the environment may result in the contamination of patient samples with bacilli or DNA fragments during processing. Thus, the application of a highly sensitive NTM DNA detection method can result in false-positive results.
ANTIMICROBIAL SUSCEPTIBILITY
No standardized procedure is available for the susceptibility testing of M. haemophilum, although a recent CLSI document includes recommendations for a disk agar elution method for M. haemophilum (24a).
The application of different culture media can result in variations in the MIC values obtained for the same isolate. Moreover, European and U.S. guidelines do not always fully agree on the critical concentrations and protocols for susceptibility testing (160). Therefore, the in vitro susceptibilities presented in Table 5 are approximations. M. haemophilum appears to be susceptible to ciprofloxacin, clarithromycin, rifabutin, and clofazimine but resistant to isoniazid and ethambutol (96, 105, 126, 141). Discrepant results have been observed for amikacin and streptomycin; our results demonstrate high MIC values, allowing us not to consider aminoglycosides for the treatment of M. haemophilum infections. Isoniazid may be more active than indicated by the in vitro test results, since hemin, used as a broth supplement, can antagonize the in vitro activity of isoniazid (9). Interesting results were obtained for cycloserine, with an MIC50 of 50 μg/ml. While macrolides and rifamycin appear to be highly active against M. haemophilum, resistance is readily acquired by a single mutation in the 23S gene and the rpoB gene, respectively (78, 110). Therefore, dual or triple therapy is advised over monotherapy.
Table 5.
Resistance of clinical isolates to antimicrobial agentsd
| Antimicrobial agent | 1993 study (n = 12)a |
2001 study (n = 16)b |
CHIMED study, 2003-2004 (n = 18)c |
|||
|---|---|---|---|---|---|---|
| MIC50 | MIC90 | Disk (μg/ml) | % sensitivity | MIC50 | MIC90 | |
| Ciprofloxacin | 2 | 8 | 2 | 100 | ≤1 | 4 |
| Clarithromycin | ≤0.25 | ≤0.25 | 3 | 100 | ≤2 | ≤2 |
| Rifabutin | ≤0.03 | ≤0.03 | NT | NT | ≤0.2 | ≤0.2 |
| Rifampin | 0.5 | 1 | 1 | 94 | 0.2 | 1 |
| Amikacin | 4 | 8 | 2 | 100 | 10 | 20 |
| Ethionamide | R* | R* | 5 | 0 | NT | NT |
| Streptomycin | NT | NT | 10 | 100 | 10 | 20 |
| Ethambutol | R* | R* | 5 | 0 | >20 | >20 |
| Isoniazid | 8 | >32 | 0.2 | 0 | ≥20 | ≥20 |
| Clofazimine | 2 | 2 | NT | NT | ≤0.5 | ≤0.5 |
| Prothionamide | NT | NT | NT | NT | 5 | 20 |
| Cycloserine | NT | NT | NT | NT | 50 | >50 |
Data from reference 9. The method applied was a microtiter array with Middlebrook 7H9 broth plus hemin. MICs are in μg/ml.
Data from reference 126. The method applied was a disk elution method on Middlebrook 7H10 agar with hemin.
The method applied was an agar dilution method on Middlebrook 7H10 medium with a hemin source. MICs are in μg/ml.
R*, tested but not active; NT, not tested.
TREATMENT
No standard guidelines are available for the treatment of M. haemophilum infection. Although no optimal therapeutic regimen and treatment duration for M. haemophilum have been established, experts generally agree that patients should be placed on multiple antibiotics that include some combination of clarithromycin, ciprofloxacin, and one of the rifamycins (123, 126) for a duration of 12 to 24 months (126). Therapy should be tailored to the individual patient based on his or her disease presentation and underlying degree of immune suppression. The contribution of antibiotics to the healing of M. haemophilum lesions is difficult to evaluate. Recovery may depend mostly on an improved immunologic state (108). Even if M. haemophilum infections are diagnosed early, adequate treatment may be complicated by an inability to reduce immune suppression, adverse reactions to the antibiotics, patient intolerance of the antibiotics, the antimicrobial resistance of M. haemophilum isolates, interactions between antimicrobials and immunosuppressive agents, and superinfection of cutaneous lesions with, for example, Staphylococcus aureus (39).
Immunocompromised Patients
Skin lesions.
Only a few M. haemophilum infections in immunocompromised children have been described (11, 21, 28). One patient was cured after antibiotics for 6 months (28), whereas the other two patients failed treatment (11, 21). One of these failing patients was cured after immune restoration, surgical drainage, and additional antibiotic treatment (11).
Numerous cases of skin infections in immunocompromised adults have been reported (Table 1). In all patients for whom therapy was reported, treatment consisted of antituberculous drugs guided by the susceptibility pattern of the cultured microorganism. The regimen usually consisted of at least three drugs: almost always clarithromycin (29) plus ciprofloxacin, ethambutol, and/or rifabutin-rifampin. The treatment duration varied between 3 and 42 months, with a median of approximately 6 months. For AIDS patients, highly active antiretroviral therapy (HAART) was usually also started. With one exception (47), all patients were cured.
To summarize, antibiotic treatment is indicated for patients with M. haemophilum skin infections. Curative surgical excision is possible in rare cases with few infected sites (100). The duration of antibiotic therapy is not well defined and depends on the clinical presentation, degree of immune suppression, and clinical course. In an earlier review the minimum recommended duration of antibiotic therapy was 12 months, but treatment may need to be extended for up to 24 months (123, 126). The exacerbation of the skin lesions shortly after the initiation of treatment, however, is not uncommon. These exacerbations most likely occur as a result of a paradoxical reaction: an immune response to the local release of products of mycobacterial cell death and lysis. These reactions tend to improve within 2 to 3 weeks (85, 104, 126). In general, patient outcomes tend to be satisfactory for M. haemophilum skin infections (126).
Disseminated infection/pulmonary infection.
Tables 2 and 3 give an overview of the reported cases of disseminated and pulmonary infections and the subsequent treatment. Five out of the 10 patients reported with disseminated disease responded to treatment. For disseminated M. haemophilum infections, a multidrug regimen combining clarithromycin, ciprofloxacin, and rifampin-rifabutin is recommended (157).
For the reported pulmonary infections (Table 3), the level of response to treatment is lower. Only three patients from the nine reported cases responded permanently to the therapeutic regimen. Although no studies of the duration of treatment for M. haemophilum infections have been conducted, American Thoracic Society guidelines recommend treatment until cultures taken during therapy are negative for 1 year (55). Whether tumor necrosis factor alpha (TNF-α) treatment can be continued during antimycobacterial treatment is a matter of debate (148). In active tuberculosis infections, treatment with TNF-α is contraindicated until patients complete a standard regimen of antituberculosis therapy. No information is available for NTM disease (148).
Pyomyositis.
The majority of the described cases of pyomyositis were successfully treated with a combination of surgery and antibiotic therapy. Surgical debridement of necrotic tissue is required when extensive inflammation is present (124). Based on limited data from individual cases (70, 82, 124), a combination of surgery and antibiotic therapy with clarithromycin, ciprofloxacin, and one of the rifamycins appears to be effective. However, the wounds did not resolve in a case reported by Shih et al. (127). The therapeutic regimen consisted of ethambutol, rifampin, clarithromycin, ciprofloxacin, and amikacin. Repeated debridement of the right thigh was performed, but the patient died of fungemia due to Candida glabrata 3 months after admission.
The duration of therapy should depend on the patient's underlying disease presentation, degree of immunosuppression, and response to therapy. Treatment should generally be continued for at least 1 year and perhaps for as long as 2 years (123, 126).
Skeletal infections/osteomyelitis.
Data from the publications on skeletal M. haemophilum infections are presented in Table 4. The clinical response to treatment varies, even when the above-mentioned antibiotics are used. Prolonged maintenance therapy lasting months, or even years, with several drugs is generally necessary, particularly for patients with sustained immunosuppression (83).
Immunocompetent Patients
Immunocompetent adults.
In adults, the most frequently reported manifestations are skin lesions with or without lymphadenitis. Success has been reported with antibiotic treatment for 4 to 6 months (8, 124, 128). Treatment in these patients consisted of clarithromycin, rifabutin, and ethambutol or ciprofloxacin. In a recent case series of 12 patients with eyebrow lesions and cervicofacial lymphadenitis, surgical excision was curative, and in the majority of the cases antibiotics were not successful (52).
Immunocompetent children.
The most common manifestation of M. haemophilum infection in immunocompetent children is cervicofacial lymphadenitis. Excisional surgery leads to a quick resolution and the best esthetic outcome (87, 88). In more advanced stages with extensive necrosis and skin discoloration, excisional surgery can be technically difficult. Whether antibiotic treatment offers benefits over observation alone in these cases is not clear. Both success and failure have been reported for antibiotic treatment (60, 88, 92, 147). In the largest reported case series, 32 children in Israel were treated by observation alone (164). Total resolution was achieved for 71% of patients within 6 months and for the remaining patients within 9 to 12 months. For children with an advanced stage of nontuberculous mycobacterial cervicofacial lymphadenitis, no significant difference in the median healing times between an observational approach and antibiotic therapy with clarithromycin and rifabutin was found (86).
Treatment Outcome
In the cases reported after 1996, almost all immunocompromised patients were cured, with the few exceptions described above (47, 104, 126, 127, 149). Thus, in summary, most immunocompromised patients will recover after prolonged antimycobacterial treatment, especially those with skin infections, but mortality can occur in patients with deep-seated infections. In these circumstances, surgery is usually not an option, leaving only an alleviation of immunosuppressive medication, if possible, or, in AIDS patients, antiretroviral treatment.
RECOMMENDATIONS AND CONCLUSION
In conclusion, M. haemophilum infection should be considered in the differential diagnosis of chronic cervicofacial lymphadenitis in young immunocompetent children and ulcerating skin lesions and/or arthritis in immunocompromised patients, especially when AFB are seen by direct microscopy and when routine mycobacterial cultures remain sterile. Detailed clinical information, adjusted mycobacterial culture procedures, and molecular techniques all contribute to the adequate and rapid diagnosis of M. haemophilum infections. The outcome of M. haemophilum lymphadenitis in immunocompetent patients favors surgical intervention rather than antibiotic treatment.
Biographies
 Jerome A. Lindeboom, M.D., D.D.S., Ph.D., is an Oral and Maxillofacial surgeon at the Department of Oral and Maxillofacial Surgery at the Academic Medical Center (AMC) in Amsterdam and the Academic Centre for Dentistry Amsterdam (ACTA), The Netherlands. He also works as an Oral and Maxillofacial surgeon at the Department of Oral and Maxillofacial Surgery at the Amstelland Hospital, Amstelveen, The Netherlands, and he is editor of the European Journal of Oral Implantology.
Jerome A. Lindeboom, M.D., D.D.S., Ph.D., is an Oral and Maxillofacial surgeon at the Department of Oral and Maxillofacial Surgery at the Academic Medical Center (AMC) in Amsterdam and the Academic Centre for Dentistry Amsterdam (ACTA), The Netherlands. He also works as an Oral and Maxillofacial surgeon at the Department of Oral and Maxillofacial Surgery at the Amstelland Hospital, Amstelveen, The Netherlands, and he is editor of the European Journal of Oral Implantology.
 Lesla E. S. Bruijnesteijn van Coppenraet is a clinical molecular microbiologist at the Department of Medical Microbiology and Infectious Diseases in the Isala Clinics, Zwolle, The Netherlands. She conducted a Ph.D. study with a special interest in Mycobacterium haemophilum at the Leiden University Medical Center, The Netherlands, and wrote her thesis about diagnostics of nontuberculous mycobacteria in 2009.
Lesla E. S. Bruijnesteijn van Coppenraet is a clinical molecular microbiologist at the Department of Medical Microbiology and Infectious Diseases in the Isala Clinics, Zwolle, The Netherlands. She conducted a Ph.D. study with a special interest in Mycobacterium haemophilum at the Leiden University Medical Center, The Netherlands, and wrote her thesis about diagnostics of nontuberculous mycobacteria in 2009.
 Dick van Soolingen, Ph.D., is the head of the Mycobacteria Reference Laboratory at the National Institute for Public Health and the Environment (RIVM) in Bilthoven, The Netherlands. In addition, he is a professor at the Department of Microbiology and of Pulmonary Diseases, Radboud University Nijmegen Medical Centre/University Lung Centre Dekkerswald, Nijmegen, The Netherlands. He produced multiple articles on the clinical relevance of nontuberculous mycobacteria in recent years.
Dick van Soolingen, Ph.D., is the head of the Mycobacteria Reference Laboratory at the National Institute for Public Health and the Environment (RIVM) in Bilthoven, The Netherlands. In addition, he is a professor at the Department of Microbiology and of Pulmonary Diseases, Radboud University Nijmegen Medical Centre/University Lung Centre Dekkerswald, Nijmegen, The Netherlands. He produced multiple articles on the clinical relevance of nontuberculous mycobacteria in recent years.
 Jan M. Prins is an infectious disease (ID) specialist and Professor of Infectious Diseases at the Department of Infectious Diseases, Tropical Medicine and AIDS, at the Academic Medical Center (AMC) in Amsterdam, The Netherlands. He is head of the Infectious Diseases Fellowship Training Program at the AMC in Amsterdam, and he is chairman of the Dutch Working Party on Antibiotic Policy (SWAB) and chairs the SWAB guideline development committee.
Jan M. Prins is an infectious disease (ID) specialist and Professor of Infectious Diseases at the Department of Infectious Diseases, Tropical Medicine and AIDS, at the Academic Medical Center (AMC) in Amsterdam, The Netherlands. He is head of the Infectious Diseases Fellowship Training Program at the AMC in Amsterdam, and he is chairman of the Dutch Working Party on Antibiotic Policy (SWAB) and chairs the SWAB guideline development committee.
 Eduard J. Kuijper, M.D., Ph.D., medical microbiologist, is the head of the Department of Experimental Microbiology at the Leiden University Medical Center. He introduced molecular biology and mass spectrometry in diagnostics of bacterial diseases and initiated research projects on Mycobacterium haemophilum and Clostridium difficile, with interest in epidemiology and pathogenesis. He is currently also leading a national workgroup on microbiological diagnostics of mycobacterial diseases.
Eduard J. Kuijper, M.D., Ph.D., medical microbiologist, is the head of the Department of Experimental Microbiology at the Leiden University Medical Center. He introduced molecular biology and mass spectrometry in diagnostics of bacterial diseases and initiated research projects on Mycobacterium haemophilum and Clostridium difficile, with interest in epidemiology and pathogenesis. He is currently also leading a national workgroup on microbiological diagnostics of mycobacterial diseases.
REFERENCES
- 1. Abbott M. R., Smith D. D. 1981. Mycobacterial infections in immunosuppressed patients. Med. J. Aust. 1:351–353 [DOI] [PubMed] [Google Scholar]
- 2. Abell F., Harrison P. B., Seldon M. 1994. Mycobacterium haemophilum infection in an elderly patient. Aust. N. Z. J. Med. 24:404. [DOI] [PubMed] [Google Scholar]
- 3. Armstrong K. L., James R. W., Dawson D. J., Francis P. W., Masters B. 1992. Mycobacterium haemophilum causing perihilar or cervical lymphadenitis in healthy children. J. Pediatr. 121:202–205 [DOI] [PubMed] [Google Scholar]
- 4. Atlas R. M., Snyder J. W. 2006. Handbook of media for clinical microbiology, 2nd ed., p. 307 CRC Press, Boca Raton, FL [Google Scholar]
- 5. Azov A. G., Koch J., Hamilton-Dutoit S. J. 2005. Improved diagnosis of mycobacterial infections in formalin-fixed and paraffin-embedded sections with nested polymerase chain reaction. APMIS 113:586–593 [DOI] [PubMed] [Google Scholar]
- 6. Bachmann S., Schnyder U., Pfyffer G. E., Lüthy R., Weber R. 1996. Mycobacterium haemophilum infection in a patient with AIDS. Dtsch. Med. Wochenschr. 121:1189–1192 [DOI] [PubMed] [Google Scholar]
- 7. Reference deleted.
- 8. Bekou V., Büchau A., Flaig M. J., Ruzicka T., Hogardt M. 2011. Cutaneous infection by Mycobacterium haemophilum and kansasii in an IgA-deficient man. BMC Dermatol. 11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bernard E. M., Edwards F. F., Kiehn T. E., Brown S. T., Armstrong D. 1993. Activities of antimicrobial agents against clinical isolates of Mycobacterium haemophilum. Antimicrob. Agents Chemother. 37:2323–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Besra G. S., et al. 1991. Structural elucidation and antigenicity of a novel glycolipid antigen from Mycobacterium haemophilum. Biochemistry 30:7772–7777 [DOI] [PubMed] [Google Scholar]
- 11. Bosma F., et al. 2004. Mycobacterium reverse hybridization line-probe assay used to diagnose disseminated Mycobacterium haemophilum infection in a child with acute lymphoblastic leukemia. Eur. J. Clin. Microbiol. Infect. Dis. 23:345–347 [DOI] [PubMed] [Google Scholar]
- 12. Branger B., et al. 1985. Mycobacterium haemophilum and Mycobacterium xenopi associated infection in a renal transplant patient. Clin. Nephrol. 23:46–49 [PubMed] [Google Scholar]
- 13. Bruijnesteijn van Coppenraet E. S., et al. 2004. Real-time PCR assay using fine-needle aspirates and tissue biopsy specimens for rapid diagnosis of mycobacterial lymphadenitis in children. J. Clin. Microbiol. 42:2644–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bruijnesteijn van Coppenraet L. E. S., Kuijper E. J., Lindeboom J. A., Prins J. M., Claas E. C. 2005. Mycobacterium haemophilum and lymphadenitis in children. Emerg. Infect. Dis. 11:62–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bruijnesteijn van Coppenraet L. E. S., Smit V. T. H. B. M., Templeton K. E., Claas E. C. J., Kuijper E. J. 2007. Application of real-time PCR to recognize atypical mycobacteria in archival skin biopsies: high prevalence of Mycobacterium haemophilum. Diagn. Mol. Pathol. 16:81–86 [DOI] [PubMed] [Google Scholar]
- 16. Bruijnesteijn van Coppenraet L. E. S., et al. 2009. Amplified fragment length polymorphism analysis of human clinical isolates of Mycobacterium haemophilum from different continents. Clin. Microbiol. Infect. 15:924–930 [DOI] [PubMed] [Google Scholar]
- 17. Buijtels P. C., Petit P. L. 2005. Comparison of NaOH-N-acetyl cysteine and sulfuric acid decontamination methods for recovery of mycobacteria from clinical specimens. J. Microbiol. Methods 62:83–88 [DOI] [PubMed] [Google Scholar]
- 18. Buijtels P. C., et al. 2008. Rapid identification of mycobacteria by Raman spectroscopy. J. Clin. Microbiol. 46:961–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Busam K. J., Kiehn T. E., Salob S. P., Myskowski P. L. 1999. Histologic reactions to cutaneous infections by Mycobacterium haemophilum. Am. J. Surg. Pathol. 23:1379–1385 [DOI] [PubMed] [Google Scholar]
- 20. Cameselle D., et al. 2007. Sporotrichoid cutaneous infection by Mycobacterium haemophilum in an AIDS patient. Actas Dermosifiliogr. 98:188–193 (In Spanish.) [PubMed] [Google Scholar]
- 21. Campbell L. B., Maroon M., Pride H., Adams D. C., Tyler W. B. 2006. Mycobacterium haemophilum in an immunosuppressed child. Pediatr. Dermatol. 23:481–483 [DOI] [PubMed] [Google Scholar]
- 22. Castro-Silva A. N., et al. 2011. Cutaneous Mycobacterium haemophilum infection in a kidney transplant recipient after acupuncture treatment. Transpl. Infect. Dis. 13:33–37 [DOI] [PubMed] [Google Scholar]
- 23. Centers for Disease Control 1991. Mycobacterium haemophilum infections—New York City metropolitan area, 1990-1991. MMWR Morb. Mortal. Wkly. Rep. 40:636–637, 643 [PubMed] [Google Scholar]
- 24. Chang C. T., Wang L. Y., Liao C. Y., Huang S. P. 2002. Identification of nontuberculous mycobacteria existing in tap water by PCR-restriction fragment length polymorphism. Appl. Environ. Microbiol. 68:3159–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a. CLSI 2005. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standard, 2nd ed. M24-A2 CLSI, Wayne, PA: [PubMed] [Google Scholar]
- 25. Cohen Y. H., et al. 2008. Mycobacterium haemophilum and lymphadenitis in immunocompetent children, Israel. Emerg. Infect. Dis. 14:1437–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cooper D. K. C., et al. 1983. Infectious complications after heart transplantation. Thorax 38:822–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Covert T. C., Rodgers M. R., Reyes A. L., Stelma G. N., Jr 1999. Occurrence of nontuberculous mycobacteria in environmental samples. Appl. Environ. Microbiol. 65:2492–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Da Mata O., et al. 2008. The diagnosis of two cases of cutaneous ulcer caused by infection with Mycobacterium haemophilum: direct identification in a clinical sample by polymerase chain reaction-restriction endonuclease analysis. Int. J. Dermatol. 47:820–823 [DOI] [PubMed] [Google Scholar]
- 29. Darling T. N., et al. 1994. Treatment of Mycobacterium haemophilum infection with an antibiotic regimen including clarithromycin. Br. J. Dermatol. 131:376–379 [DOI] [PubMed] [Google Scholar]
- 30. Davis B. R., Brumbach J., Sanders W. J., Wolinsky E. 1982. Skin lesions caused by Mycobacterium haemophilum. Ann. Intern. Med. 97:723–724 [DOI] [PubMed] [Google Scholar]
- 31. Dawson D. J., Blacklock Z. M., Kane D. W. 1981. Mycobacterium haemophilum causing lymphadenitis in an otherwise healthy child. Med. J. Aust. 2:289–290 [DOI] [PubMed] [Google Scholar]
- 32. Dawson D. J., Jennis F. F. 1980. Mycobacteria with a growth requirement for ferric ammonium citrate, identified as Mycobacterium haemophilum. J. Clin. Microbiol. 11:190–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dever L. L., Martin J. W., Seaworth B., Jorgensen J. H. 1992. Varied presentations and responses to treatment of infections caused by Mycobacterium haemophilum in patients with AIDS. Clin. Infect. Dis. 14:1195–1200 [DOI] [PubMed] [Google Scholar]
- 34. Devulder G., Pérouse de Montclos M., Flandrois J. P. 2005. A multigene approach to phylogenetic analysis using the genus Mycobacterium as a model. Int. J. Syst. Evol. Microbiol. 55:293–302 [DOI] [PubMed] [Google Scholar]
- 35. Dobos K. M., Quinn F. D., Ashford D. A., Horsburgh C. R., King C. H. 1999. Emergence of a unique group of necrotizing mycobacterial diseases. Emerg. Infect. Dis. 5:367–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dore N., Collins J. P., Mankiewicz E. 1979. A sporotrichoid-like Mycobacterium kansasii infection of the skin treated with minocycline hydrochloride. Br. J. Dermatol. 101:75–79 [DOI] [PubMed] [Google Scholar]
- 37. Elsayed S., Read R. 2006. Mycobacterium haemophilum osteomyelitis: case report and review of the literature. BMC Infect. Dis. 6:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Endo T., et al. 2001. Mycobacterium haemophilum infection in a Japanese patient with AIDS. J. Infect. Chemother. 7:186–190 [DOI] [PubMed] [Google Scholar]
- 39. Fairhurst R. M., et al. 2002. Mycobacterium haemophilum infections in heart transplant recipients: case report and review of the literature. Am. J. Transplant. 2:476–479 [DOI] [PubMed] [Google Scholar]
- 40. Falkinham J. O., III, Norton C. D., LeChevallier M. W. 2001. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other mycobacteria in drinking water distribution systems. Appl. Environ. Microbiol. 67:1225–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Falkinham J. O., III 2009. Surrounded by mycobacteria: nontuberculous mycobacteria in the human environment. J. Appl. Microbiol. 107:356–367 [DOI] [PubMed] [Google Scholar]
- 42. Feldman R. A., Hershfield E. 1974. Mycobacterial skin infection by an unidentified species: a report of 29 patients. Ann. Intern. Med. 80:445–452 [DOI] [PubMed] [Google Scholar]
- 43. Fischer L. J., Quinn F. D., White E. H., King C. H. 1996. Intracellular growth and cytotoxicity of Mycobacterium haemophilum in a human epithelial cell line (Hec-1-B). Infect. Immun. 64:269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fischer L. J., Quinn F. D., Kikuta-Oshima L., Ribot E. M., King C. H. 1996. Identification of genes specifically expressed by Mycobacterium haemophilum in association with human epithelial cells. Ann. N. Y. Acad. Sci. 797:277–279 [DOI] [PubMed] [Google Scholar]
- 45. Fordham von Reyn C., et al. 1994. Dual skin testing with Mycobacterium avium sensitin and purified protein derivate: an open study of patients with M. avium complex infection or tuberculosis. Clin. Infect. Dis. 19:15–20 [DOI] [PubMed] [Google Scholar]
- 46. Fordham von Reyn C., et al. 1998. Dual skin testing with Mycobacterium avium sensitin and purified protein derivate to discriminate pulmonary disease due to M. avium complex from pulmonary disease due to Mycobacterium tuberculosis. J. Infect. Dis. 177:730–736 [DOI] [PubMed] [Google Scholar]
- 47. Friedli A., Krischer J., Hirschel B., Saurat J. H., Pechère M. 2000. An annular plaque due to Mycobacterium haemophilum infection in a patient with AIDS. J. Am. Acad. Dermatol. 43:913–915 [DOI] [PubMed] [Google Scholar]
- 48. Gardy J. L., et al. 2011. Whole-genome sequencing and social-network analysis of a tuberculosis outbreak. N. Engl. J. Med. 364:730–739 [DOI] [PubMed] [Google Scholar]
- 49. Geisler W. M., Harrington R. D., Wallis C. K., Harnisch J. P., Liles W. C. 2002. Broad spectrum of dermatologic manifestations caused by Mycobacterium haemophilum infection. Arch. Dermatol. 138:229–230 [DOI] [PubMed] [Google Scholar]
- 50. Gey van Pittius N. C., et al. 2006. Evolution and expansion of the Mycobacterium tuberculosis PE and PPE multigene families and their association with the duplication of the ESAT-6 (esx) gene cluster regions. BMC Evol. Biol. 6:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gibson M. K., Villano S. A., Huff C. A., Cofrancesco J. 1999. Recrudescence of cutaneous Mycobacterium haemophilum lesions following tetanus immunization. Clin. Infect. Dis. 28:919–920 [DOI] [PubMed] [Google Scholar]
- 52. Giulieri S., et al. 2011. Outbreak of Mycobacterium haemophilum infections after permanent makeup of the eyebrows. Clin. Infect. Dis. 52:488–491 [DOI] [PubMed] [Google Scholar]
- 53. Goodman M. D., Elsasser G. N., Biehle J. 2004. Cutaneous ulceration in a patient with HIV. Am. Fam. Physician 70:1537–1538 [PubMed] [Google Scholar]
- 54. Gouby A., Branger B., Oules R., Ramuz M. 1988. Two cases of Mycobacterium haemophilum infection in a renal-dialysis unit. J. Med. Microbiol. 25:299–300 [DOI] [PubMed] [Google Scholar]
- 55. Griffith D. E., et al. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175:367–416 [DOI] [PubMed] [Google Scholar]
- 56. Gruschke A., Enzensberger R., Brade V. 2002. Osteomyelitis of the tibial head caused by Mycobacterium haemophilium in a patient with AIDS. Dtsch. Med. Wochenschr. 127:1947–1950 [DOI] [PubMed] [Google Scholar]
- 57. Gupta I., et al. 1992. Mycobacterium haemophilum osteomyelitis in an AIDS patient. N. J. Med. 89:201–202 [PubMed] [Google Scholar]
- 58. Gutierrez M. C., et al. 2005. Ancient origin and gene mosaicism of the progenitor of Mycobacterium tuberculosis. PLoS Pathog. 1:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Haimi-Cohen Y., Zeharia A., Mimouni M., Soukhaman M., Amir J. 2001. Skin indurations in response to tuberculin testing in patients with nontuberculous mycobacterial lymphadenitis. Clin. Infect. Dis. 33:1786–1788 [DOI] [PubMed] [Google Scholar]
- 60. Haimi-Cohen Y., et al. 2009. Chronic cheek lesions: an unusual manifestation of nontuberculous mycobacterial cervicofacial infection. J. Pediatr. 155:746–748 [DOI] [PubMed] [Google Scholar]
- 61. Hanau L. H., Leaf A., Soeiro R., Weiss L. M., Pollack S. S. 1994. Mycobacterium marinum infection in a patient with the acquired immunodeficiency syndrome. Cutis 54:103–105 [PubMed] [Google Scholar]
- 62. Harmsen D., et al. 2003. RIDOM: comprehensive and public sequence database for identification of Mycobacterium species. BMC Infect. Dis. 3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hernandez-Divers S. J., Shearer D. 2002. Pulmonary mycobacteriosis caused by Mycobacterium haemophilum and M. marinum in a royal python. J. Am. Vet. Med. Assoc. 220:1661–1663 [DOI] [PubMed] [Google Scholar]
- 64. Hirsch R., Miller S. M., Kazi S., Cate T. R., Reveille J. D. 1996. Human immunodeficiency virus-associated atypical mycobacterial skeletal infections. Semin. Arthritis Rheum. 25:347–356 [DOI] [PubMed] [Google Scholar]
- 65. Holladay K. L., Carmichael J. K. 1996. Mycobacterium haemophilum cellulitis and osteomyelitis in a man with AIDS. J. Am. Board Fam. Pract. 9:122–124 [PubMed] [Google Scholar]
- 66. Holton J., Nye P., Miller R. 1991. Mycobacterium haemophilum infection in a patient with AIDS. J. Infect. 23:303–306 [DOI] [PubMed] [Google Scholar]
- 67. Hsiao C. H., Lin Y. T., Lai C. C., Chou C. H., Hsueh P. R. 2010. Identification of nontuberculous mycobacterial infection by IS6110 and hsp65 gene analysis on lung tissues. Diagn. Microbiol. Infect. Dis. 68:241–246 [DOI] [PubMed] [Google Scholar]
- 68. Hussein Z., Landt O., Wirths B., Wellinghausen N. 2009. Detection of non-tuberculous mycobacteria in hospital water by culture and molecular methods. Int. J. Med. Microbiol. 299:281–290 [DOI] [PubMed] [Google Scholar]
- 69. Jacob B., Debey B. M., Bradway D. 2006. Spinal intradural Mycobacterium haemophilum granuloma in an American bison (Bison bison). Vet. Pathol. 43:998–1000 [DOI] [PubMed] [Google Scholar]
- 70. Jang E. Y., et al. 2007. Case of pyomyositis due to Mycobacterium haemophilum in a renal transplant recipient. J. Clin. Microbiol. 45:3847–3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Julián E., et al. 2010. Microscopic cords, a virulence-related characteristic of Mycobacterium tuberculosis, are also present in nonpathogenic mycobacteria. J. Bacteriol. 192:1751–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kamboj M., et al. 2008. Mycobacterium haemophilum infection after alemtuzumab treatment. Emerg. Infect. Dis. 14:1821–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Keller M., Mak A., Thibert L., Rene P., Klein M. B. 2008. Mycobacterium haemophilum epididymal abscess in a renal transplant patient. J. Clin. Microbiol. 46:2459–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kent M. L., et al. 2004. Mycobacteriosis in zebrafish (Danio rerio) research facilities. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 138:383–390 [DOI] [PubMed] [Google Scholar]
- 75. Kiehn T. E., White M. 1994. Mycobacterium haemophilum: an emerging pathogen. Eur. J. Clin. Microbiol. Infect. Dis. 13:925–931 [DOI] [PubMed] [Google Scholar]
- 76. Kiehn T. E., et al. 1993. A cluster of four cases of Mycobacterium haemophilum infection. Eur. J. Clin. Microbiol. Infect. Dis. 12:114–118 [DOI] [PubMed] [Google Scholar]
- 77. Kikuchi K., Bernard E. M., Kiehn T. E., Armstrong D., Riley L. W. 1994. Restriction fragment length polymorphism analysis of clinical isolates of Mycobacterium haemophilum. J. Clin. Microbiol. 32:1763–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Klein J. L., Brown T. J., French G. L. 2001. Rifampin resistance in Mycobacterium kansasii is associated with rpoB mutations. Antimicrob. Agents Chemother. 45:3056–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kristjansson M., Bieluch V. M., Byeff P. D. 1991. Mycobacterium haemophilum infection in immunocompromised patients: case report and review of the literature. Rev. Infect. Dis. 13:906–910 [DOI] [PubMed] [Google Scholar]
- 80. Kuijper E. J., Wit F. W., Veenstra J., Böttger E. C. 1997. Recovery of Mycobacterium haemophilum skin infection in an HIV-1-infected patient after the start of antiretroviral triple therapy. Clin. Microbiol. Infect. 3:584–585 [DOI] [PubMed] [Google Scholar]
- 81. Lederman C., et al. 1994. Mycobacterium haemophilum cellulites in a heart transplant recipient. J. Am. Acad. Dermatol. 30:804–805 [DOI] [PubMed] [Google Scholar]
- 82. Lee W. J., et al. 2010. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases. J. Dermatol. 37:965–972 [DOI] [PubMed] [Google Scholar]
- 83. Lefkowitz R. A., Singson R. D. 1998. Considering Mycobacterium haemophilum in the differential diagnosis for lytic bone lesions in AIDS patients who present with ulcerating skin lesions. Skeletal Radiol. 27:334–336 [DOI] [PubMed] [Google Scholar]
- 84. Lerner G. W., Safdar A., Coppel S. 1995. Mycobacterium haemophilum infection in AIDS. Infect. Dis. Clin. Pract. 4:233–236 [Google Scholar]
- 85. Lin J. H., Chen W., Lee J. Y., Yan J. J., Huang J. J. 2003. Disseminated cutaneous Mycobacterium haemophilum infection with severe hypercalcaemia in a failed renal transplant recipient. Br. J. Dermatol. 149:200–202 [DOI] [PubMed] [Google Scholar]
- 86. Lindeboom J. A. 2011. Conservative wait-and-see therapy versus antibiotic treatment for nontuberculous mycobacterial cervicofacial lymphadenitis in children. Clin. Infect. Dis. 52:180–184 [DOI] [PubMed] [Google Scholar]
- 87. Lindeboom J. A., et al. 2009. Esthetic outcome of surgical excision versus antibiotic therapy for nontuberculous mycobacterial cervicofacial lymphadenitis in children. Pediatr. Infect. Dis. J. 28:1028–1030 [DOI] [PubMed] [Google Scholar]
- 88. Lindeboom J. A., Kuijper E. J., Bruijnesteijn van Coppenraet E. S., Lindeboom R., Prins J. M. 2007. Surgical excision versus antibiotic treatment for nontuberculous mycobacterial cervicofacial lymphadenitis in children: a multicenter, randomized, controlled trial. Clin. Infect. Dis. 44:1057–1064 [DOI] [PubMed] [Google Scholar]
- 89. Lindeboom J. A., Kuijper C. F., van Furth M. 2007. Inguinal lymphadenitis caused by Mycobacterium haemophilum in an immunocompetent child. Pediatr. Infect. Dis. J. 26:84–86 [DOI] [PubMed] [Google Scholar]
- 90. Lindeboom J. A., Prins J. M., Bruijnesteijn van Coppenraet E. S., Lindeboom R., Kuijper E. J. 2005. Cervicofacial lymphadenitis in children caused by Mycobacterium haemophilum. Clin. Infect. Dis. 41:1569–1575 [DOI] [PubMed] [Google Scholar]
- 91. Lindeboom J. A., Kuijper E. J., Prins J. M., Bruijnesteijn van Coppenraet L. E. S., Lindeboom R. 2006. Tuberculin skin testing is useful in the screening for nontuberculous mycobacterial cervicofacial lymphadenitis in children. Clin. Infect. Dis. 43:1547–1551 [DOI] [PubMed] [Google Scholar]
- 92. Lindeboom J. A., Kuijper E. J., Bruijnesteijn van Coppenraet E. S., Prins J. M. 2006. First case of an oculofacial lesion due to Mycobacterium haemophilum infection in an immunocompetent child. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 101:774–776 [DOI] [PubMed] [Google Scholar]
- 93. Lomvardias S., Madge G. E. 1972. Chaetoconidium and atypical acid fast bacilli in skin ulcers. Arch. Dermatol. 106:875–876 [DOI] [PubMed] [Google Scholar]
- 94. Lott J. P., Werth V. P., Kovarik C. L. 2008. Cutaneous Mycobacterium haemophilum infection in iatrogenically immunocompromised patients without transplantation. J. Am. Acad. Dermatol. 59:139–142 [DOI] [PubMed] [Google Scholar]
- 95. Lotz A., et al. 2010. Rapid identification of mycobacterial whole cells in solid and liquid culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 48:4481–4486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Males B. M., West T. E., Bartholomew W. R. 1987. Mycobacterium haemophilum infection in a patient with acquired immune deficiency syndrome. J. Clin. Microbiol. 25:186–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Malouf M. A., Glanville A. R. 1999. The spectrum of mycobacterial infection after lung transplantation. Am. J. Respir. Crit. Care Med. 160:1611–1616 [DOI] [PubMed] [Google Scholar]
- 98. Martinelli C., et al. 2004. First case of Mycobacterium haemophilum infection in an AIDS patient in Italy. J. Eur. Acad. Dermatol. Venereol. 18:83–85 [DOI] [PubMed] [Google Scholar]
- 99. McBride M. E., et al. 1991. Diagnostic and therapeutic considerations for cutaneous Mycobacterium haemophilum infections. Arch. Dermatol. 127:276–277 [PubMed] [Google Scholar]
- 100. McGovern J., Bix B. C., Webster G. W. 1994. Mycobacterium haemophilum skin disease successfully treated with excision. J. Am. Acad. Dermatol. 30:269–270 [DOI] [PubMed] [Google Scholar]
- 101. Mezo A., et al. 1979. Unusual mycobacteria in 5 cases of opportunistic infections. Pathology 11:377–384 [DOI] [PubMed] [Google Scholar]
- 102. Millar M. J., Bulliard C., Balachandran C., Maloof A. J. 2007. Mycobacterium haemophilum infection presenting as filamentary keratopathy in an immunocompromised adult. Cornea 26:764–766 [DOI] [PubMed] [Google Scholar]
- 103. Minani T. J., Saubolle M. A., Yu E., Sussland Z. 2010. Mycobacterium haemophilum as a novel etiology of cervical lymphadenitis in an otherwise healthy adult patient. J. Clin. Microbiol. 48:2636–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Modi D., et al. 2007. Mycobacterium haemophilum: a rare cause of endophthalmitis. Retina 27:1148–1151 [DOI] [PubMed] [Google Scholar]
- 105. Moulsdale M. T., Harper J. M., Thatcher G. N., Dunn B. L. 1983. Infection by Mycobacterium haemophilum, a metabolically fastidious acid-fast bacillus. Tubercle 64:29–36 [DOI] [PubMed] [Google Scholar]
- 106. Murdoch M. E., Leigh I. M. 1989. Sporotrichoid spread of cutaneous Mycobacterium chelonei infection. Clin. Exp. Dermatol. 14:309–312 [DOI] [PubMed] [Google Scholar]
- 107. Olsen R. J., Cernoch P. L., Land G. A. 2006. Mycobacterial synovitis caused by slow-growing nonchromogenic species: eighteen cases and a review of the literature. Arch. Pathol. Lab. Med. 130:783–791 [DOI] [PubMed] [Google Scholar]
- 108. Paech V., et al. 2002. Remission of cutaneous Mycobacterium haemophilum infection as a result of antiretroviral therapy in a human immunodeficiency virus-infected patient. Clin. Infect. Dis. 34:1017–1019 [DOI] [PubMed] [Google Scholar]
- 109. Pai H. H., Chen W. C., Peng C. F. 2003. Isolation of non-tuberculous mycobacteria from hospital cockroaches (Periplaneta americana). J. Hosp. Infect. 53:224–228 [DOI] [PubMed] [Google Scholar]
- 110. Pfister P., et al. 2004. The structural basis of macrolide-ribosome binding assessed using mutagenesis of 23S rRNA positions 2058 and 2059. J. Mol. Biol. 342:1569–1581 [DOI] [PubMed] [Google Scholar]
- 111. Phowthongkum P., Puengchitprapai A., Udomsantisook N., Tumwasorn S., Suankratay C. 2008. Spindle cell pseudotumor of the brain associated with Mycobacterium haemophilum and Mycobacterium simiae mixed infection in a patient with AIDS: the first case report. Int. J. Infect. Dis. 12:421–424 [DOI] [PubMed] [Google Scholar]
- 112. Plemmons R. M., McAllister C. K., Garces M. C., Ward R. L. 1997. Osteomyelitis due to Mycobacterium haemophilum in a cardiac transplant patient: case report and analysis of interactions among clarithromycin, rifampin, and cyclosporine. Clin. Infect. Dis. 24:995–997 [DOI] [PubMed] [Google Scholar]
- 113. Pryor M., et al. 2004. Investigation of opportunistic pathogens in municipal drinking water under different supply and treatment regimes. Water. Sci. Technol. 50(1):83–90 [PubMed] [Google Scholar]
- 114. Rajpara A., Sri J., Driscoll M. 2010. Mycobacterium haemophilum: cutaneous nodules in a renal transplant patient. Dermatol. Online J. 16(7):3. [PubMed] [Google Scholar]
- 115. Richter E., Rüsch-Gerdes S., Hillemann D. 2006. Evaluation of the GenoType Mycobacterium Assay for identification of mycobacterial species from cultures. J. Clin. Microbiol. 44:1769–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ringuet H., et al. 1999. hsp65 sequencing for identification of rapidly growing mycobacteria. J. Clin. Microbiol. 37:852–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Rogers P. L., et al. 1988. Disseminated Mycobacterium haemophilum infection in two patients with the acquired immunodeficiency syndrome. Am. J. Med. 84:640–642 [DOI] [PubMed] [Google Scholar]
- 118. Roth A., et al. 2000. Novel diagnostic algorithm for identification of mycobacteria using genus-specific amplification of the 16S-23S rRNA gene spacer and restriction endonucleases. J. Clin. Microbiol. 38:1094–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Sagi L., et al. 2010. Mycobacterium haemophilum infection presenting as bilateral cellulitis and annular lesion in a heart transplant recipient. Isr. Med. Assoc. J. 12:57–58 [PubMed] [Google Scholar]
- 120. Saleeb P. G., Drake S. K., Murray P. R., Zelazny A. M. 2011. Identification of mycobacteria in solid-culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 49:1790–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sampaio J. L., et al. 2002. Mycobacterium haemophilum: emerging or underdiagnosed in Brazil? Emerg. Infect. Dis. 8:1359–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Samra Z., et al. 1999. Optimal detection and identification of Mycobacterium haemophilum in specimens from pediatric patients with cervical lymphadenopathy. J. Clin. Microbiol. 37:832–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Saubolle M. A., Kiehn T. E., White M. H., Rudinsky M. F., Armstrong D. 1996. Mycobacterium haemophilum: microbiology and expanding clinical and geographic spectra of disease in humans. Clin. Microbiol. Rev. 9:435–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Schumacher O., Dabernig J., Nenadic I., Ingianni G., Cedidi C. 2008. Severe course of a rare non-tuberculous mycobacteriosis (M. haemophilum) of the hand—case report and strategic comments. Handchir. Mikrochir. Plast. Chir. 40:342–347 [DOI] [PubMed] [Google Scholar]
- 125. Seitz C. S., Trautemann A., Bröcker E. B., Abele-Horn M., Goebeler M. 2008. Skin nodules in rheumatoid arthritis due to infection with Mycobacterium haemophilum. Acta Derm. Venereol. 88:428–429 [DOI] [PubMed] [Google Scholar]
- 126. Shah M. K., Sebti A., Kiehn T. E., Massarella S. A., Sepkowitz K. A. 2001. Mycobacterium haemophilum in immunocompromised patients. Clin. Infect. Dis. 33:330–337 [DOI] [PubMed] [Google Scholar]
- 127. Shih J. Y., et al. 1998. Pyomyositis due to Mycobacterium haemophilum in a patient with polymyositis and long-term steroid use. Clin. Infect. Dis. 26:505–507 [DOI] [PubMed] [Google Scholar]
- 128. Smith S., Taylor G. D., Fanning E. A. 2003. Chronic cutaneous Mycobacterium haemophilum infection acquired from coral injury. Clin. Infect. Dis. 37:100–101 [DOI] [PubMed] [Google Scholar]
- 129. Somoskövi A., Magyar P. 1999. Comparison of the Mycobacteria Growth Indicator Tube with MB Redox, Löwenstein-Jensen, and Middlebrook 7H11 media for recovery of mycobacteria in clinical specimens. J. Clin. Microbiol. 37:1366–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sompolinsky D., Lagziel A., Naveh D., Yankilevitz T. 1978. Mycobacterium haemophilum sp. nov., a new pathogen of humans. Int. J. Syst. Bacteriol. 28:67–75 [Google Scholar]
- 131. Sompolinsky D., Lagziel A., Rosenberg I. 1979. Further studies of a new pathogenic mycobacterium (M. haemophilum sp. nov.). Can. J. Microbiol. 25:217–226 [DOI] [PubMed] [Google Scholar]
- 132. Soubani A. O., Mohammed A., Forlenza S. 1994. Successful treatment of disseminated Mycobacterium haemophilum infection in a patient with AIDS. Clin. Infect. Dis. 18:475. [DOI] [PubMed] [Google Scholar]
- 133. Sowden D., Kemp R., Dawson D. 1993. Osteomyelitis due to Mycobacterium haemophilum in a patient with AIDS. Pathology 25:308–309 [DOI] [PubMed] [Google Scholar]
- 134. Stauffer F., et al. 1998. Genus level identification of mycobacteria from clinical specimens by using an easy-to-handle Mycobacterium-specific PCR assay. J. Clin. Microbiol. 36:614–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Straus W. L., et al. 1994. Clinical and epidemiologic characteristics of Mycobacterium haemophilum, an emerging pathogen in immunocompromised patients. Ann. Intern. Med. 120:118–125 [DOI] [PubMed] [Google Scholar]
- 136. Stürenburg E. E., et al. 2004. Disseminated Mycobacterium haemophilum infection as initial manifestation of AIDS. Tuberculosis 84:341–345 [DOI] [PubMed] [Google Scholar]
- 137. Swart R. M., et al. 2009. Nontuberculous mycobacteria infection and tumor necrosis factor-alpha antagonists. Emerg. Infect. Dis. 15:1700–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Tan H. H., Tan A., Theng C., Ng S. K. 2004. Cutaneous Mycobacterium haemophilum infections in immunocompromised patients in a dermatology clinic in Singapore. Ann. Acad. Med. Singapore 33:532–536 [PubMed] [Google Scholar]
- 139. Teh C. L., Kong K. O., Chong A. P., Badsha H. 2002. Mycobacterium haemophilum infection in an SLE patient on mycophenolate mofetil. Lupus 11:249–252 [DOI] [PubMed] [Google Scholar]
- 140. Thibert L., Lapierre S. 1993. Routine application of high-performance liquid chromatography for identification of mycobacteria. J. Clin. Microbiol. 31:1759–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Thibert L., Lebel F., Martineau B. 1990. Two cases of Mycobacterium haemophilum infection in Canada. J. Clin. Microbiol. 28:621–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tobler N. E., Pfunder M., Herzog K., Frey J. E., Altwegg M. 2006. Rapid detection and species identification of Mycobacterium spp. using real-time PCR and DNA-microarray. J. Microbiol. Methods 66:116–124 [DOI] [PubMed] [Google Scholar]
- 143. Tortoli E., et al. 2001. Performance assessment of new multiplex probe assay for identification of mycobacteria. J. Clin. Microbiol. 39:1079–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Tortoli E., Mariottini A., Mazzarelli G. 2003. Evaluation of INNO-LiPA MYCOBACTERIA v2: improved reverse hybridization multiple DNA probe assay for mycobacterial identification. J. Clin. Microbiol. 41:4418–4420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Tuohy M. J., Hall G. S., Sholtis M., Procop G. W. 2005. Pyrosequencing as a tool for the identification of common isolates of Mycobacterium sp. Diagn. Microbiol. Infect. Dis. 51:245–250 [DOI] [PubMed] [Google Scholar]
- 146. Vaerewijck M. J., Huys G., Palomino J. C., Swings J., Portaels F. 2005. Mycobacteria in drinking water distribution systems: ecology and significance for human health. FEMS Microbiol. Rev. 29:911–934 [DOI] [PubMed] [Google Scholar]
- 147. Van de Griendt E. J., Rietra P. J., van Andel R. N. 2003. Mycobacterium haemophilum as the cause of lymphadenitis in the neck in an otherwise healthy boy. Ned. Tijdschr. Geneeskd. 147:1367–1369 [PubMed] [Google Scholar]
- 148. Van Ingen J., Boeree M. J., Dekhuijzen P. N., van Soolingen D. 2008. Mycobacterial disease in patients with rheumatic disease. Nat. Clin. Pract. Rheumatol. 4:649–656 [DOI] [PubMed] [Google Scholar]
- 149. Von Stebut E., Wiest K., Braeuninger W. 2005. Chronic infiltrates and persisting ulcerations on the arms and legs. Arch. Dermatol. 141:897–902 [DOI] [PubMed] [Google Scholar]
- 150. Walder B. K., et al. 1976. The skin and immunosuppression. Aust. J. Dermatol. 17:94–97 [DOI] [PubMed] [Google Scholar]
- 151. Wang S. X., Sng L. H., Leong H. N., Tan B. H. 2004. Direct identification of Mycobacterium haemophilum in skin lesions of immunocompromised patients by PCR-restriction endonuclease analysis. J. Clin. Microbiol. 42:3336–3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Ward M. S., Lam K. V., Cannell P. K., Herrmann R. P. 1999. Mycobacterial central venous catheter tunnel infection: a difficult problem. Bone Marrow Transplant. 24:325–329 [DOI] [PubMed] [Google Scholar]
- 153. Weinstock D. M., Feinstein M. B., Sepkowitz K. A., Jakubowski A. 2003. High rates of infection and colonization by nontuberculous mycobacteria after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 31:1015–1021 [DOI] [PubMed] [Google Scholar]
- 154. Whipps C. M., Dougan S. T., Kent M. L. 2007. Mycobacterium haemophilum infections of zebrafish (Danio rerio) in research facilities. FEMS Microbiol. Lett. 270:21–26 [DOI] [PubMed] [Google Scholar]
- 155. White M. P., Bangash H., Goel K. M., Jenkins P. A. 1986. Nontuberculous mycobacterial lymphadenitis. Arch. Dis. Child. 61:368–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. White M. H., Papadopoulos E., Small T. N., Kiehn T. E., Armstrong D. 1995. Mycobacterium haemophilum infections in bone marrow transplant recipients. Transplantation 60:957–960 [PubMed] [Google Scholar]
- 157. White D. A., Kiehn T. E., Bondoc A. Y., Massarella S. A. 1999. Pulmonary nodule due to Mycobacterium haemophilum in an immunocompetent host. Am. J. Respir. Crit. Care Med. 160:1366–1368 [DOI] [PubMed] [Google Scholar]
- 158. Whittier S., Hopfer R. L., Knowles M. R., Gilligan P. H. 1993. Improved recovery of mycobacteria from respiratory secretions of patients with cystic fibrosis. J. Clin. Microbiol. 31:861–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Winter N., et al. 1995. Characterization of the gene encoding the immunodominant 35 kDa protein of Mycobacterium leprae. Mol. Microbiol. 16:865–876 [DOI] [PubMed] [Google Scholar]
- 160. Woods G. L., Warren N. G., Inderlied C. B. 2007. Susceptibility test methods: mycobacteria, nocardia, and other actinomycetes, p. 1223–1248In Murray P. R., Baron E. J., Jorgensen J. H., Landry M. L., Pfaller M. A. (ed.), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC [Google Scholar]
- 161. Xiong L., Kong F., Yang Y., Cheng J., Gilbert G. L. 2006. Use of PCR and reverse line blot hybridization macroarray based on 16S-23S rRNA gene internal transcribed spacer sequences for rapid identification of 34 Mycobacterium species. J. Clin. Microbiol. 44:3544–3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Yakrus M. A., Straus W. L. 1994. DNA polymorphisms detected in Mycobacterium haemophilum by pulsed-field gel electrophoresis. J. Clin. Microbiol. 32:1083–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Yarrish R. L., et al. 1992. Osteomyelitis caused by Mycobacterium haemophilum: successful therapy in two patients with AIDS. AIDS 6:557–561 [PubMed] [Google Scholar]
- 164. Zeharia A., et al. 2008. Management of nontuberculous mycobacteria-induced cervical lymphadenitis with observation alone. Pediatr. Infect. Dis. J. 27:920–922 [DOI] [PubMed] [Google Scholar]