Abstract
Summary: Infection with Mycobacterium tuberculosis causes a variety of clinical conditions ranging from life-long asymptomatic infection to overt disease with increasingly severe tissue damage and a heavy bacillary burden. Immune biomarkers should follow the evolution of infection and disease because the host immune response is at the core of protection against disease and tissue damage in M. tuberculosis infection. Moreover, levels of immune markers are often affected by the antigen load. We review how the clinical spectrum of M. tuberculosis infection correlates with the evolution of granulomatous lesions and how granuloma structural changes are reflected in the peripheral circulation. We also discuss how antigen-specific, peripheral immune responses change during infection and how these changes are associated with the physiology of the tubercle bacillus. We propose that a dynamic approach to immune biomarker research should overcome the challenges of identifying those asymptomatic and symptomatic stages of infection that require antituberculosis treatment. Implementation of such a view requires longitudinal studies and a systems immunology approach leading to multianalyte assays.
INTRODUCTION
The importance of diagnostic research on tuberculosis (TB) cannot be overstated. One-third of the world population carries an asymptomatic infection with Mycobacterium tuberculosis, which results in eight million new cases of TB and two million deaths every year. Identifying and treating those who progress to disease and can transmit infection to contacts are crucial to successful control. Our current diagnostic toolbox is inadequate to achieve these goals. For example, none of the tests for active TB is sufficiently accurate, timely, and appropriate for low-income and low-technology settings, where most TB cases are found (reviewed in references 44, 87, 88, 91, 143, 144, 145, and 162). The recently developed gamma interferon (IFN-γ) release assays (IGRAs) diagnose latent M. tuberculosis infection (LTBI) more accurately than the century-old tuberculin skin test (reviewed in references 36, 77, 109, and 110), but they fail to facilitate decisions concerning targeted LTBI treatment (97, 107, 108). Indeed, it is recognized that the millennium development goals set by the United Nations in the fight against TB, i.e., cutting in half the global prevalence and death rate by 2015 (42), cannot be reached without the development of new diagnostic tools (http://www.stoptb.org/globalplan).
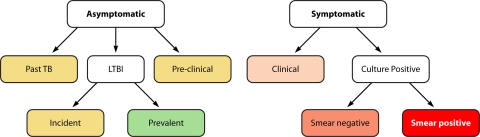
Diagnosing TB is no simple matter. Infection with M. tuberculosis has been commonly regarded as having a binary clinical outcome. One is LTBI, which is characterized by a positive tuberculin skin test (TST)/IGRA, the absence of symptoms, and a normal chest X-ray. The other outcome is active TB, which is typically defined by the detection of tubercle bacilli or bacillary products in pathological specimens, usually sputum. However, it is becoming increasingly clear that the clinical spectrum of M. tuberculosis infection is more complex than previously appreciated. The definition of LTBI includes multiple conditions, as is best recognized in nonhuman primate models (6, 162). In humans, forms of LTBI can be differentiated on the basis of risk of reactivation. For example, in immunocompetent individuals, the annual risk of developing active TB is 1.5% in the first 2 years after infection and 0.1% thereafter (96). Asymptomatic infection with a history of past TB carries a greater risk of reactivation than LTBI alone, particularly when chest X-ray findings are abnormal (104). Active pulmonary TB also presents with a spectrum of clinical manifestations, which are usually associated with increasing bacillary burden. Since active TB is diagnosed with bacteriological assays, a low bacillary burden often leads to a missed diagnosis. It emerges from the above considerations that M. tuberculosis infection results in a continuum of ill-defined, sometimes overlapping, clinical manifestations (6, 162) (Fig. 1). Since treatment decisions are based on particular criteria (for example, treatment of LTBI is warranted only when the risk of reactivation is high [3]), recognizing the spectrum of asymptomatic and symptomatic stages of M. tuberculosis infection is critical for implementing more effective TB control policies.
Fig. 1.
Clinical states of M. tuberculosis infection. This schematic is adapted from the classification of TB by the American Thoracic Society (ATS) (2). ATS class numbers are also indicated, as applicable. Infected individuals are divided into asymptomatic and symptomatic. (i) The asymptomatic group is further divided into subgroups; color codes indicate the relative risk of progression to active disease in each subgroup (green, low; yellow, high). Past TB (inactive TB; class 4) indicates either a history of a previous episode(s) of active TB or abnormal stable radiographic findings and no bacteriological and/or radiographic evidence of current disease. LTBI (class 1) indicates a positive TST/IGRA and no clinical, bacteriological, or radiographic evidence of active disease. LTBI is further divided into incident/recent (<2 years after infection) or prevalent/remote (>2 years postinfection). The preclinical TB/incipient TB group includes asymptomatic individuals found to have developed active disease when examined at later (short-term) times. (ii) The symptomatic group is also further divided into subgroups; here, color codes indicate bacillary load (orange, low; red, high). Clinical TB indicates symptoms and/or radiographic findings suggestive of active TB but no bacteriological evidence of disease. Culture-confirmed TB (class 3) indicates bacteriological evidence of active TB. These patients are further subdivided into smear-negative and smear-positive groups based on sputum smear microscopy (It is noted that the extent of radiographic lung involvement, such as cavitary and noncavitary disease, is often also used to classify patients.).
Immunological biomarkers should best distinguish the stages of M. tuberculosis infection from one another. Immunological events are at the core of TB pathogenesis, since they are responsible for both tissue damage and protection (41). Thus, various phases and outcomes of M. tuberculosis infection should be associated with particular immunological events. Moreover, assessing immune responses circumvents the need to detect tubercle bacilli or their products, both of which are currently inaccessible during most of the asymptomatic infection and even during early symptomatic stages. Despite these considerations, no immunodiagnostic test exists that can accurately diagnose active TB, distinguish LTBI from active TB, or tell apart asymptomatic forms of infection that are associated with a high risk of disease progression (the shortcomings of TB immunodiagnostics have been extensively reviewed [36, 38, 134, 142, 143]). It is time to translate the complexities of the clinical spectrum of M. tuberculosis infection into new paradigms for TB immunodiagnosis.
The present review explores parallels between the clinical and pathological development of M. tuberculosis infection and the evolution of immune responses. Previous considerations of the immunological spectrum in TB have usually been limited to clinical manifestations of disease (see, for example, references 29 and 81), typically in search of a parallel with leprosy (100, 119). These have been guided primarily by the Th1/Th2 paradigm, which is now seen as an oversimplification of opposite immunophenotypes (61). Here we examine the evolution of histopathological events, immune mediators, and cell types in peripheral blood and their relationship to the physiology of the tubercle bacillus (Fig. 2) (we do not review the effects of coinfection with HIV, which relate primarily to loss of immune control during latent M. tuberculosis infection). We then discuss how recognizing the immunological spectrum of M. tuberculosis infection impacts the development of new immunodiagnostics. The present article emphasizes the need for “combinatorial” systems approaches to biomarker selection rather than the diagnostic characteristics of individual biomarkers. For excellent meta-analyses of immunological biomarkers and their reported accuracy in the diagnosis of LTBI and/or active TB, the reader is referred to recent reviews (162, 163).
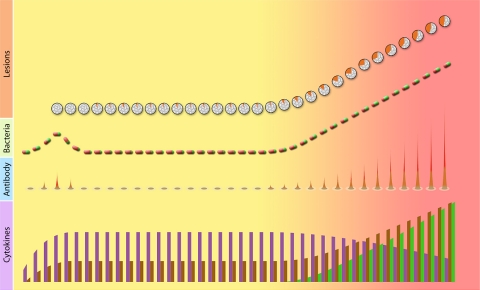
Fig. 2.
Schematic representation of bacteriological, histopathological, and immunological changes during M. tuberculosis infection. The background color reflects the clinical spectrum of infection, progressing from asymptomatic (yellow) to symptomatic (red). The changes in the granulomatous lesions are shown relative to the number of lesions (vertical axis) and the quality of the granuloma (color composition). In each granuloma icon, the gray, granular area represents cells, while the solid orange color represents caseum. For tubercle bacilli, bacterial numbers (vertical axis) and phenotype (color composition of the bacilli) are depicted. In each icon, the color indicates the growth phase (green, growing bacilli; red, nongrowing bacilli). For antibody levels, the icon, which represents antibody responses to the entire proteome, is roughly divided into a nonreactive, dominant area of the proteome (outer, colorless) an and inner, reactive area (red gradient). The gradient of red indicates rarely reactive (orange) and commonly reactive (dark red) proteins. The height of the reactive proteome area (vertical axis) represents the frequency of reactive TB sera. The early, transient peak shown in the antibody curve is derived from monkey and human data. A similar course of the bacillary curve is inferred from the likelihood that tubercle bacilli multiply before immunity is expressed and bacillary growth is controlled. For cytokine levels, three hypothetical patterns are shown, with levels (vertical axis) decreasing (purple) or increasing (brown) with disease progression or being detected only during active disease (green). Each pattern may be characteristic of one or more cytokines.
THE GRANULOMATOUS LESIONS
Tubercle bacilli interact with immune cells and various host cell types interact with each other to form the granuloma, a dynamic structure that is the histopathological hallmark of M. tuberculosis infection. The granuloma acts as a site of immune cell priming. The systemic immune response should reflect changes in the local immune compartments due to the recirculation of immune cells and the release of soluble mediators that reach the periphery.
The Tuberculous Granuloma
The tuberculous granuloma is a dynamic multicellular aggregate. Macrophages predominate in the inner cellular layer as multinucleated giant cells, epithelioid cells, or foamy cells (127). T lymphocytes expressing αβ T-cell receptors (TCR) are also present. CD4+ T cells are localized in both inner and outer cellular layers, while CD8+ T cells are found mostly in the outer cellular layer. The outer cellular layer also contains B cell-rich aggregates, which include bacillus-laden antigen-presenting cells (APCs) and T lymphocytes. Granulomas also contain atypical lymphocytes such as γδ T cells, which do not require major histocompatibility complex (MHC) class I or II molecules for antigen recognition, and regulatory T cells with immunosuppressive properties (12, 58). The typical caseous granuloma includes a central necrotic core. Some bacilli are found in the necrotic core, but most are located at the interface between the necrotic core and the macrophage-rich, inner cellular layer (126, 156, 158). As disease progresses, granulomas tend to be less organized and may become cavitary (157). When it occurs, cavitation connects the caseous center with the bronchial tree. Loss of organization in the granuloma alters the interactions between immune cells (75) and correlates with tissue destruction, as indicated in active TB by increased serum levels of matrix metalloproteinase-9 (65), a protein that degrades extracellular matrix (115). Granuloma progression is associated with increased bacterial numbers, particularly at the luminal surface of the cavity. This may be favored by the local selective reduction of T cells, which diminishes T-cell–macrophage interactions (7, 75, 157). Disorganization of advanced granulomas may also facilitate bacterial dissemination to other areas of the lung and to other organs, with formation of new granulomas (157).
There are many variants of the tuberculous granuloma (86), which differ in cellular composition and structure. Some granulomas are rich in granulocytes (86). Others vary with regard to T-cell composition. For example, it was reported that while most granulomas stained positive for CD4+ T cells, only half were positive for CD8+ T cells (46). Consequently, each granuloma may exhibit distinct profiles of cytokine production (45, 46). When a necrotic center is present, its characteristics may also vary (e.g., central necrosis may be suppurative). Healing of granulomas is associated with fibrotic changes, mineralization (15, 86), and presumably the presence of few, if any, tubercle bacilli. Differences between granulomas are also seen in the pattern of distribution in the lung, which may be focal, multifocal, coalescing, or invasive. While the architecture of the granuloma typically changes with the evolution of infection, different types of granulomatous lesions are also found in the same individual, either with active disease (47) or with latent infection (6).
Studies on experimentally infected macaques have greatly contributed to understanding the evolution of the granuloma structure with disease progression (15, 86). In these animals, latent infection is usually associated with one or few localized granulomas, with or without minimal involvement of thoracic lymph nodes. Active disease is characterized by large numbers of highly disseminated granulomas, which tend to invade nearby vessels and airways. Granulomas in animals with latent infection differ from those found in animals with active disease with regard to structure and immune cell composition: active disease is associated with higher numbers of caseous granulomas, more numerous CD4+ and CD8+ T cells (up to 100-fold), and higher proportions of T cells expressing the chemokine receptors CCR5 and CXCR3 (86). Differences are also seen with antigen-specific immune responses, as the number of cells producing IFN-γ in response to the M. tuberculosis secreted proteins ESAT-6 and CFP-10 is higher in the lungs of monkeys with active disease than in those with latent infection (86).
Priming in the Granuloma
The granuloma is a site of immunological priming, which occurs at the interface between the macrophage-rich inner layer and the surrounding T-cell-rich outer layer (126, 156, 158). Since T cells in this layer are predominantly CD4+ cells, they are more likely to interact with APCs than CD8+ T cells, which are found mostly in the outer cellular layer. Additional priming sites for B cells and T cells include the follicle-like structures found in the outer cellular layer, which are rich in B cells, CD4+ and CD8+ T cells, and infected APCs. In these follicles, B cells are found at different states of differentiation (naïve cells, memory cells, and antibody-secreting plasma cells). An additional route of priming may exist in caseous granulomas, where mycobacterial antigens presumably released from dead bacilli in the necrotic center (45, 66) reach the surrounding APC-rich areas.
Multiple factors at the site of priming affect the immune response. The relative local abundance of a particular cell type targeted for priming is important for the strength and persistence of the immune responses induced. The nature of the bacterial antigen is also significant. For example, B-cell priming is favored in areas containing extracellular bacteria, since B cells react preferentially with particulate antigens. Additionally, the intracellular location of bacteria has a role in the endogenous processing of antigens. For the majority of intracellular mycobacteria, which are found in the phagosome, antigen presentation is directed to MHC class II molecules (32). Tubercle bacilli have also been found in the cytoplasm of phagocytes (93, 159), a location that triggers presentation by MHC class I molecules. Although it is controversial, a possibility also exists that bacterial proteins secreted by the bacteria in the phagosome enter the surrounding host cell cytoplasm (21, 150). Priming of CD8+ T cells may also involve blebs, which are generated by apoptosis of infected macrophages (130), or exosomes, which are membrane-bound vesicles secreted by infected macrophages (8, 10, 51). Yet another factor affecting priming and immune responses is the local concentration of bacterial antigen. This may vary not only in relation to the absolute bacterial load but also in relation to the relative bacterial antigen expression patterns that reflect microenvironmental variations between granulomas or even between different areas of the same granuloma (45, 47, 116). The relationship between bacterial growth phenotypes and bacterial antigen expression is discussed below.
Bacterial Phenotypes and Antigen Expression
That tubercle bacilli multiply during active disease and remain dormant during LTBI is an oversimplified scenario, since several lines of evidence suggest that multiple bacillary phenotypes are concurrently found during infection. In the case of active TB, the need for prolonged chemotherapy is partially attributable to the phenotypic drug resistance associated with the presence of nonreplicating mycobacteria (24). Indeed, nonreplicating tubercle bacilli most likely exist in granulomatous lesions irrespective of stage and structural integrity, since prolonged anti-TB chemotherapy is required even in HIV-coinfected individuals, where granulomas tend to be less organized (24). Another line of evidence for mixed bacillary phenotypes in active TB derives from microbiological studies of sputum. Up to 85% of bacilli found in sputum samples from active TB patients can be laden with neutral lipids, which are a trait of nonreplicating M. tuberculosis (49). Moreover, sputum samples from most TB patients contain predominantly resuscitation-promoting factor (RFP)-dependent bacteria (101). These bacteria, which are not detected by standard CFU enumeration, require treatment with RFP proteins for growth or for regrowth following dormancy (101). RFP-dependent bacilli are tolerant to rifampin and are eliminated by antibiotic treatment more slowly than colony-forming bacilli (101).
Mixed bacterial phenotypes are also likely to be present during latent infection. For example, in vitro culturing of granulomatous tissue from autoptic lungs of individuals with LTBI who died from TB-unrelated pathology has yielded mycobacteria exhibiting different rates of regrowth (43). Moreover, the ability of isoniazid, a drug effective only against multiplying bacteria, to reduce the risk of disease reactivation in LTBI (23) indirectly supports the presence of multiplying bacilli during latent infection. The possibility of gathering direct evidence on the physiological state of tubercle bacilli during latent infection has been hampered primarily by the failure to determine their location. Staining of acid-fast bacilli (AFB) may not be the detection method of choice, since nonreplicating mycobacteria exhibit reduced acid-fastness due to cell wall remodeling (28). Alternative methods, such as in situ PCR methods, have detected mycobacterial DNA in alveolar and interstitial macrophages present in “normal-appearing” lung tissue (62), suggesting locations of tubercle bacilli other than the classical granuloma. Due to the difficulties described above, whether the entire bacterial population is not growing during latent infection or a growing subset of bacilli exists remains a point of debate (43).
The bacillary growth state is likely to be important for stimulation of the immune response because actively growing and dormant tubercle bacilli probably express different antigen sets. These two types of bacilli differ in major metabolic pathways, protein secretory pathways, and cell wall composition (28, 30, 137, 152, 166). Moreover, protein secretion increases with bacillary growth rate (4), and secreted proteins are favored antigen targets (26). Murine studies have shown that expression of immunodominant antigens varies with bacillary growth phase (in mice, tubercle bacilli grow exponentially in the acute phase of mouse lung infection and then stop growing during chronic infection [135]). For example, during chronic infection, expression of the gene encoding the antigen 85 (Ag85) complex, which is involved in the biosynthesis of mycolic acids (9), is downregulated (136), while expression of hspX (encoding α-crystallin), which is part of the so-called dormancy (devR/dosR) regulon (160), is induced (135). This dichotomy fits with mycolic acid synthesis being a cellular activity associated with bacterial multiplication and expression of the devR/dosR regulon being associated with M. tuberculosis growth arrest (120, 135). Similar inferences were made from human studies in which differences in bacterial gene expression patterns were seen between tubercle bacilli grown in vitro and those found in sputa of TB patients (49) or in human tuberculous lung tissue (116).
Local and Systemic Immune Responses
While the study of the infection site reveals interactions between the pathogen and immune cells (reviewed in references 72 and 132), it is the characterization of the peripheral immune response that should help identify stage-specific immune markers that are suitable for diagnostic development. Thus, it is imperative to understand how well the peripheral response reflects events at the infection site. Little doubt exists that immune responses expressed at the infection site and in the periphery differ quantitatively and qualitatively. Immune cells are localized at the site of infection due to clonal expansion and migration from the peripheral circulation and from lymphoid organs after priming (35, 146, 155). As a result, the number of antigen-specific lymphocytes at the infection site is, on average, 10 times greater than that in peripheral blood (70, 71, 103). Qualitative differences may also occur. For example, CD4+/CD8+ T-cell ratios have been found to be higher in the bronchoalveolar lavage fluid than in peripheral blood during active disease (154). Moreover, a broader repertoire of epitopes recognized by T cells was found in the pleural fluid than in peripheral blood (168). On the other end, communication between the infection site and the periphery undoubtedly exists, since lymphocytes circulate between lymphoid and nonlymphoid tissues and soluble immune mediators released by these cells may reach the periphery. Immune cells in the granuloma presumably enter the bloodstream through the blood vessels found at the granulomatous site. Indeed granulomas are highly vascularized, probably due to the vascular endothelial growth factor secreted by activated macrophages at the site of infection (126). In addition, lymph nodes draining pulmonary lesions are connected to the systemic circulation. These communication lines make it possible to follow the events occurring at the site of infection by sampling peripheral blood, as demonstrated by primate studies. Indeed, the frequency of antigen-specific, IFN-γ-producing cells found in granulomas and peripheral blood of macaques is greater with active disease than with latent infection (86).
IMMUNE MARKERS
Many studies on T- and B-cell responses during infection with M. tuberculosis have been conducted. T lymphocytes are the effectors of the protective immune response. Thus, these cells have been extensively investigated in terms of expression of antigen-specific TCR, functional markers, and cytokine production in association with controlled infection (latent infection) versus loss of immune control (active TB), most typically in relation to vaccine research. M. tuberculosis antigens that evoke T-cell responses have also been studied, with particular attention to T-cell antigens produced by virulent tubercle bacilli but not by other, nonpathogenic mycobacterial species. An important result of that work has been the development of novel immunodiagnostics for LTBI (114, 117). The next diagnostic challenge is to distinguish between stages of M. tuberculosis infection by identification of stage-specific cellular immune markers. Expression of these markers may reflect (i) changes in antigen burden and (ii) acquisition by T cells of effector functions that are causally associated with infection outcome. In the field of antibody responses, which do not confer protection, most research has been on identifying serodominant antigens for diagnosis of active TB. Serological studies have shown pronounced variability in antibody profiles between TB patients. This has made it difficult to utilize antibodies as accurate biomarkers of active TB. Nevertheless, antibody levels do reflect bacillary burden and may track relative changes in the burden of particular bacterial antigens during the course of infection. The dynamic characteristics of the antibody response have been most clearly seen with the serological interrogation of the entire M. tuberculosis proteome. The challenge in this field is how to utilize the dynamic properties of the antibody response for diagnostics. In this section, we first review the key mediators of the immune response. We then present work describing the immunological differences between active TB and LTBI and showing how immune responses change with the evolution of infection. We discuss the two arms of immunity separately, since research on cellular and humoral immune markers has been typically conducted with different goals and different study designs (only a few studies have investigated humoral and T-cell responses concurrently; see, for example, references 89 and 92).
Mediators of Antigen-Specific Immune Responses
As shown in animal models, CD4+ T cells are initially primed in the draining lymph nodes, where the bacilli are transported by dendritic cells (169). In a mouse model, priming/activation occurs about 1 week following infection; activated cells reach the lung in 2 to 4 weeks postinfection (169). The critical role of CD4+ T cells in controlling M. tuberculosis infection has been shown by murine studies (105) and by the dramatic increase of the incidence of active TB associated with HIV coinfection (48). CD8+ T cells have the same kinetics of appearance in tissues as CD4+ T cells in mice. CD4+ and CD8+, MHC class Ia-restricted T cells expressing αβ TCR recognize protein antigens (M. tuberculosis proteins recognized by these cell types have been listed [106]). In contrast, lipids and glycolipids are presented in the context of MHC class Ib (CD1d) molecules. Unconventional CD8+ T cells, which carry a γδ T-cell receptor, lack the fine antigen specificity of αβ TCR. These cells respond to a wide variety of pathogen-derived antigens, such as lipids, phospho- and lipoproteins, and even nonpeptide phosphorylated oligonucleotides.
Many cellular immune responses are mediated by cytokines secreted by the immune cells. Cytokines are typically classified into proinflammatory Th1 cytokines and anti-inflammatory Th2 cytokines. Their relative levels determine the outcome of some mycobacterial infections, such as leprosy. In the case of TB, the association between Th1/Th2 balance and infection outcome has been actively investigated, but the results remain inconclusive (94). Many Th1 cytokines are critical for controlling infection with M. tuberculosis (reviewed in references 25, 48, and 105). One is IFN-γ, as shown by studies with individuals defective in genes for IFN-γ or its receptor and in animal models. Another is interleukin-12 (IL-12), which stimulates IFN-γ production. A lack of this cytokine increases susceptibility to mycobacterial infections in humans. Tumor necrosis factor alpha (TNF-α), a proinflammatory cytokine, is also required for control of the infection, as shown by the increased incidence of TB reactivation in individuals receiving anti-TNF-α antibody treatment for unrelated disease. Other examples of proinflammatory responses are the production of IL-2, which is involved in the clonal expansion of antigen-specific T cells (125), and of the chemokine CXCL10 (IP-10), which is important in trafficking monocytes and Th1 cells to the site of inflammation. Examples of Th2 cytokines induced by mycobacterial infection include IL-4, which has an anti-inflammatory role, and IL-10 and transforming growth factor β (TGF-β), which suppress T-cell responses (reviewed in references 48 and 122). Recently, a new Th cell population (Th17) has been recognized, which produces IL-17, IL17F, IL-21, and IL-22 (153). In mice, most of the IL-17 response is generated by γδ T cells (25). It appears that IL-17 and Th17 cells mediate immune pathology and may have a detrimental effect in TB (153).
B lymphocytes are central to the humoral adaptive immunity. They function as APCs and may have immune regulatory roles in TB (90). The production of antibody has been investigated almost exclusively in the context of biomarker research (reviewed in references 1, 112, and 142), since a protective role against TB for antibodies is generally dismissed, with few exceptions (see, for example, reference 151). Among antibody isotypes, IgG molecules have been mainly investigated, owing to their specificity for antigen. This isotype, which is T-cell dependent, is a predominant component of secondary immune responses. The relative production of the various subclasses of IgG antibodies is influenced by the presence of particular cytokines and B-cell activators (32, 67, 140). For example, the presence of IFN-γ favors IgG2, IL-4 and IL-13 favor IgG4, while IL-10 favors IgG1 and IgG3. In addition, subclasses depend on the biochemical nature of the antigen (32, 140). For example, most antibodies against protein antigens are of the IgG1 and IgG3 subclasses.
Cellular Immune Responses and Infection State
The investigation of antigen-specific, T-cell-mediated immune responses relative to infection state has followed at least two approaches. By far dominant, one has been the use of ex vivo stimulation of immune cells obtained from distinct study populations (active TB patients, LTBI cases, household contacts, and uninfected controls) with one or few immunodominant M. tuberculosis antigens (for example, ESAT-6, the 19-kDa lipoprotein, and Ag85B). These studies have investigated multiple characteristics of the cells responding to antigen stimulation, such as cell surface markers and production of cytokines and chemokines, to characterize immune function in relation to infection state. This methodology can be characterized as “one/few antigens, many read-outs.” In a second approach, one or a few read-outs have been utilized (e.g., the secretion of one or a few cytokines) to assess the response of immune cells to various antigens that, for example, are presumed to be differentially expressed at various stages of infection (“many antigens, one/few read-outs”). Together, the two approaches have shown that different stages of M. tuberculosis infection are associated with quantitative and/or qualitative differences in immune cell types and released immune mediators. These are reviewed below.
Cellular responses in latent infection and active TB.
Cellular responses to M. tuberculosis antigens have been investigated mostly by flow cytometry analysis. One area of research has been the characterization of memory phenotypes by measuring production of IFN-γ and IL-2, given that effector cells secrete predominantly IFN-γ, effector-memory cells secrete both IFN-γ and IL-2, and central memory cells are known to secrete only IL-2 (99). In active TB, most antigen-specific T cells are effector cells, while central memory cells predominate in LTBI (16, 129). Antibiotic treatment causes a relative decrease of cells expressing effector phenotypes and a concurrent increase of memory cells in treated TB patients (16, 99). These results suggest that the relative frequencies of memory cells and effector cells are associated with changes in antigen burden, as described for viral infections (111). Another line of investigation has characterized multifunctional T cells (34, 133). Multifunctional T cells secreting IFN-γ, TNF-α, and IL-2 are more frequent in TB patients (85 to 90%) than in LTBI cases (10 to 15%) (14, 147). Their frequency decreases during antituberculosis treatment (14, 171). Thus, also the abundance of multifunctional T cells may track increased bacterial load associated with development of active disease (14). In another study, the frequency of T cells producing only TNF-α was sufficient to distinguish active TB from LTBI (59). Together, these studies indicate that the responses of circulating antigen-specific T cells differ between active TB and latent infection.
Even though flow cytometry analysis is highly informative, methods detecting soluble immune mediators as solid-phase antigen are more suitable for diagnostic development. The cytokine most investigated in TB research has been IFN-γ. The success of IGRAs in diagnosing LTBI has encouraged new effort to determine whether the presence of active TB correlates with increased levels of IFN-γ relative to those detected in LTBI. Studies with experimentally infected animals support this possibility (5). Indeed, many studies conducted with IGRAs have shown higher IFN-γ responses in active TB patients than in LTBI cases (17, 73, 74, 164). Discordant results, however, have also been obtained (113). The disease-associated increase seems to be more evident with the enzyme-linked immunospot (ELISPOT) assay, an assay enumerating IFN-γ-producing cells, than with enzyme-linked immunosorbent assay (ELISA), which measures the levels of IFN-γ secreted by the cultured cells (17, 18, 39, 40, 79, 80, 118). Thus, the two assays may have different sensitivities. Despite these encouraging results, it is difficult to distinguish active TB from latent infection on the basis of IFN-γ levels alone (97), due to individual variability of IFN-γ levels and the resulting overlap between the two states. Moreover, the finding that IGRA-negative contacts of TB cases can progress to active TB in less than 2 years after exposure (170) suggests perhaps that either the antigens used or the cytokine measured in the IGRAs may not cover the entire population. In addition, the sensitivity of IGRAs appears to be lower in active TB than in LTBI (see, for example, references 124 and 125), presumably due to immunosuppression accompanying active disease or to sequestration of IFN-γ-producing cells at the infection site (78). Thus, IGRAs cannot be used to rule out active TB (95). It has been suggested that the use of particular ESAT-6-derived peptides in lieu of full-length protein may enhance discrimination between active disease and latent infection (53, 56). Together with the observation that responses to ex vivo stimulation with purified protein derivative (PPD) do not differ between active TB and LTBI (64), the results obtained with ESAT-6 peptides suggest that the choice of epitope is a critical factor in the evaluation of biomarker levels in response-to-antigen assays.
Measurement of additional cytokines secreted by peripheral blood mononuclear cells (PBMCs) in response to M. tuberculosis antigens may also help distinguish active TB from LTBI. In one study, levels of IL-2 measured after prolonged incubation (72 h) of PBMCs with the antigens used in IGRAs were higher in LTBI than in active disease (11). The diagnostic potential of IP-10 (125) also has been evaluated, with mixed results (54, 149, 167). Since secretion of IP-10 is less affected than that of IFN-γ by immunosuppression (55) and is age independent (85), IP-10 has been proposed as an adjunct marker of LTBI diagnosis in pediatric and HIV-coinfected populations (85, 124). In other studies, the levels of epidermal growth factor (EGF), sCD40L, vascular endothelial growth factor (VEGF), TGF-α, and IL-1α released by antigen-stimulated PBMCs in a commercial IGRA distinguished TB patients from household contacts more accurately than any single marker alone (19). Release of multiple cytokines in response to bacterial antigens other than those used for the commercial IGRAs may also increase diagnostic accuracy (see, for example, references 20 and 148). Indeed, cellular responses vary with the antigen used to stimulate the cells ex vivo (60, 123). While of limited scope, these studies show that multiple host markers can help discriminate between infection states.
Cellular responses and antigen expression.
Few studies have investigated the correlation between cellular responses and bacterial antigen expression. Murine studies have shown that in the peripheral blood of infected animals, the number of CD4+ T cells reacting to ESAT-6 is higher than that of Ag85B-specific CD4+ T cells; this correlates with greater abundance of the ESAT-6 transcript relative to the Ag85B transcript in lung RNA (121). The evidence is indirect in humans, where only few studies have investigated this correlation. Immune recognition of bacterial antigens associated with the nonreplicating bacterial state (“dormancy” antigens) is stronger in latent infection than in active disease, presumably correlating with higher expression of these antigens during latent infection. For example, individuals having latent infection express stronger responses to antigens encoded by the dosR regulon than active TB patients (83). A protein encoded by the dosR regulon (Rv3407) has been proposed as a latent infection marker (131). Furthermore, the cellular immune response to Rv2628, another product of the dosR regulon, was associated with cured TB and remote infection (52). Moreover, cellular responses to α-crystallin, which is encoded by another dosR-regulated gene, are stronger in LTBI than in active TB (33). Additionally, IFN-γ production in response to Rv2659 and Rv2660, which are two proteins encoded by the starvation stimulon, was more frequent in LTBI than in active TB (57). Collectively, these results strongly indicate that antigen recognition by T cells tracks antigen profiles expressed by tubercle bacilli in association with growth phase. It is worth mentioning, however, that one study found no difference in the response to α-crystallin between active TB and LTBI (64). One possible explanation is that strong differences in absolute bacillary load between latent infection and multibacillary forms of active TB overcome the effect of growth-phase-associated antigen expression.
Cellular responses and disease progression.
Very few studies have investigated the evolution of cellular immune responses over time. One such study showed that antigen- and mitogen-induced IFN-γ/IL-10 ratios were higher in household contacts of TB cases who did not develop disease than in those who did (68). In a more common study design, baseline levels of immune markers have been analyzed in relation to onset of disease at a later date. In these studies, higher baseline levels of IFN-γ measured by IGRAs correlated with increased risk of disease progression (31, 37, 63, 84, 98). Similar results were obtained when tuberculin skin test responses were investigated (5). Thus, there is a correlation between levels of antigen-specific responses and bacillary burden, as proposed largely on the basis of animal studies (5). However, it has also been reported that increased IFN-γ and decreased IL-4 production by αβ and γδ CD8+ T cells negatively correlate with development of disease in health care workers (105a). These results seem applicable to the general population, since similar conclusions were drawn in comparisons between subjects with LTBI and active TB patients (120a, 165). The basis of the apparent contradiction between the two sets of conclusions reported above is unclear, though it may lie in the cytokine-expressing T-cell population examined. The ratio between the Th2 cytokine IL-4 and the IL-4 splice variant IL-4δ2, which exhibits an expression pattern similar to that of IFN-γ (165), has been proposed as another indicator of TB reactivation risk (38a, 162). Collectively, the results of these studies indicate that the imbalance between proinflammatory and anti-inflammatory responses associated with disease progression is reflected in the peripheral blood.
The levels of immune markers can also change with severity of active disease. In one study, severe tuberculosis was associated with reduced IFN-γ production by antigen-stimulated PBMCs (139), while in another study the number of antigen-specific T cells was higher in cavitary disease than in noncavitary disease (118a). The observed divergence likely results from the use of different antigens and assay types in the two studies.
Humoral Immune Responses and Infection State
While studies of T-cell immunity in TB have most typically focused on small numbers of immunodominant antigens, as mentioned above, the antibody response has been explored in relation to many antigenic targets. The evaluation of many antibody specificities has provided the opportunity to extensively assess the relationship between bacillary (i.e., antigen) burden and antibody response. Various aspects of this relationship are reviewed in this section, with an emphasis on results obtained with the serological interrogation of the full proteome of M. tuberculosis. We also review longitudinal studies investigating antibody responses during progression of the infection. Since the antibody response varies substantially from one patient to another, we also discuss the potential causes of antibody heterogeneity in tuberculosis.
Antibody responses and bacterial metabolic status.
A recent interrogation of the M. tuberculosis proteome (∼4,000 proteins) with ∼500 sera from TB suspects from countries where TB is endemic showed that approximately 10% of the bacterial proteome generates human antibody responses (76). These results define the immunoproteome, which contains predominantly membrane-associated and secreted proteins. Within the immunoproteome, a much smaller pool of proteins (<1% of the proteome) were preferentially recognized by sera from active TB patients. These were predominantly secreted proteins. These conclusions agree with much of the earlier serological work utilizing culture filtrates and purified secreted proteins (75a, 138, 139a, 142, 163a). The immunoproteome data strongly suggest that membrane-associated proteins (which might derive from low numbers of live bacilli, dead bacilli, or macrophage-secreted exosomes) are occasionally targeted during latent infection or paucibacillary disease. In either condition, the extracellular proteins are underrepresented, either because dormant bacilli do not secrete (latent infection) or because the numbers of metabolically active (and secreting) mycobacteria are low (paucibacillary disease). As bacillary burden increases with disease, metabolically active bacilli secrete proteins, which become the favored targets. Thus, antibody responses are dynamic and reflect the bacterial metabolic state during infection.
Antibody responses and bacterial burden.
The strongest evidence that antibody responses reflect bacillary burden derives from the many studies showing that antibody responses tend to be much stronger in sputum smear-positive than in smear-negative pulmonary TB (13a, 138, 142, 167a). The positive correlation between antibody levels and bacillary load was first described with use of purified or semipurified proteins, such as the 38-kDa antigen and Ag85 (13a, 167a), and it has now been seen at the immunoproteome level, indicating this to be a general characteristic of the antibody response in TB. With a few antigens, such as the 19-kDa lipoprotein, antibody levels have been reported as being higher in sera from smear-negative TB patients than in those from smear-positive patients, perhaps in correlation with particular HLA phenotypes (13). Hypotheses linking antigen recognition by antibody and HLA have not been tested.
The strong correlation between antibody levels and bacillary burden constitutes a double-edged sword with regard to the usefulness of antibody as a biomarker of active TB. On one hand, conditions associated with low bacillary burden, such as stable asymptomatic infection and Mycobacterium bovis BCG vaccination, are by and large seronegative. Thus, antibodies can differentiate active TB from asymptomatic infection states that require no medical intervention. Moreover, seroconversion could be used as an early indication of disease progression, since activation of TB is presumably accompanied by an increased bacillary burden (see below). On the other hand, antibody-based assays have performed poorly when used to diagnose sputum smear-negative TB (1, 112, 138, 142), suggesting that antibody responses are ill-suited as markers of paucibacillary forms of active TB. A correlation between bacillary burden and antibody responses is also suggested by the observation that some subjects with a history of past TB are seropositive (29a, 76). These subjects may harbor a larger (or metabolically more active) bacterial population than the general latently infected population, as suggested by the observed association of past TB with increased risk of TB reactivation (58a, 104). While suggesting the intriguing possibility that increased antibody levels may help identify past TB cases in the process of reactivating TB, the occasional seropositivity seen in the past TB group poses an additional challenge to serodiagnosis of active TB.
Antibody responses and disease progression.
The association between the antibody response, bacillary load, and bacterial metabolic state suggests that antibody responses track disease progression. This possibility was tested in macaques, which respond to experimental M. tuberculosis infection with approximately equal probabilities of asymptomatic containment (latent infection) and active disease (15, 86). Moreover, some asymptomatic monkeys undergo spontaneous reactivation. It was found that in asymptomatic animals, antibody levels remained at preinfection levels or returned to preinfection levels after a transient increase (S. Kunnath, J. L. Flynn, and M. L. Gennaro, unpublished results). In contrast, antibody responses to the M. tuberculosis proteome increased in animals exhibiting active disease. The rise of antibody levels occurred at later times in the spontaneously reactivating animals relative to those classified as having acute active disease. Moreover, the number of antigenic targets increased with antibody levels in active disease, indicating that the number of antigens reaching threshold concentration levels for immune activation increases with antigen load. The findings in macaques agree with results of studies conducted on HIV-infected cohorts, where the levels of some antibodies were seen to increase prior to the diagnosis of active TB (16a, 50a, 76a, 138a). The fact that antibodies to some antigens but not to others increase with progression to disease (50a) again points to threshold levels for antibody production varying among immunodominant antigens. Whether these differences are attributable to regulatory mechanisms remains to be investigated.
Diversity of antibody responses.
The serum antibody profiles obtained from active TB patients differ from each other in terms of antigenic targets and titers. This has seriously hampered the development of TB serological diagnosis (as reviewed in many meta-analyses [1, 112, 142, 143]), since the requirement for multiple antigens as diagnostic reagents to increase the number of true-positive test results has been typically accompanied by increased false-positive test results. Host factors are almost certainly at play in determining serological diversity, since macaques infected by the same route with the same number of tubercle bacilli from the same strain develop varied antibody profiles (Kunnath et al., unpublished results). Moreover, antibodies from different murine strains recognize different antigen sets (69). In humans, the levels of some antibodies have been reported to be associated with HLA type (13, 13b). Variation may also be introduced by infecting bacterial strains differing in antigen composition. Variation most likely occurs at the level of expression of immunodominant antigens among clinical isolates (48a, 113a), since little diversity exists in genes encoding antigenic targets (102) and most antigenic epitopes are hyperconserved (22). For antigens in the PE/PPE family, strain-to-strain variation of gene expression has been proposed to provide tubercle bacilli with a dynamic antigenic profile (161). In addition to “intrinsic” host- and pathogen-derived factors, relative antigen burden can be viewed as a main source of antibody variability. It is likely that variability among antibody profiles results from the bacterial load and bacterial metabolic state at the time of testing and the relative immunodominance of each protein. Thus, the relative frequency at which the antibody response “samples” each immunodominant antigen will vary from one patient to another. Moreover, depending on relative antibody avidity, the effect of antigen load on the frequency of sampling will be greater for some antigens than for others. Furthermore, due to the chronic nature of tuberculosis, these events occur over considerable periods of time; thus, cross-sectional analyses may further accentuate variation. It should also be noted that, given that a small subset of secretory proteins are specifically targeted during active TB (76), antibody responses to tuberculosis may appear homogeneous (128) under particular patient selection and testing conditions.
NEW PRINCIPLES FOR BIOMARKER DISCOVERY
The following set of conclusions emerges from the work described above.
Clinically, infection with M. tuberculosis does not have a simple, binary outcome, i.e., latent infection and active disease. Each condition covers a spectrum of “subconditions.” Latent infection may be a stable state, it may be associated with a high risk of progression to disease, or it may represent a preclinical stage of disease. Active disease may be minimal, i.e., asymptomatic or accompanied by low-grade symptoms, or it may exhibit various degrees of severity in terms of symptoms, bacterial burden, and tissue damage. Thus, M. tuberculosis infection presents with a spectrum of multiple, often poorly separated, clinical conditions.
Histopathologically, the tuberculous granuloma is a dynamic structure. Host-pathogen interactions in the granuloma over the course of infection lead to adaptive changes of tubercle bacilli, of the phenotypes of the host immune cells, and of the levels of the immune mediators they produce. Recirculation of the immune cells and release of soluble mediators establish a link between local and systemic immune compartments.
Immunologically, the levels of some immune markers vary during infection because their expression is directly linked to immune function (e.g., protective or suppressive) and its regulation, others vary because they reflect changes in bacterial antigen composition and bacterial burden during infection, and yet others vary for both reasons.
These considerations lead to the view that the spectrum of the clinical manifestations is accompanied by a corresponding spectrum of tissue damage and immune responses. If so, particular stages of the M. tuberculosis infection are associated with specific cellular phenotypes, cytokine levels, and/or antibody profiles. However, the association between biomarkers and infection stages is not simple, because it results from the interaction/intersection of multiple covariates that are host and pathogen derived (as it can be inferred from Fig. 2). Moreover, genetic and epigenetic diversity exists among hosts and among infecting strains, which further complicates the picture. Elements of diversity can be found even within the same host, since not all granulomas evolve at the same time and bacteria can be found at different growth phases within the same granuloma. Thus, for immunodiagnosis to be an effective tool for TB control, at least two conditions must be met. One is that immune markers associated with each particular clinical condition are found, and the other is that (at least some of) these markers are robust enough to withstand the heterogeneity associated with host- and pathogen-derived sources of variation.
A Paradigm Shift
The notion that M. tuberculosis infection is associated with a spectrum of ill-separated clinical conditions has become increasingly clear (see references 6 and 162 for two recent reports). Given that the host immune response is at the heart of TB pathogenesis, the presence of a concurrent immunological spectrum is almost evident. However, biomarker research has often shied away from the complexity of the immune response to M. tuberculosis infection, implementing instead a “reductionist” approach aiming at finding a marker (or marker set) that would diagnose “infection, yes/no” or “active TB, yes/no.” As a result, reactivity to an “active TB, yes” marker in the absence of active disease has been taken as a “false-positive” result rather than as the consequence of a simplistic case classification. Underestimating the consequences of the immunological spectrum and trying to force boundaries between states where boundaries barely exist seem to constitute an example of “forcing a square peg into a round hole.” It is our view that TB immunodiagnostic research is moving toward an inevitable paradigm shift, in which the presence (and even the absence) of immune markers exhibiting various degrees of association with a particular infection state provides an immune signature or “code” that is much more predictive of a particular infection state than any of the components of the code separately. Moreover, it may be expected that a complex signature is more robust than single markers with respect to person-to-person variation. Changes in the code (or a different code) would reflect transitions from one infection state to another.
Systems Immunology
The realization that no single marker identifies a particular M. tuberculosis infection stage with adequate diagnostic accuracy has led to the search for multiple markers. Thus far, this principle has been applied to markers of the same kind. For example, since serological recognition of M. tuberculosis antigens varies among TB patients, serodiagnostic research has been oriented toward multiantigen tests to reach suitable diagnostic sensitivity. Moreover, IGRAs for the diagnosis of LTBI have included two or three antigens for better sensitivity, even though large proportions of infected individuals exhibit cellular responses to immunodominant antigens such as ESAT-6 and CFP-10. However, the failure of serology to provide accurate diagnostics for active TB and of the current IGRAs to reliably distinguish between stable and progressive LTBI raises the possibility that the immunological signature of each M. tuberculosis infection state rests on a combination of immune markers of different types. For example, detectable antibody levels are strongly associated with active TB; however, the need for multiple antibodies to boost sensitivity reduces diagnostic specificity due to accumulating positive results in the population without TB disease. In contrast, the cellular response detected by the current IGRAs can be considered “almost universal” in infected individuals. It is not infrequent, however, that responses to IGRAs wane in TB patients, due to the immunosuppression associated with active disease. It is conceivable, therefore, that the diagnostic association of antibody to active disease might be strengthened by the concurrent absence of a particular cellular response that is reduced in active disease due to immunoregulatory mechanisms. Additional diagnostic insight should result from chemokines or cytokines produced by peripheral blood cells when infection is contained rather than when immune control has failed. Moreover, it is conceivable that relative ratios of IgG isotypes, which reflect the cytokine environment, may also skew the diagnostic decision toward the presence or absence of an active disease process. All of the above could be further refined by taking into account antigen specificity of the responses, given the possibility that production of immunodominant antigens changes with the growth phase of the infecting bacilli.
At least two alternative approaches can be envisioned for finding the diagnostic immune signatures of M. tuberculosis infection states. The first would take advantage of the large body of data sketched in the sections above. In this approach, combinatorial biomarkers would be identified from the investigation of known immune responses to known bacterial antigens. An alternative approach would take advantage of high-throughput technologies, which can generate information on thousands of “conditions” at once. Such technologies are already mature for some immune markers (e.g., antibodies to proteins and peptides). For others, such as the simultaneous detection of antigen-specific T-cell responses (58b, 103a), they are still being developed. High-throughput methods should make it feasible to characterize the immunological spectrum of M. tuberculosis infection by assessing large numbers of mediators of the cellular and humoral response to many (or all) antigens of the tubercle bacillus. Either approach would have to be used in longitudinal studies to assess how transitions from one infection state to another are associated with qualitative and quantitative changes in immune markers.
The paradigm shift and the systems approach proposed above should also apply to pediatric TB, which differs from TB in adults with regard to risk of disease progression, pathophysiology, and clinical presentation (27). However, children infected or diseased with M. tuberculosis might express different immunological biomarkers than their adult counterparts, because critical differences exist between the innate and acquired responses of young children and adults (82) and because pediatric TB is often paucibacillary (141).
CONCLUSIONS
Characterizing the immunological spectrum of M. tuberculosis infection for immunodiagnostic purposes requires retooling TB immunodiagnostic research to better accommodate a dynamic, rather than static, view of biomarker discovery. The challenges are many. Longitudinal studies require large sample sizes, lengthy data collection periods, and costly resources. High-throughput methods are usually expensive. Assessment of combinatorial markers requires sophisticated analytical methods and extensive computing resources. The downstream development of multianalyte diagnostic assays that interrogate diverse components of the immune responses constitutes an area of research in its own right, particularly if the need for point-of-care tests drives the field toward lab-on-chip methodologies. However, each challenge can be turned into a new opportunity for research on TB immunodiagnostics, as was discussed at an international conference in 2008 (50). If the will exists, with the help of international funding, advocacy, and political collaboration, these challenges can be met. The success will be worth the effort because, besides LTBI diagnosis by IGRAs, our current knowledge warns us that unless we break the code, no substantial advance will be made in immunodiagnosis for TB.
ACKNOWLEDGMENTS
We thank Yuri Bushkin, Karl Drlica, and Richard Pine for critical reading of the manuscript and C&M Consulting, Denville, NJ, for help with Fig. 2.
Research in the Gennaro laboratory has been supported by the National Institutes of Health, the Foundation for New and Innovative Diagnostics, the European Union, and the Futura Foundation.
Biographies
 Shajo Kunnath-Velayudhan, M.B.B.S., M.M.S.T., obtained his medical degree from the Trichur Medical College, Kerala, India, and his master's degree in medical science and technology from the Indian Institute of Technology, Kharagpur, India. As a postdoctoral fellow in the Gennaro laboratory, he studied proteome-scale antibody responses to Mycobacterium tuberculosis infection.
Shajo Kunnath-Velayudhan, M.B.B.S., M.M.S.T., obtained his medical degree from the Trichur Medical College, Kerala, India, and his master's degree in medical science and technology from the Indian Institute of Technology, Kharagpur, India. As a postdoctoral fellow in the Gennaro laboratory, he studied proteome-scale antibody responses to Mycobacterium tuberculosis infection.
 Maria Laura Gennaro, M.D., is a Professor of Medicine at the Public Health Research Institute, New Jersey Medical School, Newark, NJ. Over the years, she has investigated bacterial pathogens, including enterotoxic Escherichia coli, Salmonella spp., Vibrio cholerae, Staphylococcus aureus, and Mycobacterium tuberculosis. The main areas of Dr. Gennaro's tuberculosis research have been the antibody response to M. tuberculosis infection and the remodeling of the M. tuberculosis transcriptome during infection. She is currently leading a systems biology approach to the study of M. tuberculosis physiology during infection and of the interaction between the tubercle bacillus and the host macrophage.
Maria Laura Gennaro, M.D., is a Professor of Medicine at the Public Health Research Institute, New Jersey Medical School, Newark, NJ. Over the years, she has investigated bacterial pathogens, including enterotoxic Escherichia coli, Salmonella spp., Vibrio cholerae, Staphylococcus aureus, and Mycobacterium tuberculosis. The main areas of Dr. Gennaro's tuberculosis research have been the antibody response to M. tuberculosis infection and the remodeling of the M. tuberculosis transcriptome during infection. She is currently leading a systems biology approach to the study of M. tuberculosis physiology during infection and of the interaction between the tubercle bacillus and the host macrophage.
REFERENCES
- 1. Abebe F., Holm-Hansen C., Wiker H. G., Bjune G. 2007. Progress in serodiagnosis of Mycobacterium tuberculosis infection. Scand. J. Immunol. 66:176–191 [DOI] [PubMed] [Google Scholar]
- 2. American Thoracic Society 2000. Diagnostic standards and classification of tuberculosis in adults and children. Am. J. Respir. Crit. Care Med. 161:1376–1395 [DOI] [PubMed] [Google Scholar]
- 3. American Thoracic Society 2000. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Recommend. Rep. 49:1–51 [PubMed] [Google Scholar]
- 4. Andersen P., Askgaard D., Ljungqvist L., Bennedsen J., Heron I. 1991. Proteins released from Mycobacterium tuberculosis during growth. Infect. Immun. 59:1905–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andersen P., Doherty T. M., Pai M., Weldingh K. 2007. The prognosis of latent tuberculosis: can disease be predicted? Trends Mol. Med. 13:175–182 [DOI] [PubMed] [Google Scholar]
- 6. Barry C. E., III, et al. 2009. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat. Rev. Microbiol. 7:845–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barry S., Breen R., Lipman M., Johnson M., Janossy G. 2009. Impaired antigen-specific CD4(+) T lymphocyte responses in cavitary tuberculosis. Tuberculosis (Edinb.) 89:48–53 [DOI] [PubMed] [Google Scholar]
- 8. Beatty W. L., et al. 2000. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic 1:235–247 [DOI] [PubMed] [Google Scholar]
- 9. Belisle J. T., et al. 1997. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science 276:1420–1422 [DOI] [PubMed] [Google Scholar]
- 10. Bhatnagar S., Shinagawa K., Castellino F. J., Schorey J. S. 2007. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 110:3234–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Biselli R., et al. 2010. Detection of interleukin-2 in addition to interferon-gamma discriminates active tuberculosis patients, latently infected individuals, and controls. Clin. Microbiol. Infect. 16:1282–1284 [DOI] [PubMed] [Google Scholar]
- 12. Boom W. H. 1999. Gammadelta T cells and Mycobacterium tuberculosis. Microbes Infect. 1:187–195 [DOI] [PubMed] [Google Scholar]
- 13. Bothamley G. H., Schreuder G. M., de Vries R. R., Ivanyi J. 1993. Association of antibody responses to the 19-kDa antigen of Mycobacterium tuberculosis and the HLA-DQ locus. J. Infect. Dis. 167:992–993 [DOI] [PubMed] [Google Scholar]
- 13a. Bothamley G. H., Rudd R., Festenstein F., Ivanyi J. 1992. Clinical value of the measurement of Mycobacterium tuberculosis specific antibody in pulmonary tuberculosis. Thorax 47:270–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13b. Bothamley G. H., et al. 1989. Association of tuberculosis and M. tuberculosis-specific antibody levels with HLA. J. Infect. Dis. 159:549–555 [DOI] [PubMed] [Google Scholar]
- 14. Caccamo N., et al. 2010. Multifunctional CD4(+) T cells correlate with active Mycobacterium tuberculosis infection. Eur. J. Immunol. 40:2211–2220 [DOI] [PubMed] [Google Scholar]
- 15. Capuano S. V., III, et al. 2003. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect. Immun. 71:5831–5844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Casey R., et al. 2010. Enumeration of functional T-cell subsets by fluorescence-immunospot defines signatures of pathogen burden in tuberculosis. PLoS One 5:e15619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a. Cavalcante S., et al. 1997. Association between an early humoral response to Mycobacterium tuberculosis antigens and later development of tuberculosis in human immunodeficiency virus-infected individuals. Int. J. Tuberc. Lung Dis. 1:170–174 [PubMed] [Google Scholar]
- 17. Chee C. B., Barkham T. M., Khinmar K. W., Gan S. H., Wang Y. T. 2009. Quantitative T-cell interferon-gamma responses to Mycobacterium tuberculosis-specific antigens in active and latent tuberculosis. Eur. J. Clin. Microbiol. Infect. Dis. 28:667–670 [DOI] [PubMed] [Google Scholar]
- 18. Chee C. B., et al. 2008. Comparison of sensitivities of two commercial gamma interferon release assays for pulmonary tuberculosis. J. Clin. Microbiol. 46:1935–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chegou N. N., Black G. F., Kidd M., van Helden P. D., Walzl G. 2009. Host markers in QuantiFERON supernatants differentiate active TB from latent TB infection: preliminary report. BMC Pulm. Med. 9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen J., et al. 2009. Novel recombinant RD2- and RD11-encoded Mycobacterium tuberculosis antigens are potential candidates for diagnosis of tuberculosis infections in BCG-vaccinated individuals. Microbes Infect. 11:876–885 [DOI] [PubMed] [Google Scholar]
- 21. Clemens D. L., Lee B. Y., Horwitz M. A. 2002. The Mycobacterium tuberculosis phagosome in human macrophages is isolated from the host cell cytoplasm. Infect. Immun. 70:5800–5807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Comas I., et al. 2010. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat. Genet. 42:498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Comstock G. W., Baum C., Snider D. E., Jr 1979. Isoniazid prophylaxis among Alaskan Eskimos: a final report of the bethel isoniazid studies. Am. Rev. Respir. Dis. 119:827–830 [DOI] [PubMed] [Google Scholar]
- 24. Connolly L. E., Edelstein P. H., Ramakrishnan L. 2007. Why is long-term therapy required to cure tuberculosis? PLoS Med. 4:e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cooper A. M. 2009. Cell-mediated immune responses in tuberculosis. Annu. Rev. Immunol. 27:393–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Covert B. A., Spencer J. S., Orme I. M., Belisle J. T. 2001. The application of proteomics in defining the T cell antigens of Mycobacterium tuberculosis. Proteomics 1:574–586 [DOI] [PubMed] [Google Scholar]
- 27. Cruz A. T., Starke J. R. 2010. Pediatric tuberculosis. Pediatr. Rev. 31:13–26 [DOI] [PubMed] [Google Scholar]
- 28. Cunningham A. F., Spreadbury C. L. 1998. Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and localization of the 16-kilodalton alpha-crystallin homolog. J. Bacteriol. 180:801–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Daniel T. M., Oxtoby M. J., Pinto E., Moreno E. 1981. The immune spectrum in patients with pulmonary tuberculosis. Am. Rev. Respir. Dis. 123:556–559 [DOI] [PubMed] [Google Scholar]
- 29a. Davidow A., et al. 2005. Antibody profiles characteristic of Mycobacterium tuberculosis infection state. Infect. Immun. 73:6846–6851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deb C., et al. 2009. A novel in vitro multiple-stress dormancy model for Mycobacterium tuberculosis generates a lipid-loaded, drug-tolerant, dormant pathogen. PLoS One 4:e6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. del Corral H., et al. 2009. IFNgamma response to Mycobacterium tuberculosis, risk of infection and disease in household contacts of tuberculosis patients in Colombia. PLoS One 4:e8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Delves P., Martin S., Burton D., Roitt I. (ed.). 2006. Roitt's essential immunology. Blackwell Publishing, Malden, MA [Google Scholar]
- 33. Demissie A., et al. 2006. Recognition of stage-specific mycobacterial antigens differentiates between acute and latent infections with Mycobacterium tuberculosis. Clin. Vaccine Immunol. 13:179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Derrick S. C., Yabe I. M., Yang A., Morris S. L. 2011. Vaccine-induced anti-tuberculosis protective immunity in mice correlates with the magnitude and quality of multifunctional CD4 T cells. Vaccine 29:2902–2909 [DOI] [PubMed] [Google Scholar]
- 35. Dheda K., et al. 2008. Gene expression of IL17 and IL23 in the lungs of patients with active tuberculosis. Thorax 63:566–568 [DOI] [PubMed] [Google Scholar]
- 36. Diel R., et al. 2011. Interferon-gamma release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systematic review and meta-analysis. Eur. Respir. J. 37:88–99 [DOI] [PubMed] [Google Scholar]
- 37. Diel R., Loddenkemper R., Niemann S., Meywald-Walter K., Nienhaus A. 2011. Negative and positive predictive value of a whole-blood interferon-γ release assay for developing active tuberculosis: an update. Am. J. Respir. Crit. Care Med. 183:88–95 [DOI] [PubMed] [Google Scholar]
- 38. Diel R., Loddenkemper R., Nienhaus A. 2010. Evidence-based comparison of commercial interferon-gamma release assays for detecting active TB: a metaanalysis. Chest 137:952–968 [DOI] [PubMed] [Google Scholar]
- 38a. Doherty M., Wallis R. S., Zumia A., and the WHO-Tropical Disease Research/European Commission Joint Expert Consultation Group 2009. Biomarkers for tuberculosis disease status and diagnosis. Curr. Opin. Pulm. Med. 15:181–187 [DOI] [PubMed] [Google Scholar]
- 39. Dominguez J., et al. 2009. T-cell responses to the Mycobacterium tuberculosis-specific antigens in active tuberculosis patients at the beginning, during, and after antituberculosis treatment. Diagn. Microbiol. Infect. Dis. 63:43–51 [DOI] [PubMed] [Google Scholar]
- 40. Dominguez J., et al. 2008. Comparison of two commercially available gamma interferon blood tests for immunodiagnosis of tuberculosis. Clin. Vaccine Immunol. 15:168–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dorhoi A., Reece S. T., Kaufmann S. H. 2011. For better or for worse: the immune response against Mycobacterium tuberculosis balances pathology and protection. Immunol. Rev. 240:235–251 [DOI] [PubMed] [Google Scholar]
- 42. Dye C., Watt C. J., Bleed D. M., Hosseini S. M., Raviglione M. C. 2005. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. JAMA 293:2767–2775 [DOI] [PubMed] [Google Scholar]
- 43. Ehlers S. 2009. Lazy, dynamic or minimally recrudescent? On the elusive nature and location of the mycobacterium responsible for latent tuberculosis. Infection 37:87–95 [DOI] [PubMed] [Google Scholar]
- 44. Eichbaum Q., Rubin E. J. 2002. Tuberculosis. Advances in laboratory diagnosis and drug susceptibility testing. Am. J. Clin. Pathol. 118(Suppl.):S3–S17 [DOI] [PubMed] [Google Scholar]
- 45. Fenhalls G., et al. 2002. Localisation of mycobacterial DNA and mRNA in human tuberculous granulomas. J. Microbiol. Methods 51:197–208 [DOI] [PubMed] [Google Scholar]
- 46. Fenhalls G., et al. 2002. Distribution of IFN-gamma, IL-4 and TNF-alpha protein and CD8 T cells producing IL-12p40 mRNA in human lung tuberculous granulomas. Immunology 105:325–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fenhalls G., et al. 2002. In situ detection of Mycobacterium tuberculosis transcripts in human lung granulomas reveals differential gene expression in necrotic lesions. Infect. Immun. 70:6330–6338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Flynn J. L., Chan J. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93–129 [DOI] [PubMed] [Google Scholar]
- 48a. Gao Q., et al. 2005. Gene expression diversity among Mycobacterium tuberculosis clinical isolates. Microbiology 151:5–14 [DOI] [PubMed] [Google Scholar]
- 49. Garton N. J., et al. 2008. Cytological and transcript analyses reveal fat and lazy persister-like bacilli in tuberculous sputum. PLoS Med. 5:e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gennaro M. L., Doherty T. M. (ed.). 2010. Immunodiagnosis of Tuberculosis: New questions, New Tools conference, 2008. Proceedings. BMC Proc. 4(Suppl. 3). http://www.tb-conference.com [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50a. Gennaro M. L., et al. 2007. Antibody markers of incident tuberculosis among HIV-infected adults in the USA: a historical prospective study. Int. J. Tuberc. Lung Dis. 11:624–631 [PubMed] [Google Scholar]
- 51. Giri P. K., Schorey J. S. 2008. Exosomes derived from M. Bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and in vivo. PLoS One 3:e2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Goletti D., et al. 2010. Response to Rv2628 latency antigen associates with cured tuberculosis and remote infection. Eur. Respir. J. 36:135–142 [DOI] [PubMed] [Google Scholar]
- 53. Goletti D., et al. 2006. Accuracy of an immune diagnostic assay based on RD1 selected epitopes for active tuberculosis in a clinical setting: a pilot study. Clin. Microbiol. Infect. 12:544–550 [DOI] [PubMed] [Google Scholar]
- 54. Goletti D., et al. 2010. IFN-gamma, but not IP-10, MCP-2 or IL-2 response to RD1 selected peptides associates to active tuberculosis. J. Infect. 61:133–143 [DOI] [PubMed] [Google Scholar]
- 55. Goletti D., et al. 2010. Is IP-10 an accurate marker for detecting M. tuberculosis-specific response in HIV-infected persons? PLoS One 5:e12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Goletti D., et al. 2005. Selected RD1 peptides for active tuberculosis diagnosis: comparison of a gamma interferon whole-blood enzyme-linked immunosorbent assay and an enzyme-linked immunospot assay. Clin. Diagn. Lab. Immunol. 12:1311–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Govender L., et al. 2010. Higher human CD4 T cell response to novel Mycobacterium tuberculosis latency associated antigens Rv2660 and Rv2659 in latent infection compared with tuberculosis disease. Vaccine 29:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Green A. M., et al. 2010. CD4(+) regulatory T cells in a cynomolgus macaque model of Mycobacterium tuberculosis infection. J. Infect. Dis. 202:533–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58a. Grzybowski, Fishaut S. H., Rowe J., Brown A. 1971. Tuberculosis among patients with various radiologic abnormalities, followed by the chest clinic service. Am. Rev. Respir. Dis. 104:605–608 [DOI] [PubMed] [Google Scholar]
- 58b. Hadrup S. R., et al. 2009. Parallel detection of antigen-specific T-cell responses by multidimensional encoding of MHC multimers. Nat. Methods 6:520–526 [DOI] [PubMed] [Google Scholar]
- 59. Harari A., et al. 2011. Dominant TNF-alpha(+) Mycobacterium tuberculosis-specific CD4(+) T cell responses discriminate between latent infection and active disease. Nat. Med. 17:372–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hasan Z., et al. 2009. Differential live Mycobacterium tuberculosis-, M. bovis BCG-, recombinant ESAT6-, and culture filtrate protein 10-induced immunity in tuberculosis. Clin. Vaccine Immunol. 16:991–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hegazy A. N., et al. 2010. Interferons direct Th2 cell reprogramming to generate a stable GATA-3(+)T-bet(+) cell subset with combined Th2 and Th1 cell functions. Immunity 32:116–128 [DOI] [PubMed] [Google Scholar]
- 62. Hernandez-Pando R., et al. 2000. Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet 356:2133–2138 [DOI] [PubMed] [Google Scholar]
- 63. Higuchi K., Harada N., Fukazawa K., Mori T. 2008. Relationship between whole-blood interferon-gamma responses and the risk of active tuberculosis. Tuberculosis (Edinb.) 88:244–248 [DOI] [PubMed] [Google Scholar]
- 64. Hinks T. S., et al. 2009. Frequencies of region of difference 1 antigen-specific but not purified protein derivative-specific gamma interferon-secreting T cells correlate with the presence of tuberculosis disease but do not distinguish recent from remote latent infections. Infect. Immun. 77:5486–5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hrabec E., Strek M., Zieba M., Kwiatkowska S., Hrabec Z. 2002. Circulation level of matrix metalloproteinase-9 is correlated with disease severity in tuberculosis patients. Int. J. Tuberc. Lung Dis. 6:713–719 [PubMed] [Google Scholar]
- 66. Humphrey D. M., Weiner M. H. 1987. Mycobacterial antigen detection by immunohistochemistry in pulmonary tuberculosis. Hum. Pathol. 18:701–708 [DOI] [PubMed] [Google Scholar]
- 67. Hussain R., Shahid F., Zafar S., Dojki M., Dockrell H. M. 2004. Immune profiling of leprosy and tuberculosis patients to 15-mer peptides of Mycobacterium leprae and M. tuberculosis GroES in a BCG vaccinated area: implications for development of vaccine and diagnostic reagents. Immunology 111:462–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hussain R., Talat N., Shahid F., Dawood G. 2007. Longitudinal tracking of cytokines after acute exposure to tuberculosis: association of distinct cytokine patterns with protection and disease development. Clin. Vaccine Immunol. 14:1578–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Huygen K., et al. 1993. Influence of genes from the major histocompatibility complex on the antibody repertoire against culture filtrate antigens in mice infected with live Mycobacterium bovis BCG. Infect. Immun. 61:2687–2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jafari C., et al. 2006. Rapid diagnosis of smear-negative tuberculosis by bronchoalveolar lavage enzyme-linked immunospot. Am. J. Respir. Crit. Care Med. 174:1048–1054 [DOI] [PubMed] [Google Scholar]
- 71. Jafari C., et al. 2008. Local immunodiagnosis of pulmonary tuberculosis by enzyme-linked immunospot. Eur. Respir. J. 31:261–265 [DOI] [PubMed] [Google Scholar]
- 72. Jafari C., Lange C. 2008. Suttons's law: local immunodiagnosis of tuberculosis. Infection 36:510–514 [DOI] [PubMed] [Google Scholar]
- 73. Janssens J. P., et al. 2007. Quantitative scoring of an interferon-gamma assay for differentiating active from latent tuberculosis. Eur. Respir. J. 30:722–728 [DOI] [PubMed] [Google Scholar]
- 74. Kanunfre K. A., Leite O. H., Lopes M. I., Litvoc M., Ferreira A. W. 2008. Enhancement of diagnostic efficiency by a gamma interferon release assay for pulmonary tuberculosis. Clin. Vaccine Immunol. 15:1028–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kaplan G., et al. 2003. Mycobacterium tuberculosis growth at the cavity surface: a microenvironment with failed immunity. Infect. Immun. 71:7099–7108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75a. Kumar G., et al. 2008. Diagnostic potential of Ag85C in comparison to various secretory antigens for childhood tuberculosis. Scand. J. Immunol. 68:177–183 [DOI] [PubMed] [Google Scholar]
- 76. Kunnath-Velayudhan S., et al. 2010. Dynamic antibody responses to the Mycobacterium tuberculosis proteome. Proc. Natl. Acad. Sci. U. S. A. 107:14703–14708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76a. Laal S., et al. 1997. Surrogate marker of preclinical tuberculosis in human immunodeficiency virus infection: antibodies to an 88-kDa secreted antigen of Mycobacterium tuberculosis. J. Infect. Dis. 176:133–143 [DOI] [PubMed] [Google Scholar]
- 77. Lalvani A., Pareek M. 2010. A 100 year update on diagnosis of tuberculosis infection. Br. Med. Bull. 93:69–84 [DOI] [PubMed] [Google Scholar]
- 78. Lange C., Pai M., Drobniewski F., Migliori G. B. 2009. Interferon-gamma release assays for the diagnosis of active tuberculosis: sensible or silly? Eur. Respir. J. 33:1250–1253 [DOI] [PubMed] [Google Scholar]
- 79. Latorre I., et al. 2009. Quantitative evaluation of T-cell response after specific antigen stimulation in active and latent tuberculosis infection in adults and children. Diagn. Microbiol. Infect. Dis. 65:236–246 [DOI] [PubMed] [Google Scholar]
- 80. Lee J. Y., et al. 2006. Comparison of two commercial interferon-gamma assays for diagnosing Mycobacterium tuberculosis infection. Eur. Respir. J. 28:24–30 [DOI] [PubMed] [Google Scholar]
- 81. Lenzini L., Rottoli P., Rottoli L. 1977. The spectrum of human tuberculosis. Clin. Exp. Immunol. 27:230–237 [PMC free article] [PubMed] [Google Scholar]
- 82. Lewinsohn D. A., Gennaro M. L., Scholvinck L., Lewinsohn D. M. 2004. Tuberculosis immunology in children: diagnostic and therapeutic challenges and opportunities. Int. J. Tuberc. Lung Dis. 8:658–674 [PubMed] [Google Scholar]
- 83. Leyten E. M., et al. 2006. Human T-cell responses to 25 novel antigens encoded by genes of the dormancy regulon of Mycobacterium tuberculosis. Microbes Infect. 8:2052–2060 [DOI] [PubMed] [Google Scholar]
- 84. Lienhardt C., et al. 2010. Evaluation of the prognostic value of IFN-gamma release assay and tuberculin skin test in household contacts of infectious tuberculosis cases in Senegal. PLoS One 5:e10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lighter J., Rigaud M., Huie M., Peng C. H., Pollack H. 2009. Chemokine IP-10: an adjunct marker for latent tuberculosis infection in children. Int. J. Tuberc. Lung Dis. 13:731–736 [PubMed] [Google Scholar]
- 86. Lin P. L., et al. 2009. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect. Immun. 77:4631–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ling D. I., Flores L. L., Riley L. W., Pai M. 2008. Commercial nucleic-acid amplification tests for diagnosis of pulmonary tuberculosis in respiratory specimens: meta-analysis and meta-regression. PLoS One 3:e1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ling D. I., Zwerling A. A., Pai M. 2008. GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: a meta-analysis. Eur. Respir. J. 32:1165–1174 [DOI] [PubMed] [Google Scholar]
- 89. Macedo G. C., Bozzi A., Weinreich H. R., Bafica A., Teixeira H. C., Oliveira S. C. 2011. Human T cell and antibody-mediated responses to the Mycobacterium tuberculosis recombinant 85A, 85B, and ESAT-6 antigens. Clin. Dev. Immunol. 2011:351573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Maglione P. J., Chan J. 2009. How B cells shape the immune response against Mycobacterium tuberculosis. Eur. J. Immunol. 39:676–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mase S. R., et al. 2007. Yield of serial sputum specimen examinations in the diagnosis of pulmonary tuberculosis: a systematic review. Int. J. Tuberc. Lung Dis. 11:485–495 [PubMed] [Google Scholar]
- 92. Masungi C., et al. 2002. Differential T and B cell responses against Mycobacterium tuberculosis heparin-binding hemagglutinin adhesin in infected healthy individuals and patients with tuberculosis. J. Infect. Dis. 185:513–520 [DOI] [PubMed] [Google Scholar]
- 93. McDonough K. A., Kress Y., Bloom B. R. 1993. Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect. Immun. 61:2763–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mendez A., Hernandez-Pando R., Contreras S., Aguilar D., Rook G. A. 2011. CCL2, CCL18 and sIL-4R in renal, meningeal and pulmonary TB; a 2 year study of patients and contacts. Tuberculosis (Edinb.) 91:140–145 [DOI] [PubMed] [Google Scholar]
- 95. Menzies D. 2008. Using tests for latent tuberculous infection to diagnose active tuberculosis: can we eat our cake and have it too? Ann. Intern. Med. 148:398–399 [DOI] [PubMed] [Google Scholar]
- 96. Menzies D., Gardiner G., Farhat M., Greenaway C., Pai M. 2008. Thinking in three dimensions: a web-based algorithm to aid the interpretation of tuberculin skin test results. Int. J. Tuberc. Lung Dis. 12:498–505 [PubMed] [Google Scholar]
- 97. Menzies D., Pai M., Comstock G. 2007. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann. Intern. Med. 146:340–354 [DOI] [PubMed] [Google Scholar]
- 98. Metcalfe J. Z., et al. 2010. Evaluation of quantitative IFN-gamma response for risk stratification of active tuberculosis suspects. Am. J. Respir. Crit. Care Med. 181:87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Millington K. A., et al. 2007. Dynamic relationship between IFN-gamma and IL-2 profile of Mycobacterium tuberculosis-specific T cells and antigen load. J. Immunol. 178:5217–5226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Montoya D., Modlin R. L. 2010. Learning from leprosy: insight into the human innate immune response. Adv. Immunol. 105:1–24 [DOI] [PubMed] [Google Scholar]
- 101. Mukamolova G. V., Turapov O., Malkin J., Woltmann G., Barer M. R. 2010. Resuscitation-promoting factors reveal an occult population of tubercle bacilli in sputum. Am. J. Respir. Crit. Care Med. 181:174–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Musser J. M., Amin A., Ramaswamy S. 2000. Negligible genetic diversity of mycobacterium tuberculosis host immune system protein targets: evidence of limited selective pressure. Genetics 155:7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Nemeth J., et al. 2009. Recruitment of Mycobacterium tuberculosis specific CD4+ T cells to the site of infection for diagnosis of active tuberculosis. J. Intern. Med. 265:163–168 [DOI] [PubMed] [Google Scholar]
- 103a. Newell E. W., Klein L. O., Yu W., Davis M. M. 2009. Simultaneous detection of many T-cell specificities using combinatorial tetramer staining. Nat. Methods 6:497–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Nolan C. M., Elarth A. M. 1988. Tuberculosis in a cohort of Southeast Asian refugees. A five-year surveillance study. Am. Rev. Respir. Dis. 137:805–809 [DOI] [PubMed] [Google Scholar]
- 105. North R. J., Jung Y. J. 2004. Immunity to tuberculosis. Annu. Rev. Immunol. 22:599–623 [DOI] [PubMed] [Google Scholar]
- 105a. Ordway D. J., et al. 2004. Increased interleukin-4 production by CD8 and gammadelta T cells in health-care workers is associated with the subsequent development of active tuberculosis. J. Infect. Dis. 190:756–766 [DOI] [PubMed] [Google Scholar]
- 106. Ottenhoff T. H. M., Lewinsohn D. A., Lewinsohn D. M. 2008. Human CD4 and CD8 T cell responses to Mycobacterium tuberculosis: antigen specificity, function, implications and applications, p. 119–155 In Kaufmann S. H. E., Britton W. J. (ed.), Handbook of tuberculosis: immunology and cell biology. Wiley-VCH Verlag GmbH & co KGaA, Weinheim, Germany [Google Scholar]
- 107. Pai M. 2010. Spectrum of latent tuberculosis—existing tests cannot resolve the underlying phenotypes. Nat. Rev. Microbiol. 8:242 (Author reply, 8:242.) [DOI] [PubMed] [Google Scholar]
- 108. Pai M., Dheda K., Cunningham J., Scano F., O'Brien R. 2007. T-cell assays for the diagnosis of latent tuberculosis infection: moving the research agenda forward. Lancet Infect. Dis. 7:428–438 [DOI] [PubMed] [Google Scholar]
- 109. Pai M., Riley L. W., Colford J. M., Jr 2004. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect. Dis. 4:761–776 [DOI] [PubMed] [Google Scholar]
- 110. Pai M., Zwerling A., Menzies D. 2008. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann. Intern. Med. 149:177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Pantaleo G., Harari A. 2006. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat. Rev. Immunol. 6:417–423 [DOI] [PubMed] [Google Scholar]
- 112. Parkash O., Singh B. P., Pai M. 2009. Regions of differences encoded antigens as targets for immunodiagnosis of tuberculosis in humans. Scand. J. Immunol. 70:345–357 [DOI] [PubMed] [Google Scholar]
- 113. Pathan A. A., et al. 2001. Direct ex vivo analysis of antigen-specific IFN-gamma-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: associations with clinical disease state and effect of treatment. J. Immunol. 167:5217–5225 [DOI] [PubMed] [Google Scholar]
- 113a. Pheiffer C., Betts J. C., Flynn H. R., Lukey P. T., van Helden P. 2005. Protein expression by a Beijing strain differs from that of another clinical isolate and Mycobacterium tuberculosis H37Rv. Microbiology 151:1139–1150 [DOI] [PubMed] [Google Scholar]
- 114. Pollock J. M., Andersen P. 1997. The potential of the ESAT-6 antigen secreted by virulent mycobacteria for specific diagnosis of tuberculosis. J. Infect. Dis. 175:1251–1254 [DOI] [PubMed] [Google Scholar]
- 115. Price N. M., Gilman R. H., Uddin J., Recavarren S., Friedland J. S. 2003. Unopposed matrix metalloproteinase-9 expression in human tuberculous granuloma and the role of TNF-alpha-dependent monocyte networks. J. Immunol. 171:5579–5586 [DOI] [PubMed] [Google Scholar]
- 116. Rachman H., et al. 2006. Unique transcriptome signature of Mycobacterium tuberculosis in pulmonary tuberculosis. Infect. Immun. 74:1233–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ravn P., et al. 1999. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J. Infect. Dis. 179:637–645 [DOI] [PubMed] [Google Scholar]
- 118. Ravn P., et al. 2005. Prospective evaluation of a whole-blood test using Mycobacterium tuberculosis-specific antigens ESAT-6 and CFP-10 for diagnosis of active tuberculosis. Clin. Diagn. Lab Immunol. 12:491–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118a. Ribeiro S., et al. 2009. T-SPOT. TB responses during treatment of pulmonary tuberculosis. BMC Infect. Dis. 9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ridley D. S. 1974. Histological classification and the immunological spectrum of leprosy. Bull. World Health Organ. 51:451–465 [PMC free article] [PubMed] [Google Scholar]
- 120. Roberts D. M., Liao R. P., Wisedchaisri G., Hol W. G., Sherman D. R. 2004. Two sensor kinases contribute to the hypoxic response of Mycobacterium tuberculosis. J. Biol. Chem. 279:23082–23087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120a. Roberts T., Beyers N., Aguirre A., Walzl G. 2007. Immunosuppression during active tuberculosis is characterized by decreased interferon-gamma production and CD25 expression with elevated forkhead box P3, transforming growth factor-beta, and interleukin-4 mRNA levels. J. Infect. Dis. 195:870–888 [DOI] [PubMed] [Google Scholar]
- 121. Rogerson B. J., et al. 2006. Expression levels of Mycobacterium tuberculosis antigen-encoding genes versus production levels of antigen-specific T cells during stationary level lung infection in mice. Immunology 118:195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Rook G. A. 2007. Th2 cytokines in susceptibility to tuberculosis. Curr. Mol. Med. 7:327–337 [DOI] [PubMed] [Google Scholar]
- 123. Rueda C. M., Marin N. D., Garcia L. F., Rojas M. 2010. Characterization of CD4 and CD8 T cells producing IFN-gamma in human latent and active tuberculosis. Tuberculosis (Edinb.) 90:346–353 [DOI] [PubMed] [Google Scholar]
- 124. Ruhwald M., Bjerregaard-Andersen M., Rabna P., Eugen-Olsen J., Ravn P. 2009. IP-10, MCP-1, MCP-2, MCP-3, and IL-1RA hold promise as biomarkers for infection with M. tuberculosis in a whole blood based T-cell assay. BMC Res. Notes 2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ruhwald M., et al. 2007. CXCL10/IP-10 release is induced by incubation of whole blood from tuberculosis patients with ESAT-6, CFP10 and TB7.7. Microbes Infect. 9:806–812 [DOI] [PubMed] [Google Scholar]
- 126. Russell D. G. 2007. Who puts the tubercle in tuberculosis? Nat. Rev. Microbiol. 5:39–47 [DOI] [PubMed] [Google Scholar]
- 127. Russell D. G., Cardona P. J., Kim M. J., Allain S., Altare F. 2009. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat. Immunol. 10:943–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Samanich K., Belisle J. T., Laal S. 2001. Homogeneity of antibody responses in tuberculosis patients. Infect. Immun. 69:4600–4609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sargentini V., et al. 2009. Cytometric detection of antigen-specific IFN-gamma/IL-2 secreting cells in the diagnosis of tuberculosis. BMC Infect. Dis. 9:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Schaible U. E., et al. 2003. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat. Med. 9:1039–1046 [DOI] [PubMed] [Google Scholar]
- 131. Schuck S. D., et al. 2009. Identification of T-cell antigens specific for latent Mycobacterium tuberculosis infection. PLoS One 4:e5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Schwander S., Dheda K. 2011. Human lung immunity against Mycobacterium tuberculosis: insights into pathogenesis and protection. Am. J. Respir. Crit. Care Med. 183:696–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Seder R. A., Darrah P. A., Roederer M. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8:247–258 [DOI] [PubMed] [Google Scholar]
- 134. Sester M., et al. 2011. Interferon-gamma release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur. Respir. J. 37:100–111 [DOI] [PubMed] [Google Scholar]
- 135. Shi L., Jung Y. J., Tyagi S., Gennaro M. L., North R. J. 2003. Expression of Th1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence. Proc. Natl. Acad. Sci. U. S. A. 100:241–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Shi L., North R., Gennaro M. L. 2004. Effect of growth state on transcription levels of genes encoding major secreted antigens of Mycobacterium tuberculosis in the mouse lung. Infect. Immun. 72:2420–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Shi L., et al. 2005. Changes in energy metabolism of Mycobacterium tuberculosis in mouse lung and under in vitro conditions affecting aerobic respiration. Proc. Natl. Acad. Sci. U. S. A. 102:15629–15634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Silva V. M., Kanaujia G., Gennaro M. L., Menzies D. 2003. Factors associated with humoral response to ESAT-6, 38 kDa and 14 kDa in patients with a spectrum of tuberculosis. Int. J. Tuberc. Lung Dis. 7:478–484 [PubMed] [Google Scholar]
- 138a. Simonney N., et al. 2007. B-cell immune responses in HIV positive and HIV negative patients with tuberculosis evaluated with an ELISA using a glycolipid antigen. Tuberculosis (Edinb.) 87:109–122 [DOI] [PubMed] [Google Scholar]
- 139. Sodhi A., Gong J., Silva C., Qian D., Barnes P. F. 1997. Clinical correlates of interferon gamma production in patients with tuberculosis. Clin. Infect. Dis. 25:617–620 [DOI] [PubMed] [Google Scholar]
- 139a. Sonnenberg M. G., Belisle J. T. 1997. Definition of Mycobacterium tuberculosis culture filtrate proteins by two-dimensional polyacrylamide gel electrophoresis, N-terminal amino acid sequencing, and electrospray mass spectrometry. Infect. Immun. 65:4515–4524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Sousa A. O., et al. 1998. IgG subclass distribution of antibody responses to protein and polysaccharide mycobacterial antigens in leprosy and tuberculosis patients. Clin. Exp. Immunol. 111:48–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Starke J. R. 2007. New concepts in childhood tuberculosis. Curr. Opin. Pediatr. 19:306–313 [DOI] [PubMed] [Google Scholar]
- 142. Steingart K. R., et al. 2009. Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin. Vaccine Immunol. 16:260–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Steingart K. R., et al. 2007. Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review. PLoS Med. 4:e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Steingart K. R., et al. 2006. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect. Dis. 6:570–581 [DOI] [PubMed] [Google Scholar]
- 145. Steingart K. R., et al. 2006. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect. Dis. 6:664–674 [DOI] [PubMed] [Google Scholar]
- 146. Strassburg A., Jafari C., Ernst M., Lotz W., Lange C. 2008. Rapid diagnosis of pulmonary TB by BAL enzyme-linked immunospot assay in an immunocompromised host. Eur. Respir. J. 31:1132–1135 [DOI] [PubMed] [Google Scholar]
- 147. Sutherland J. S., Adetifa I. M., Hill P. C., Adegbola R. A., Ota M. O. 2009. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur. J. Immunol. 39:723–729 [DOI] [PubMed] [Google Scholar]
- 148. Sutherland J. S., de Jong B. C., Jeffries D. J., Adetifa I. M., Ota M. O. 2010. Production of TNF-alpha, IL-12(p40) and IL-17 can discriminate between active TB disease and latent infection in a West African cohort. PLoS One 5:e12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Syed Ahamed Kabeer B., Raman B., Thomas A., Perumal V., Raja A. 2010. Role of QuantiFERON-TB gold, interferon gamma inducible protein-10 and tuberculin skin test in active tuberculosis diagnosis. PLoS One 5:e9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Teitelbaum R., et al. 1999. Mycobacterial infection of macrophages results in membrane-permeable phagosomes. Proc. Natl. Acad. Sci. U. S. A. 96:15190–15195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Teitelbaum R., et al. 1998. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc. Natl. Acad. Sci. U. S. A. 95:15688–15693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Timm J., et al. 2003. Differential expression of iron-, carbon-, and oxygen-responsive mycobacterial genes in the lungs of chronically infected mice and tuberculosis patients. Proc. Natl. Acad. Sci. U. S. A. 100:14321–14326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Torrado E., Cooper A. M. 2010. IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev. 21:455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Tsao T. C., et al. 2002. Shifts of T4/T8 T lymphocytes from BAL fluid and peripheral blood by clinical grade in patients with pulmonary tuberculosis. Chest 122:1285–1291 [DOI] [PubMed] [Google Scholar]
- 155. Tully G., et al. 2005. Highly focused T cell responses in latent human pulmonary Mycobacterium tuberculosis infection. J. Immunol. 174:2174–2184 [DOI] [PubMed] [Google Scholar]
- 156. Ulrichs T., Kaufmann S. H. 2006. New insights into the function of granulomas in human tuberculosis. J. Pathol. 208:261–269 [DOI] [PubMed] [Google Scholar]
- 157. Ulrichs T., et al. 2005. Differential organization of the local immune response in patients with active cavitary tuberculosis or with nonprogressive tuberculoma. J. Infect. Dis. 192:89–97 [DOI] [PubMed] [Google Scholar]
- 158. Ulrichs T., et al. 2004. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. J. Pathol. 204:217–228 [DOI] [PubMed] [Google Scholar]
- 159. van der Wel N., et al. 2007. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 129:1287–1298 [DOI] [PubMed] [Google Scholar]
- 160. Voskuil M. I., et al. 2003. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 198:705–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Voskuil M. I., Visconti K. C., Schoolnik G. K. 2004. Mycobacterium tuberculosis gene expression during adaptation to stationary phase and low-oxygen dormancy. Tuberculosis (Edinb.) 84:218–227 [DOI] [PubMed] [Google Scholar]
- 162. Wallis R. S., et al. 2010. Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet 375:1920–1937 [DOI] [PubMed] [Google Scholar]
- 163. Walzl G., Ronacher K., Hanekom W., Scriba T. J., Zumla A. 2011. Immunological biomarkers of tuberculosis. Nat. Rev. Immunol. 11:343–354 [DOI] [PubMed] [Google Scholar]
- 163a. Wang B. L., et al. 2005. Antibody response to four secretory proteins from Mycobacterium tuberculosis and their complex antigen in TB patients. Int. J. Tuberc. Lung Dis. 9:1327–1334 [PubMed] [Google Scholar]
- 164. Wang J. Y., et al. 2007. Diagnosis of tuberculosis by an enzyme-linked immunospot assay for interferon-gamma. Emerg. Infect. Dis. 13:553–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Wassie L., et al. 2008. Ex vivo cytokine mRNA levels correlate with changing clinical status of Ethiopian TB patients and their contacts over time. PLoS One 3:e1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Wayne L. G., Sohaskey C. D. 2001. Nonreplicating persistence of Mycobacterium tuberculosis. Annu. Rev. Microbiol. 55:139–163 [DOI] [PubMed] [Google Scholar]
- 167. Whittaker E., Gordon A., Kampmann B. 2008. Is IP-10 a better biomarker for active and latent tuberculosis in children than IFNgamma? PLoS One 3:e3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167a. Wiker H. G., Harboe M. 1992. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol. Rev. 56:648–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Wilkinson K. A., et al. 2005. Ex vivo characterization of early secretory antigenic target 6-specific T cells at sites of active disease in pleural tuberculosis. Clin. Infect. Dis. 40:184–187 [DOI] [PubMed] [Google Scholar]
- 169. Wolf A. J., et al. 2008. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J. Exp. Med. 205:105–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Yoshiyama T., Harada N., Higuchi K., Sekiya Y., Uchimura K. 2010. Use of the QuantiFERON-TB Gold test for screening tuberculosis contacts and predicting active disease. Int. J. Tuberc. Lung Dis. 14:819–827 [PubMed] [Google Scholar]
- 171. Young J. M., Adetifa I. M., Ota M. O., Sutherland J. S. 2010. Expanded polyfunctional T cell response to mycobacterial antigens in TB disease and contraction post-treatment. PLoS One 5:e11237. [DOI] [PMC free article] [PubMed] [Google Scholar]