Abstract
One hundred three isolates of Carnobacterium spp. from raw meat were analyzed by random amplification of polymorphic DNA (RAPD) and PCR and were identified by 16S rRNA gene sequencing. Forty-five strains of Carnobacterium maltaromaticum were characterized for their growth capabilities at different temperatures, NaCl concentrations, and pH values and for in vitro lipolytic and proteolytic activities. Moreover, their spoilage potential in meat was investigated by analyzing the release of volatile organic compounds (VOCs) in meat stored in air or vacuum packs. Almost all the strains were able to grow at 4, 10, and 20°C, at pH values of 6 to 9, and in the presence of 2.5% NaCl. The release of VOCs by each strain in beef stored at 4°C in air and vacuum packs was evaluated by headspace solid-phase microextraction (HS-SPME)-gas chromatography-mass spectrometry (GC-MS) analysis. All the meat samples inoculated and stored in air showed higher numbers of VOCs than the vacuum-packed meat samples. Acetoin, 1-octen-3-ol, and butanoic acid were the compounds most frequently found under both storage conditions. The contaminated meat samples were evaluated by a sensory panel; the results indicated that for all sensory odors, no effect of strain was significant (P > 0.05). The storage conditions significantly affected (P < 0.05) the perception of dairy, spoiled-meat, and mozzarella cheese odors, which were more intense in meat stored in air than in vacuum packs but were never very intense. In conclusion, different strains of C. maltaromaticum can grow efficiently in meat stored at low temperatures both in air and in vacuum packs, producing volatile molecules with low sensory impacts, with a negligible contribution to meat spoilage overall.
INTRODUCTION
Carnobacteria are ubiquitous lactic acid bacteria (LAB) occurring in different environments, including fish, meat, and a variety of other foods (15, 27, 35, 36). The genus Carnobacterium is currently divided into 10 species: Carnobacterium alterfunditum, C. divergens, C. funditum, C. gallinarum, C. inhibens, C. maltaromaticum, C. mobile, C. viridans, C. pleistocenium, and C. jeotgali, a new species isolated from a traditional Korean fermented food (21). Only two species, C. divergens and C. maltaromaticum, are frequently isolated from various foods, including fish, meat, and some dairy products (27). The occurrence of Carnobacterium in dairy products and other foods is most likely underestimated, since its growth is inhibited by the acetate contained in MRS agar and Rogosa agar, which are commonly used for the enumeration of LAB (7, 39, 42). C. divergens and C. maltaromaticum can also grow in meat products at low temperatures (19, 39) and are frequently predominant members of the microbial community of raw meat (beef, pork, lamb, and poultry). They are found in meat products stored aerobically, in vacuum packaging, or in modified-atmosphere packaging (MAP) (25, 44). Although in the studies cited above C. maltaromaticum and C. divergens were the prevalent species among lactic acid bacteria, the results showed no potential role as spoilage bacteria.
Carnobacterium species are currently the subject of research interest particularly aimed at exploring protective cultures to inhibit pathogenic and spoilage microorganisms in meat and fish (25, 26).
Carnobacterium spp. are psychrotrophic bacteria that are able to grow in meat products at temperatures as low as 1.5°C (19), which is why they may be responsible for rapid spoilage during the storage of meat. Little is known regarding the ability of Carnobacterium spp. to produce flavor-associated metabolites in chilled foods. One strain of C. maltaromaticum has been found to produce flavor-associated metabolites in vacuum-packed beef (12), while other Carnobacterium spp. have been found to influence the sensory characteristics of MAP-stored shrimp in association with Brochothrix thermosphacta (26), even though few strains were studied. Most of the studies describe the isolation and characterization of C. divergens and C. maltaromaticum from meat and seafood (1, 20, 27, 37). Others report the ability of these species to produce volatile organic compounds in laboratory media (22, 23), while data on the production of spoilage-associated molecules in food by a large number of different strains are lacking. The development of organoleptic spoilage is related to microbial consumption of meat nutrients, such as sugars and free amino acids, and the release of undesired volatile metabolites that affect the sensory quality of the meat.
The aim of this study was to identify and characterize Carnobacterium sp. isolates from meat and to investigate the spoilage potential of the strains identified in air-stored and vacuum-packed meat.
MATERIALS AND METHODS
Isolation of bacteria.
Beef samples (longissimus dorsi) before and after storage in air or vacuum packs for 20 days (34) were used as sources for the isolation on cresol red thallium acetate sucrose inulin (CTSI) agar (46) of Carnobacterium spp. after incubation at 20°C for 10 days. One hundred three isolates were obtained and characterized in this study. The purified isolates were characterized by microscopic observation, Gram staining, and catalase reactions. Moreover, three strains of C. maltaromaticum (2M, 7M, and 9P) previously identified (12) were also characterized in this study, while C. maltaromaticum strain DSM20342 was from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ). The strains used in this study were routinely grown in tryptone soy broth (TSB), and the working cultures were maintained in TSB with 25% glycerol at −20°C.
DNA extraction.
DNA was extracted using the Wizard DNA purification kit (Promega, Madison, WI) according to the manufacturer's instructions. After DNA precipitation with 0.7 volume of isopropanol, the resulting pellet was washed with 70% ethanol. Finally, 5 μl of 10× RNase buffer and 0.5 μl of RNase (Promega) were added, and the solution was incubated at 37°C for 30 min. The DNA extracted was stored at −20°C.
Molecular typing by RAPD-PCR and cluster analysis.
Random amplification of polymorphic DNA (RAPD)-PCR was used for molecular typing of the 107 isolates. Primers XD5 (5′-CTGGCGGCTG-3′) (12) and 239 (5′-CTGAAGCGGA-3′) (32) were used. PCRs were carried out in 25 μl of reaction mixture with 20 ng of the template DNA as described previously (12). The amplified products were resolved by electrophoresis on a 1.5% (wt/vol) agarose gel with Tris-borate-EDTA (TBE) at 7 V cm−1 for 2.5 h. A 1-kb DNA ladder (Invitrogen) was used as the molecular weight marker. The gels were acquired using a Gel Doc apparatus (Bio-Rad).
A database of fingerprints was created by using Bionumerics software, version 5.1 (Applied Maths, St. Martens Latem, Belgium). A combined data matrix of all the fingerprints obtained using two different RAPD-PCR conditions was defined, and a similarity dendrogram was created by using the Dice coefficient and the UPGMA (unweighted-pair group method using arithmetic means) clustering algorithm (43).
Sequencing.
In order to amplify the 16S rRNA gene, oligonucleotide primers fD1 (5′-AGAGTTTGATCCTGGCTCAG-3′) and rD1 (5′-AAGGAGGTGATCCAGCC-3′), described by Weisburg et al. (47), were used. 16S rRNA gene sequencing for identification was performed on representative isolates chosen on the basis of molecular typing by RAPD-PCR. The PCR mixture and conditions have been reported previously (12). PCR products from the 16S rRNA gene (1.6 kb) were purified with the QIAquick PCR purification kit (Qiagen) according to the supplier's instructions. The DNA sequence was determined by the dideoxy chain termination method by using the primer fD1 (47). DNA sequence alignments were performed using the National Center for Biotechnology Information (NCBI) online GenBank tools (2).
Effects of temperature, NaCl, and pH on the growth of C. maltaromaticum.
Forty-five strains were selected for characterization as representative of clusters obtained after the comparison of RAPD-PCR fingerprints. In order to evaluate the effects of temperature, NaCl, and pH on the growth of C. maltaromaticum, the strains used in this study were grown in TSB at 4, 10, 20, or 40°C, in TSB supplemented with 2.5%, 5.5%, or 8.5% (wt/vol) NaCl at 20°C, or in TSB adjusted to pH 2 to 11 at 20°C. TSB medium was adjusted to the appropriate pH by using either 0.1 N HCl or 1 N NaOH. Strains with an initial optical density (OD) of 0.1 were inoculated in triplicate in a 0.2-ml volume of each medium. Growth curves at different NaCl concentrations and at different pH values were obtained at the optimal growth temperature of 20°C for 40 h by using the 96-well BioTek Elx808 microtiter plate reader at 600 nm (BioTek Instruments, Inc., Winooski, VT), with readings performed every 30 min. Spectrophotometric data were processed by a modified Gompertz equation (48) in order to calculate the growth curve parameters, i.e., lag time, maximum growth rate (μmax), and maximum population level. Results were calculated as the means of three determinations. Carbohydrate utilization patterns were determined by using API 50CHL kit (bio-Mérieux, Marcy l'Etoile, France) according to the manufacturer's instructions.
Proteolytic and lipolytic activities.
Beef sarcoplasmic proteins were extracted as described by Mauriello et al. (30). The proteolytic activities of the 45 C. maltaromaticum strains were determined according to the work of Villani et al. (45). Lipolytic activity was tested on Spirit Blue agar plates supplemented with a mixture of olive oil and Tween 80 according to the supplier's instructions (Sigma, Milan, Italy) with Staphylococcus aureus ATCC 25923 as a positive control. Duplicate plates were inoculated with streaks of the C. maltaromaticum strains and were incubated for 3 days at 20°C and 4°C, respectively. After the incubation, lipolytic activity was detected by the formation of a clear zone around the colony (18).
Meat contamination by C. maltaromaticum and storage in air and vacuum packs.
The 45 strains of C. maltaromaticum were further characterized for their spoilage potential in meat that was stored in air or vacuum packed. Beef muscles (longissimus dorsi) were superficially decontaminated as described previously (12), and meat chops (about 40 g) were cut from the internal part of the muscle and were spiked with suspensions of each of the strains mentioned above in quarter-strength Ringer's solution (Oxoid) to reach a final concentration of 103 to 104 CFU g−1. To obtain this concentration, 1 ml of each overnight culture was used to make 3 decimal dilutions in quarter-strength Ringer's solution, and 1 ml of the 10−3 dilution was used to inoculate the meat chops. The microbial load of the inoculated meat was routinely checked to ensure that the desired inoculum was always reached.
The strains were inoculated singly, and noninoculated meat chops were included as controls; the experiments were performed in duplicate (the same strain was inoculated into two different pieces of meat; the two pieces of meat were stored under identical conditions and were analyzed separately). In each experiment, two meat chops were inoculated with a single strain of C. maltaromaticum; one chop was stored in air, and the other was packed under a vacuum. For air storage, the contaminated meat was placed in glass bottles (500 ml) and was incubated at 4°C for 7 days (11). Another portion of the contaminated meat was vacuum packed using a bag (200 by 300 mm) of plastic barrier film (low-density polyethylene; oxygen transmission rate, 0.83 cm−3 m−2 h−1 at 23°C; Cryovac BB3050, provided by Cryovac Sealed Air S.r.l., Milan, Italy) and was stored under a vacuum at 4°C for 7 days. After 0 and 7 days of storage in air and vacuum packs, viable counts were performed on CTSI agar.
Determination of volatile organic compounds (VOCs) by gas chromatography-mass spectrometry (GC-MS).
Chemical analyses of contaminated meat were performed after 0 and 7 days of storage at 4°C in air or vacuum packs.
For the headspace solid-phase microextraction (HS-SPME)-GC-MS analysis of meat samples stored in air, the fiber was inserted through a septum directly into a 500-ml bottle used to store the meat in air. For the HS-SPME-GC-MS analysis of vacuum-packaged meat, the samples were transferred to the 500-ml bottles and were analyzed under the same conditions used for samples stored in air. The SPME fiber (carboxen [CAR]-divinylbenzene [DVB]-polydimethylsiloxane [PDMS]; Supelco, Sigma-Aldrich, Bornem, Belgium) was immersed in the HS of the glass for 1 h at room temperature. Thermal desorption of the analytes from the fiber inside the GC injection port was carried out in the split mode (1/10) at a desorption temperature of 250°C for 1 min. All samples were analyzed with an HP7890A GC coupled to an HP5975C quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA). The gas chromatograph was equipped with an HP-5ms capillary column (length, 30 m; inner diameter, 0.25 mm), and the carrier gas used was helium (1 ml min−1). For the analysis of volatile compounds, the GC oven temperature was programmed from 40°C (held for 7 min) to 180°C at 5°C/min. The masses were scanned on an m/z range of 45 to 350 atomic mass units (amu). For the identification of volatile components, the NIST library and comparison with the spectra and retention times of standards were used.
The compounds identified by the use of standards purchased from Sigma-Aldrich (St. Louis, MO) were acetoin, 2-heptanone, 3-octanone, hexanal, heptanal, 2-heptenal, 2-octenal, nonanal, decanal, 1-hexanol, 3-methyl-1-butanol, 2-butoxyethanol, heptanol, 1-octen-3-ol, 1-octanol, 2-octen-1-ol, phenylethyl alcohol, 2-ethyl-1-hexanol, butanoic acid, hexanoic acid, nonanoic acid, and tetradecanal. The remaining compounds were identified by library comparison of the mass spectra.
Calibration was performed by spiking sterile meat samples (meat matrix; in fact, possible interactions between volatiles and meat texture can modify the partitioning of molecules between the headspace and the meat surface) with γ-butyrolactone (2 μg for all samples) and with the standards listed above at 5 concentration levels in ranges where the goodness of fit of linear regression was evaluated (r2, ≥0.95). As many as 3 replicates of determinations were performed.
The matrix formed by the mean values of the VOCs across the 45 samples was analyzed by means of hierarchical cluster analysis (average linkage method) to identify groups of strains based on the degrees of similarity among their volatile components. Similarity dendrograms were obtained both for strains after 7 days of storage in air and for strains after 7 days of storage in vacuum packs.
All the statistical analyses were performed using Systat software, version 5.2.1 for Mac.
Sensory analysis.
Samples of meat contaminated with one of nine C. maltaromaticum strains (2M, D1202, Ic75, I175, B1201, H173, I173, B175, I174), which represented the similarity groups based on VOC profile analysis, were used for sensory analysis along with noncontaminated control samples (stored both in air and in vacuum packs) in order to determine whether there were differences between strains in terms of perceived odors. Sensory analysis was carried out after storage of meat at 4°C for 7 days in air or vacuum packs. Eight judges participated in the panel to evaluate the odor profiles of the samples. The judges were selected for their sensory abilities and their experience in evaluation of the odors of spoiled meat from a previous work (11). The samples were presented in bottles in such a way that the nature of the samples could not be seen; they were equilibrated for 30 min at room temperature and were then served individually to the judges. During each session, four inoculated samples and one control sample were evaluated; each was tested in a randomized design with 3 replications, identified by 3 random-digit codes. Thirteen attributes were evaluated by using continuous 10-cm scales anchored by scores of 0 (absent) and 10 (extremely intense).
Data were collected by means of FIZZ Acquisition software (Biosystèmes, Couternon, France) and were analyzed by two-way analysis of variance (ANOVA) (SPSS, version 13.0) in order to evaluate the effects of both the strain and the storage condition.
RESULTS
Isolation of Carnobacterium spp. from meat and molecular typing.
After purification and preliminary characterization, the Gram-positive and catalase-negative bacterial isolates were analyzed by RAPD-PCR. A considerable portion of the 103 isolates were grouped in clusters with a very high level of similarity (data not shown). After the first screening, all the strains displaying a similarity value below 95% with any of the others were selected for identification by 16S rRNA gene sequencing. Three strains were identified as C. divergens (99% similarity; closest relative, GenBank accession number AM179875) and 41 were identified as C. maltaromaticum (99% similarity; closest relative, accession number AY543035). A dendrogram showing the sequence similarities among the strains and with the 16S rRNA gene sequences of C. maltaromaticum ATCC 27865 (DSM20342) and C. divergens ATCC 35677 is shown in Fig. S1 in the supplemental material.
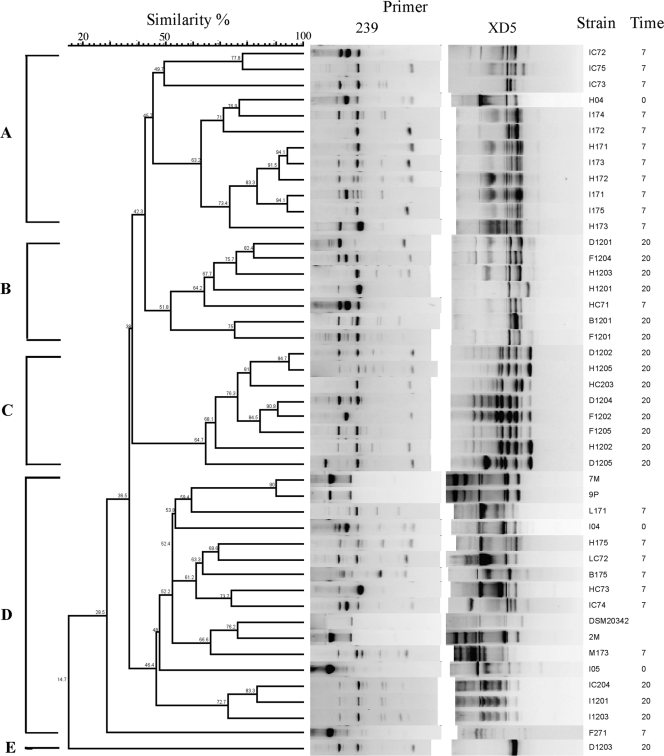
RAPD-PCR fingerprints and a similarity dendrogram of the 41 C. maltaromaticum isolates and the 4 reference strains are reported in Fig. 1, where the isolation time is also indicated. It was possible to obtain four subclusters: subcluster A grouped strains isolated from the same meat samples stored for 7 days; subcluster C grouped eight strains with the same isolation time (20 days); subclusters B and D included strains from different sources and with different isolation times; and strain D1203 could not be included in the other subclusters.
Fig. 1.
Similarity dendrogram generated by RAPD-PCR fingerprints. A combined data matrix of all the fingerprints was defined, and the similarity dendrogram was obtained by using the UPGMA (unweighted-pair group method using arithmetic means) clustering algorithm (43). The strain designations and times of isolation are given on the right (0, beef at time zero; 7, beef after 7 days of storage at 4°C; 20, beef after 20 days of storage at 4°C).
Effects of temperature, NaCl, and pH on the growth of C. maltaromaticum.
The 45 strains of C. maltaromaticum were characterized by the analysis of growth curves under different conditions. Growth occurred between 4 and 40°C in the presence of 2.5 and 5.5% (wt/vol) NaCl and at pH 6 to 10. Spectrophotometric growth data were processed by a modified Gompertz equation to calculate the lag time, μmax, and maximum population level. The results of growth at 4, 10, and 40°C could not be modeled by the Gompertz equation. All the strains tested were able to grow at 20°C; 40 strains grew at 4 and 10°C; and only 10 strains grew at 40°C (data not shown). The values of the growth curve parameters at different pH values and different NaCl concentrations are plotted as heat maps in Fig. S2 and S3 in the supplemental material, respectively. The growth curve parameters were affected by both pH values (see Fig. S2) and salinity (see Fig. S3).
All the strains produced acid from ribose, d-glucose, d-fructose, d-mannose, N-acetylglucosamine, amygdalin, arbutin, esculin, cellobiose, saccharose, trehalose, and β-gentiobiose. However, D1201 and H171 produced acid from maltose and melezitose, whereas Ic204, Ic73, and DSM20342 produced acid from lactose (data not shown). None of the strains analyzed in this study showed lipolytic or proteolytic activity at either 20°C or 4°C.
Production of volatile organic compounds (VOCs) in beef.
The initial viable counts of contaminated meat samples on CTSI agar ranged from 103 to 104 CFU g−1, and the load increased to 107 CFU g−1 after 1 week of incubation at 4°C both in air and under a vacuum (Table 1). By GC-MS analysis, 33 and 24 VOCs were identified in the HS of meat samples inoculated with the 45 C. maltaromaticum strains stored in air and vacuum packs, respectively.
Table 1.
Viable counts on CTSI agar of Carnobacterium spp. in inoculated and uninoculateda meat samples after 0 and 7 days of storage at 4°C in air and vacuum packs
| Sample | Viable count (CFU g−1)b in: |
|||
|---|---|---|---|---|
| Air |
Vacuum pack |
|||
| Day 0 | Day 7 | Day 0 | Day 7 | |
| Control | 0 | 0 | 0 | 0 |
| H04 | 2.8 × 104 | 3.5 × 107 | 1.1 × 104 | 2.0 × 107 |
| I04 | 1.8 × 104 | 6.3 × 107 | 5.8 × 103 | 1.0 × 107 |
| I05 | 5.8 × 104 | 1.5 × 107 | 2.1 × 104 | 1.0 × 107 |
| B175 | 5.0 × 104 | 3.6 × 107 | 1.5 × 104 | 4.6 × 107 |
| F271 | 1.4 × 104 | 1.7 × 107 | 1.9 × 104 | 5.9 × 107 |
| Hc71 | 2.3 × 104 | 8.8 × 106 | 1.1 × 104 | 4.8 × 107 |
| Hc73 | 1.2 × 104 | 7.8 × 106 | 6.1 × 103 | 6.7 × 107 |
| H171 | 1.2 × 104 | 1.8 × 107 | 1.6 × 104 | 1.6 × 107 |
| H172 | 1.5 × 104 | 1.0 × 107 | 1.7 × 104 | 6.8 × 107 |
| H173 | 2.2 × 104 | 4.0 × 107 | 8.3 × 103 | 4.1 × 107 |
| H175 | 1.6 × 104 | 2.8 × 107 | 4.4 × 103 | 4.8 × 107 |
| Ic72 | 2.8 × 104 | 5.0 × 107 | 1.7 × 104 | 3.2 × 107 |
| Ic73 | 3.3 × 104 | 9.5 × 106 | 2.3 × 104 | 8.4 × 107 |
| Ic74 | 3.8 × 104 | 3.5 × 107 | 1.2 × 104 | 5.5 × 107 |
| Ic75 | 4.2 × 104 | 3.1 × 107 | 4.2 × 103 | 1.6 × 107 |
| I171 | 8.7 × 103 | 1.3 × 107 | 1.3 × 104 | 6.3 × 107 |
| I172 | 1.8 × 104 | 7.5 × 107 | 3.1 × 104 | 1.9 × 107 |
| I173 | 1.3 × 104 | 2.3 × 107 | 1.2 × 104 | 1.0 × 107 |
| I174 | 3.3 × 104 | 2.1 × 107 | 2.7 × 104 | 8.6 × 107 |
| I175 | 1.0 × 104 | 4.1 × 107 | 2.5 × 104 | 4.4 × 107 |
| Lc72 | 3.3 × 104 | 2.1 × 107 | 4.7 × 104 | 8.1 × 107 |
| L171 | 1.3 × 104 | 5.8 × 107 | 2.5 × 104 | 6.0 × 107 |
| M173 | 6.2 × 104 | 3.2 × 107 | 8.4 × 103 | 3.3 × 107 |
| B1201 | 1.1 × 104 | 8.2 × 107 | 5.5 × 104 | 6.0 × 107 |
| D1201 | 2.2 × 104 | 3.4 × 107 | 1.1 × 103 | 3.6 × 107 |
| D1202 | 2.9 × 104 | 6.2 × 107 | 2.6 × 103 | 1.6 × 107 |
| D1203 | 1.3 × 104 | 9.1 × 107 | 1.1 × 104 | 1.0 × 107 |
| D1204 | 2.2 × 104 | 8.7 × 107 | 2.0 × 104 | 7.6 × 106 |
| D1205 | 1.7 × 104 | 7.8 × 107 | 3.0 × 103 | 1.8 × 107 |
| F1201 | 1.3 × 103 | 1.2 × 107 | 2.6 × 103 | 2.4 × 107 |
| F1202 | 2.0 × 104 | 1.0 × 107 | 6.4 × 103 | 1.0 × 107 |
| F1204 | 4.6 × 104 | 1.0 × 107 | 8.6 × 103 | 1.0 × 107 |
| F1205 | 2.6 × 104 | 6.3 × 107 | 7.7 × 103 | 2.0 × 107 |
| Hc203 | 3.1 × 104 | 8.0 × 106 | 1.9 × 104 | 8.2 × 107 |
| H1201 | 3.7 × 104 | 1.2 × 107 | 1.2 × 104 | 9.2 × 107 |
| H1202 | 2.2 × 104 | 7.1 × 107 | 2.4 × 104 | 1.3 × 107 |
| H1203 | 2.8 × 104 | 6.3 × 107 | 9.5 × 103 | 1.8 × 107 |
| H1205 | 2.4 × 104 | 1.6 × 107 | 2.6 × 104 | 1.5 × 107 |
| Ic204 | 1.5 × 104 | 5.9 × 107 | 1.9 × 104 | 1.8 × 107 |
| I1203 | 4.3 × 104 | 1.0 × 107 | 2.2 × 104 | 1.6 × 107 |
| 2M | 2.0 × 104 | 8.2 × 107 | 1.7 × 104 | 3.0 × 107 |
| 7M | 3.0 × 103 | 7.4 × 107 | 4.0 × 104 | 2.9 × 107 |
| 9P | 2.6 × 103 | 8.0 × 107 | 9.0 × 103 | 1.5 × 107 |
| DSM20342 | 1.2 × 104 | 5.2 × 107 | 2.7 × 104 | 2.2 × 107 |
Ten samples of uninoculated meat were used as negative controls both in air and in vacuum packs.
Expressed as a mean based on duplicate experiments. Standard deviations were always lower than 20% of the means.
The volatile metabolites listed in Tables 2 to 5 were absent in sterile (control) samples; therefore, the VOCs were detected only when samples were inoculated with C. maltaromaticum strains. Experimental replicates were performed for 40% of the strains and failed to show qualitative differences, since the type and number of VOCs for each strain were the same; however, quantitative differences, ranging from 2 to 250%, were detected (data not shown).
Table 2.
Numbers of C. maltaromaticum strains associated with the production of specific VOCs in meat samples after 7 days of storage at 4°C in air and vacuum packs
| Compound | No. of strainsa in: |
|
|---|---|---|
| Air | Vacuum pack | |
| Sulfur compounds | ||
| Thiopheneb | 12 | 7 |
| Dimethyl sulfideb | 5 | 1 |
| 4-Methylthiophenolb | 12 | 19 |
| 2-Ethylthiopheneb | 3 | 3 |
| Dimethyl sulfoneb | 3 | 18 |
| Ketones | ||
| Acetoinc | 37 | 38 |
| 2-Heptanonec | 36 | 1 |
| 6-Methyl-2-heptanoneb | 2 | 0 |
| 3-Octanonec | 38 | 0 |
| Aldehydes | ||
| Hexanalc | 22 | 0 |
| Heptanalc | 1 | 0 |
| 2-Heptenalc | 6 | 0 |
| Benzaldehydec | 4 | 2 |
| 2-Octenalc | 15 | 4 |
| Nonanalc | 19 | 2 |
| 3,5-Dimethylbenzaldehydeb | 13 | 3 |
| Decanalc | 8 | 3 |
| Tetradecanalc | 14 | 2 |
| Alcohols | ||
| 3-Methyl-1-butanolc | 32 | 0 |
| 1-Hexanolc | 21 | 0 |
| 2-Butoxyethanolc | 12 | 0 |
| Heptanolc | 26 | 0 |
| 1-Octen-3-olc | 42 | 25 |
| 2-Ethyl-1-hexanolc | 31 | 19 |
| 1-Octanolc | 16 | 1 |
| 2-Octen-1-olc | 29 | 4 |
| Phenylethyl alcoholc | 8 | 4 |
| 3-Phenoxy-1-propanolb | 8 | 5 |
| Tridecanolb | 8 | 5 |
| Carboxylic acids | ||
| Butanoic acidc | 41 | 32 |
| Hexanoic acidc | 30 | 7 |
| Nonanoic acidc | 1 | 25 |
| Furan compound (2-pentylfuran)b | 31 | 2 |
Data are relative to the quantitative determination of the different compounds, including those occurring in traces.
Identified by comparison of the mass spectrum with the NIST library.
Identified by comparison with the mass spectrum and retention time of a known standard.
Table 5.
Mean levels of ketones, aldehydes, acids, and alcohols detected in the headspace of meat samples inoculated with 45 strains of C. maltaromaticum after 7 days of storage at 4°C in vacuum packs
| Strain | Amt of compound (μg)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ketone (acetoin) | Aldehydes |
Acids |
Alcohols |
|||||||
| Nonanal | Tetradecanal | Butanoic | Hexanoic | Nonanoic | 1-Octen-3-ol | 2-Ethyl-1-hexanol | 2-Octen-1-ol | Phenylethyl alcohol | ||
| H04 | 104.33 ± 0.70 | — | — | 1,890 ± 11 | — | — | — | — | — | — |
| I04 | 115.0 ± 2.2 | — | — | 363 ± 20 | — | — | — | — | — | — |
| I05 | 74.52 ± 0.56 | — | — | 1,705 ± 13 | — | — | — | — | — | — |
| B175 | 477.3 ± 2.2 | — | — | 25,853 ± 60 | — | — | 365.8 ± 3.6 | 6.86 ± 0.23 | — | — |
| F271 | 112.5 ± 1.4 | — | — | — | — | — | 92.08 ± 0.67 | 2.15 ± 0.13 | — | — |
| Hc71 | 126.39 ± 0.54 | — | — | 382.2 ± 1.2 | — | — | 142.3 ± 1.3 | 0.690 ± 0.020 | — | — |
| Hc73 | 43.57 ± 0.54 | — | — | 539.18 ± 0.89 | — | — | 17.54 ± 0.52 | — | — | — |
| H171 | 113.57 ± 0.71 | — | — | 3,400.0 ± 1.4 | — | — | — | 3.040 ± 0.090 | — | — |
| H172 | 126.8 ± 1.2 | — | — | 2,074 ± 14 | — | — | 69.15 ± 0.67 | — | — | — |
| H173 | 263.7 ± 4.4 | — | — | 8,599.3 ± 8.2 | 9,138.7 ± 8.6 | 34.44 ± 0.52 | 99.19 ± 0.66 | 1.22 ± 0.12 | — | — |
| H175 | 530.62 ± 0.61 | — | — | 3,681.0 ± 1.0 | — | — | — | — | — | 328.4 ± 1.6 |
| Ic72 | 306.2 ± 1.1 | 9.59 ± 0.23 | — | — | — | — | 361.1 ± 1.1 | 1.26 ± 0.18 | — | — |
| Ic73 | 10,933 ± 10 | — | — | 45,671 ± 15 | — | — | 211.9 ± 1.0 | — | — | — |
| Ic74 | — | — | — | 7,157.4 ± 3.0 | 327.8 ± 4.8 | — | — | — | — | 460.9 ± 1.0 |
| Ic75 | 68.58 ± 0.54 | — | — | 1,872.2 ± 2.8 | — | — | 137.61 ± 0.62 | — | — | — |
| I171 | 0.160 ± 0.030 | — | — | 7.78 ± 0.17 | — | — | 40.92 ± 0.94 | 0.640 ± 0.060 | — | — |
| I172 | 0.0800 ± 0.0010 | — | — | — | — | — | — | — | — | — |
| I173 | 323.7 ± 1.6 | — | — | 15,298.0 ± 2.6 | — | — | — | — | — | — |
| I174 | 14,644 ± 25 | — | — | 37,905.5 ± 5.2 | — | — | — | — | — | — |
| I175 | 651.0 ± 2.6 | — | — | 4,090.2 ± 2.0 | — | — | 36.85 ± 0.24 | 1.15 ± 0.14 | — | — |
| Lc72 | — | — | — | 6,446.7 ± 6.8 | — | — | 33.32 ± 0.60 | 0.830 ± 0.060 | 12.31 ± 0.59 | — |
| L171 | 0.230 ± 0.050 | 2.970 ± 0.080 | 0.310 ± 0.050 | 7,175 ± 22 | 2,450.2 ± 1.0 | — | — | 0.700 ± 0.020 | — | — |
| M173 | 1,451 ± 13 | — | — | 8,790.4 ± 2.4 | 4,486.2 ± 5.3 | 40.56 ± 0.59 | 319.36 ± 0.65 | — | — | — |
| B1201 | 396.0 ± 3.7 | — | — | 6,306.2 ± 6.2 | — | — | — | 6.070 ± 0.070 | — | — |
| D1201 | 224.5 ± 2.4 | — | — | 3,814 ± 15 | — | — | — | — | — | — |
| D1202 | 245.5 ± 9.7 | — | — | — | — | — | — | 1.89 ± 013 | — | — |
| D1203 | 36.56 ± 0.55 | — | — | 559.5 ± 3.2 | — | — | 86.2 ± 1.1 | 1.920 ± 0.040 | — | — |
| D1204 | 41.2 ± 1.1 | — | — | 926.9 ± 2.7 | — | — | — | 3.09 ± 0.17 | — | — |
| D1205 | 212.6 ± 8.7 | — | — | — | — | — | 252.6 ± 2.2 | — | — | — |
| F1201 | — | — | — | — | — | — | 141.64 ± 0.66 | 4.38 ± 0.34 | — | — |
| F1202 | 500.2 ± 2.0 | — | — | — | — | — | — | — | — | — |
| F1204 | 229.6 ± 1.6 | — | — | — | — | — | — | — | — | — |
| F1205 | 15.19 ± 0.26 | — | — | 1,088.4 ± 2.8 | — | — | 31.64 ± 0.57 | 0.890 ± 0.020 | — | — |
| Hc203 | 1,202 ± 17 | — | — | 5,208.6 ± 8.4 | — | — | 511.8 ± 1.5 | — | — | — |
| H1201 | 767.0 ± 3.6 | — | — | 5,993.7 ± 6.8 | 10,282 ± 16 | — | 258.7 ± 1.2 | — | — | — |
| H1202 | 373.5 ± 7.2 | — | 0.570 ± 0.050 | (339 ± 21)·10 | — | — | 239.41 ± 0.54 | — | 136.4 ± 4.1 | — |
| H1203 | 96.5 ± 1.0 | 8.89 ± 0.20 | — | — | — | — | 295.8 ± 1.0 | — | 1.84 ± 0.12 | — |
| H1205 | 92.1 ± 1.1 | — | — | — | — | — | 192.64 ± 0.48 | — | 140.0 ± 1.0 | — |
| Ic204 | — | — | — | 18,097 ± 22 | — | — | 267.1 ± 1.0 | — | — | 60.67 ± 0.60 |
| I1201 | — | — | — | — | — | — | — | — | — | — |
| I1203 | 241.4 ± 1.5 | — | — | 14,086 ± 16 | — | — | 255.7 ± 1.1 | 5.16 ± 0.22 | — | — |
| 2M | 73.76 ± 0.73 | — | — | — | — | — | — | — | — | — |
| 7M | 31.6 ± 1.2 | — | — | 14,362 ± 11 | 978.0 ± 2.7 | — | — | 0.440 ± 0.040 | — | — |
| 9P | 59.0 ± 1.0 | — | — | 12,695 ± 17 | 1,939 ± 19 | — | — | 18.25 ± 0.36 | — | 63.8 ± 1.6 |
| DSM20342 | 91.5 ± 2.7 | — | — | 3,756.8 ± 6.0 | — | — | 27.48 ± 0.52 | — | — | — |
Quantities were determined in the headspace of meat samples analyzed as described in Materials and Methods. The results are expressed as mean values for volatile metabolites from 3 “technical repeats” ± standard deviations. The volatile metabolites listed in this table were absent from sterile (control) samples. —, under the detection/quantification limit.
The volatile fraction of contaminated meat samples included esters, aldehydes, ketones, alcohols, carboxylic acids, and sulfur compounds (Table 2). All the meat samples inoculated and stored in air showed higher numbers of VOCs than the samples stored under a vacuum.
Compounds such as 3-methyl-1-butanol, heptanol, 3-octanone, and hexanal, which were detected in inoculated meat stored in air for 7 days at 4°C, were not detected in vacuum packages. The compounds detected from the majority of the inoculated meat samples in vacuum packaging were acetoin, butanoic acid, and 1-octen-3-ol (Table 2). Acetoin, 2-heptanone, 3-octanone, 3-methyl-1-butanol, heptanol, 1-octen-3-ol, 2-ethyl-1-hexanol, 2-octen-1-ol, butanoic acid, hexanoic acid, and 2-pentylfuran were produced in air by more than 50% of the strains (Table 2).
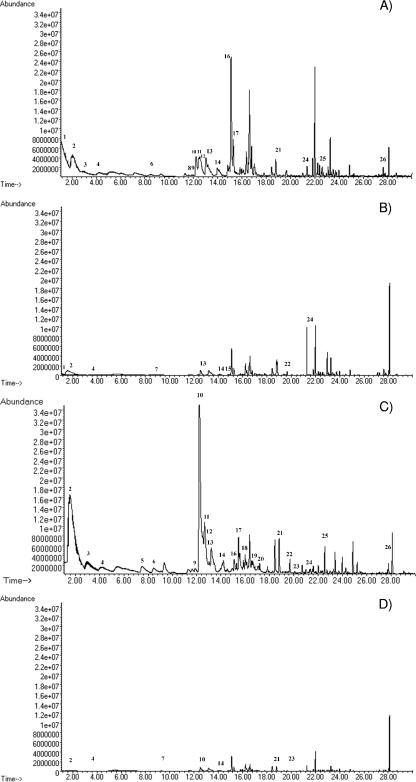
Representative chromatograms obtained for meat samples contaminated with two strains of C. maltaromaticum (D1203 and L171, which showed the highest numbers of metabolites in air and under a vacuum, respectively) after storage in air and vacuum packs at 4°C are reported in Fig. 2. The profiles clearly indicated that the occurrence of VOCs was influenced by the storage conditions.
Fig. 2.
Total ion current chromatograms obtained by HS-SPME-GC-MS analysis for meat samples contaminated with C. maltaromaticum D1203 and L171. The contaminated meat samples had initial viable counts of 104 CFU g−1 on CTSI agar, and the load increased to 107 CFU g−1 after 1 week of incubation at 4°C both in air and under a vacuum (Table 1). (A) D1203 after 7 days of storage in air; (B) D1203 after 7 days of storage in a vacuum pack; (C) L171 after 7 days of storage in air; (D) L171 after 7 days of storage in a vacuum pack. The numbers given alongside the spectra correspond to the different compounds detected, as follows: 1, thiophene; 2, acetoin; 3, 3-methyl-1-butanol; 4, butanoic acid; 5, 1-hexanol; 6, 2-heptanone; 7, dimethyl sulfide; 8, benzaldehyde; 9, heptanol; 10, 1-octen-3-ol; 11, 3-octanone; 12, 2-pentylfuran; 13, hexanoic acid; 14, 2-ethyl-1-hexanol; 15, 2-ethylthiophene; 16, 1-octanol; 17, 2-octenal; 18, 2-octen-1-ol; 19, nonanal; 20, phenylethyl alcohol; 21, 3,5-dimethylbenzaldehyde; 22, decanal; 23, 4-methylthiophenol; 24, 3-phenoxy-1-propanol; 25, tridecanol; 26, tetradecanal.
The quantities of VOCs in meat samples inoculated with 45 strains of C. maltaromaticum in air and vacuum packs are reported in Tables 3, 4, and 5. The highest number of VOCs was found in meat samples inoculated with strain D1203, I174, H1203, or L171 after 7 days of storage in air (Table 3). In contrast, about 93% of the strains showed fewer than six VOCs in stored vacuum-packed meat.
Table 3.
Mean levels of ketones, aldehydes, and acids detected in the headspace of meat samples inoculated with 45 strains of C. maltaromaticum after 7 days of storage at 4°C in air
| Strain | Amt of compound (μg)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ketones |
Aldehydes |
Acids |
||||||||
| Acetoin | 2-Heptanone | 3-Octanone | Hexanal | 2-Heptenal | 2-Octenal | Nonanal | Tetradecanal | Butanoic | Hexanoic | |
| H04 | 105.9 ± 2.9 | 4.43 ± 0.59 | — | — | — | — | 0.300 ± 0.020 | — | 3,433.3 ± 4.6 | — |
| I04 | — | — | — | — | — | — | 0.810 ± 0.040 | — | 10,149.6 ± 2.0 | — |
| I05 | 334.9 ± 3.6 | — | — | — | — | 1.37 ± 0.25 | — | — | 1,069.8 ± 1.2 | — |
| B175 | — | — | — | — | — | — | — | — | 15,548.6 ± 5.2 | 13,172.9 ± 3.8 |
| F271 | 41.1 ± 1.2 | — | — | — | — | 0.190 ± 0.070 | 0.0300 ± 0.0020 | — | 485.1 ± 3.0 | — |
| Hc71 | — | 132.1 ± 1.4 | 911.7 ± 2.9 | 4.19 ± 0.34 | — | — | — | 0.130 ± 0.030 | 5,698.8 ± 5.8 | 19,556.3 ± 3.7 |
| Hc73 | 1,357.8 ± 7.1 | — | — | — | — | — | — | — | 11,497.7 ± 4.4 | 16,017.4 ± 3.1 |
| H171 | 1,462.3 ± 9.2 | 70.1 ± 1.4 | 532.0 ± 3.2 | — | — | 3.10 ± 0.19 | — | — | 7,683.7 ± 2.1 | 12,333.4 ± 6.9 |
| H172 | 1,636.5 ± 4.5 | 38.6 ± 1.5 | 191.7 ± 1.0 | 0.140 ± 0.050 | — | — | 0.1100 ± 0.0020 | 0.150 ± 0.010 | 3,307.9 ± 4.6 | 4,080.4 ± 6.1 |
| H173 | 3,390.1 ± 9.2 | 171.1 ± 2.3 | 48.94 ± 0.95 | 8.17 ± 0.43 | — | — | 0.480 ± 0.050 | — | 21,817.0 ± 3.7 | — |
| H175 | 924.2 ± 5.5 | 38.9 ± 1.8 | 329.5 ± 2.0 | 3.12 ± 0.22 | — | 1.17 ± 0.29 | 0.290 ± 0.020 | — | 8,513.5 ± 3.6 | 7,273.3 ± 4.2 |
| Ic72 | 358.6 ± 3.2 | 139.2 ± 1.1 | 1,463.0 ± 1.0 | 6.16 ± 0.44 | — | — | — | — | 5,922.4 ± 4.3 | 23,291.1 ± 4.0 |
| Ic73 | 1,350.3 ± 6.2 | — | 281.7 ± 1.7 | — | — | — | — | — | 7,281.3 ± 3.4 | — |
| Ic74 | 208.7 ± 9.2 | 12.13 ± 0.65 | 58.57 ± 0.70 | — | — | 1.06 ± 0.12 | — | — | 2,129.7 ± 1.4 | — |
| Ic75 | 2.87 ± 0.70 | 0.830 ± 0.050 | 1.44 ± 0.45 | — | — | — | — | — | 128.8 ± 1.7 | — |
| I171 | — | — | — | — | — | 22.1 ± 1.1 | — | 0.060 ± 0.010 | 8,690.4 ± 4.7 | 3,853.0 ± 8.5 |
| I172 | 86.3 ± 1.5 | — | 22.33 ± 0.67 | 2.720 ± 0.040 | — | — | 0.150 ± 0.030 | — | 3,077.9 ± 2.1 | 8,960.0 ± 3.3 |
| I173 | 47.2 ± 2.0 | 8.32 ± 0.57 | 36.67 ± 0.86 | 1.09 ± 0.11 | — | 0.330 ± 0.030 | 0.0300 ± 0.0010 | — | 813.2 ± 4.9 | 1,044.0 ± 2.7 |
| I174 | 956.0 ± 3.6 | 579.4 ± 1.3 | 3,689 ± 14 | 49.90 ± 0.97 | — | — | 3.120 ± 0.050 | — | 30,054.6 ± 5.8 | 86,835.5 ± 4.4 |
| I175 | 90.3 ± 2.1 | 10.55 ± 0.53 | 30.54 ± 0.71 | — | — | 2.98 ± 0.11 | — | 0.0600 ± 0.0010 | 5,307.9 ± 2.7 | 1,359.6 ± 5.5 |
| Lc72 | 1,210.3 ± 9.7 | 160.7 ± 1.1 | 119.5 ± 1.5 | 21.38 ± 0.51 | — | — | — | — | 5,424.9 ± 7.8 | 26,646.0 ± 6.4 |
| L171 | 180.9 ± 1.9 | 161.7 ± 2.3 | 1,084.0 ± 4.5 | — | — | — | — | 0.170 ± 0.010 | 6,686.8 ± 5.6 | 20,244.9 ± 7.6 |
| M173 | 273.5 ± 1.4 | — | 71.1 ± 2.1 | — | — | — | 0.110 ± 0.020 | — | 7,256.0 ± 5.5 | 8,282.7 ± 4.6 |
| B1201 | — | — | 151.0 ± 4.1 | — | — | — | — | — | 25,127.6 ± 3.8 | 34,896.1 ± 5.4 |
| D1201 | 697.2 ± 6.6 | 313.6 ± 2.7 | 281.3 ± 1.6 | — | — | — | — | — | 18,880.5 ± 8.6 | — |
| D1202 | 2,198.0 ± 9.6 | 379.9 ± 1.8 | 181.9 ± 1.5 | 17.89 ± 0.54 | — | — | — | 0.0900 ± 0.0010 | 18,668.2 ± 8.9 | 38,609.92 ± 8.14 |
| D1203 | 2,102.6 ± 6.7 | 329.69 ± 0.91 | 147.70 ± 0.62 | — | — | 0.440 ± 0.070 | 0.170 ± 0.040 | 0.0600 ± 0.0020 | 5,820.9 ± 7.5 | 21,660.8 ± 8.5 |
| D1204 | 425.71 ± 0.89 | 133.3 ± 1.9 | 90.9 ± 1.1 | 5.09 ± 0.48 | — | — | 0.0400 ± 0.0040 | — | 3,039.1 ± 3.6 | 13,042.0 ± 2.8 |
| D1205 | 514.2 ± 1.3 | 1,439.2 ± 8.5 | 235.78 ± 0.90 | — | — | 1.67 ± 0.11 | 0.4200 ± 0.0040 | — | — | 30,974 ± 11 |
| F1201 | — | 5.03 ± 0.49 | 60.87 ± 0.87 | — | — | — | — | 0.0200 ± 0.0010 | 1,108.8 ± 3.1 | — |
| F1202 | 5.31 ± 0.54 | 97.75 ± 0.74 | 394.2 ± 4.2 | — | — | — | — | — | — | 12,392.8 ± 4.2 |
| F1204 | — | 409.1 ± 1.5 | 1,856 ± 12 | — | — | — | — | 0.5300 ± 0.0040 | — | — |
| F1205 | 28.2 ± 1.3 | 24.9 ± 1.0 | 51.9 ± 1.4 | 7.72 ± 0.45 | 0.390 ± 0.020 | — | — | — | 787.6 ± 2.3 | 13,077.5 ± 7.4 |
| Hc203 | — | 468.46 ± 0.91 | 1,816 ± 14 | — | — | — | — | 0.9800 ± 0.0040 | — | 81,470 ± 17 |
| H1201 | — | 84.4 ± 1.0 | 256.2 ± 4.7 | — | — | — | — | — | 5,620.4 ± 4.2 | 14,828.4 ± 3.4 |
| H1202 | 189.6 ± 3.2 | 103.03 ± 0.48 | 176.5 ± 1.7 | 29.42 ± 0.65 | 0.850 ± 0.090 | 0.530 ± 0.050 | 0.4200 ± 0.0010 | — | 2,853.8 ± 5.3 | 27,715 ± 15 |
| H1203 | 48.2 ± 1.0 | 53.86 ± 0.65 | 293.7 ± 2.8 | 22.02 ± 0.36 | 0.950 ± 0.060 | — | 0.4400 ± 0.0040 | 0.1200 ± 0.0040 | 1,222.7 ± 4.8 | 19,618.2 ± 8.5 |
| H1205 | 22.8 ± 1.2 | 32.17 ± 0.49 | 200.7 ± 3.6 | 5.20 ± 0.24 | 0.060 ± 0.020 | — | — | 0.0700 ± 0.0020 | 811.9 ± 3.6 | 7,377.8 ± 8.4 |
| Ic204 | 643.8 ± 5.1 | 162.85 ± 0.81 | 255.4 ± 3.4 | 5.13 ± 0.23 | 3.15 ± 0.27 | — | 0.180 ± 0.030 | 0.0700 ± 0.0010 | 501.5 ± 3.8 | 7,159.7 ± 5.6 |
| I1201 | 18.6 ± 1.0 | 41.57 ± 0.57 | 501.6 ± 2.2 | 94.43 ± 0.86 | 2.22 ± 0.38 | — | 1.09 ± 0.10 | 0.0800 ± 0.0040 | 897.6 ± 4.5 | — |
| I1203 | — | 293.9 ± 1.2 | 1,856 ± 14 | — | — | — | — | — | — | — |
| 2M | 12,991.4 ± 8.7 | 1,441.4 ± 3.3 | 5,874 ± 19 | 56.63 ± 0.59 | — | 2.040 ± 0.080 | — | — | 33,577 ± 16 | — |
| 7M | — | 9.90 ± 0.39 | 12.2 ± 1.5 | 1.19 ± 0.26 | — | 0.53 ± 0.11 | — | — | 547.7 ± 6.0 | 648.5 ± 3.2 |
| 9P | 5,672.9 ± 2.4 | — | — | — | — | — | — | — | 7,377.4 ± 8.1 | 4,450 ± 15 |
| DSM20342 | 70.3 ± 1.5 | 23.7 ± 1.7 | 174.8 ± 2.0 | 0.460 ± 0.080 | — | — | — | — | 3,663.6 ± 5.8 | — |
Quantities were determined in the headspace of meat samples analyzed as described in Materials and Methods. The results are expressed as mean values for volatile metabolites from 3 “technical repeats” ± standard deviations. The volatile metabolites listed in this table were absent from sterile (control) samples. —, under the detection/quantification limit.
Table 4.
Mean levels of alcohols detected in the headspace of meat samples inoculated with 45 strains of C. maltaromaticum after 7 days of storage at 4°C in air
| Strain | Amt of alcohol (μg)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3-Methyl-1-butanol | 1-Hexanol | 2-Butoxyethanol | Heptanol | 1-Octen-3-ol | 2-Ethyl-1-hexanol | 2-Octen-1-ol | 1-Octanol | Phenylethyl alcohol | |
| H04 | — | — | 41.63 ± 0.69 | — | — | — | — | — | — |
| I04 | — | — | 311 ± 11 | — | — | — | — | — | — |
| I05 | — | — | 13.53 ± 0.53 | — | 2.56 ± 0.51 | — | — | — | — |
| B175 | 90.6 ± 1.6 | 33.01 ± 0.46 | 17.22 ± 0.48 | 10.11 ± 0.33 | — | — | — | — | — |
| F271 | — | — | 3.37 ± 0.49 | — | 0.610 ± 0.030 | — | — | — | — |
| Hc71 | 142.2 ± 7.4 | — | — | — | 203.83 ± 0.40 | 4.18 ± 0.32 | 4.46 ± 0.42 | — | — |
| Hc73 | 117.5 ± 4.6 | 34.93 ± 0.13 | 60.72 ± 0.56 | 16.61 ± 0.53 | 206.75 ± 0.67 | 1.52 ± 0.32 | — | — | — |
| H171 | 93.1 ± 5.2 | 26.58 ± 0.53 | 127.94 ± 0.35 | 11.07 ± 0.53 | 144.5 ± 1.2 | — | 2.84 ± 0.26 | — | — |
| H172 | 30.7 ± 4.7 | 7.23 ± 0.41 | — | — | 48.58 ± 0.54 | 5.20 ± 0.20 | 1.54 ± 0.32 | — | — |
| H173 | 160.4 ± 8.5 | 41.61 ± 0.55 | 40.60 ± 0.57 | 15.63 ± 0.53 | 249.73 ± 0.75 | — | 6.030 ± 0.540 | — | — |
| H175 | 38.6 ± 1.5 | 11.61 ± 0.67 | 46.72 ± 0.76 | 3.930 ± 0.080 | 53.54 ± 0.80 | 1.94 ± 0.16 | 1.14 ± 0.14 | — | — |
| Ic72 | 161.7 ± 8.4 | — | — | — | 239.31 ± 0.63 | 20.47 ± 0.56 | 11.00 ± 0.24 | 17.17 ± 0.35 | 1,004.5 ± 3.0 |
| Ic73 | 39.40 ± 0.98 | 12.62 ± 0.65 | 81.00 ± 0.17 | — | 43.3 ± 1.2 | 0.590 ± 0.030 | 1.62 ± 0.21 | — | — |
| Ic74 | 22.1 ± 1.6 | — | — | — | 17.67 ± 0.74 | 1.77 ± 0.20 | — | — | — |
| Ic75 | — | — | — | 3.42 ± 0.70 | 0.940 ± 0.080 | 0.090 ± 0.010 | — | — | — |
| I171 | 52.5 ± 2.4 | — | — | — | 1.23 ± 0.25 | 0.44 ± 0.13 | — | — | — |
| I172 | 41.2 ± 1.6 | 10.62 ± 0.65 | — | 3.25 ± 0.43 | 66.3 ± 1.3 | 14.3 ± 1.1 | 2.79 ± 0.21 | 0.290 ± 0.030 | 15.12 ± 0.66 |
| I173 | 7.07 ± 0.85 | 1.26 ± 0.37 | — | — | 8.99 ± 0.45 | 1.08 ± 0.14 | 0.550 ± 0.070 | 0.150 ± 0.040 | 6.43 ± 0.40 |
| I174 | 481.6 ± 7.6 | 179.20 ± 0.91 | — | 36.67 ± 0.56 | 919.56 ± 0.97 | 46.9 ± 1.9 | 26.990 ± 0.080 | 1.930 ± 0.080 | 113.9 ± 8.6 |
| I175 | — | — | — | — | 49.08 ± 0.40 | — | — | — | — |
| Lc72 | 129.8 ± 8.4 | — | — | 18.34 ± 0.57 | 3.82 ± 0.24 | 0.810 ± 0.030 | — | — | — |
| L171 | 101.5 ± 5.2 | — | — | 10.45 ± 0.51 | 105.25 ± 0.24 | 0.91 ± 0.14 | 1.31 ± 0.33 | 19.62 ± 0.64 | 1,204.1 ± 7.7 |
| M173 | 72.8 ± 6.1 | 21.85 ± 0.77 | 12.99 ± 0.22 | 4.57 ± 0.51 | 128.10 ± 0.32 | 0.910 ± 0.070 | 4.73 ± 0.23 | 0.300 ± 0.010 | 18.67 ± 0.39 |
| B1201 | 242.2 ± 8.4 | 149.97 ± 0.47 | — | 30.03 ± 0.45 | 786.2 ± 1.1 | 24.65 ± 0.33 | 29.75 ± 0.51 | 6.43 ± 0.89 | 431 ± 11 |
| D1201 | 159 ± 11 | 96.41 ± 0.57 | — | 15.81 ± 0.33 | 475.98 ± 0.16 | — | 18.05 ± 0.59 | 0.810 ± 0.050 | 45.0 ± 1.5 |
| D1202 | 185 ± 11 | 92.25 ± 0.53 | — | 18.42 ± 0.38 | 480.93 ± 0.32 | 6.47 ± 0.31 | 12.86 ± 0.26 | 1.04 ± 0.22 | 77.2 ± 2.6 |
| D1203 | 195.3 ± 9.5 | 151.93 ± 0.65 | — | 13.19 ± 0.32 | 426.4 ± 1.0 | 1.070 ± 0.080 | 23.17 ± 0.33 | 0.930 ± 0.080 | 49.95 ± 0.15 |
| D1204 | 61.5 ± 1.3 | 76.17 ± 0.40 | — | 7.37 ± 0.69 | 171.70 ± 0.84 | 5.84 ± 0.37 | 13.03 ± 0.22 | 0.650 ± 0.030 | — |
| D1205 | 120 ± 10 | 74.24 ± 0.51 | — | 7.01 ± 0.20 | 275.83 ± 0.92 | 0.660 ± 0.070 | 10.78 ± 0.27 | 5.16 ± 0.47 | 339 ± 12 |
| F1201 | 5.4 ± 2.9 | — | 28.3 ± 2.6 | — | 11.17 ± 0.39 | 0.44 ± 0.12 | — | — | — |
| F1202 | — | — | — | — | 172.8 ± 1.1 | 6.58 ± 0.42 | 9.60 ± 0.62 | — | — |
| F1204 | 28.0 ± 3.0 | — | — | — | 905.40 ± 0.83 | 66.51 ± 0.58 | — | — | — |
| F1205 | 16.9 ± 5.8 | — | — | 2.03 ± 0.16 | 48.42 ± 0.52 | — | 3.39 ± 0.34 | — | — |
| Hc203 | — | — | — | 148.5 ± 9.8 | 1254.5 ± 1.2 | 47.63 ± 0.64 | 62.12 ± 0.31 | — | — |
| H1201 | 162.3 ± 9.7 | — | — | — | 103.11 ± 0.96 | 5.11 ± 0.14 | 0.950 ± 0.070 | — | — |
| H1202 | 53.8 ± 5.5 | 65.1 ± 2.3 | — | 5.15 ± 0.37 | 137.61 ± 0.38 | — | 11.080 ± 0.080 | — | — |
| H1203 | 45.8 ± 2.5 | 39.8 ± 1.3 | — | 5.24 ± 0.40 | 125.54 ± 0.51 | — | 12.71 ± 0.39 | 0.190 ± 0.020 | 12.10 ± 0.30 |
| H1205 | 31.9 ± 4.3 | — | 4.86 ± 0.23 | 4.18 ± 0.33 | 91.38 ± 0.47 | — | 9.48 ± 0.47 | 0.160 ± 0.020 | 10.21 ± 0.30 |
| Ic204 | 110.0 ± 8.6 | 1,398.6 ± 2.3 | — | 50.45 ± 0.59 | 288.72 ± 0.94 | — | 22.16 ± 0.34 | 1.37 ± 0.18 | 93.8 ± 5.2 |
| I1201 | 29.5 ± 2.5 | 55.44 ± 0.78 | — | 4.90 ± 0.37 | 92.990 ± 0.030 | 0.98 ± 0.12 | 11.67 ± 0.41 | 0.54 ± 0.10 | 38.57 ± 0.61 |
| I1203 | — | — | — | — | 524.77 ± 0.31 | 13.97 ± 0.11 | 30.050 ± 0.330 | — | — |
| 2M | 604 ± 10 | — | — | 198.7 ± 6.8 | 2,997.5 ± 3.4 | 36.8 ± 1.0 | — | — | — |
| 7M | — | — | — | — | 40.25 ± 0.78 | 0.310 ± 0.030 | 0.59 ± 0.16 | — | — |
| 9P | — | — | — | — | 254.60 ± 0.55 | 9.14 ± 0.25 | — | — | — |
| DSM20342 | — | — | — | 2.95 ± 0.15 | 22.06 ± 0.99 | 0.300 ± 0.030 | — | — | — |
Quantities were determined in the headspace of meat samples analyzed as described in Materials and Methods. The results are expressed as mean values for volatile metabolites from 3 “technical repeats” ± standard deviations. The volatile metabolites listed in this table were absent from sterile (control) samples. —, under the detection/quantification limit.
Five aldehydes were detected during air storage, with hexanal showing the highest quantity (Table 3). Conversely, in vacuum-packed meat, only nonanal and tetradecanal were detected, and their concentrations were low (Table 5). Acetoin was the ketone most commonly found in the headspace of meat samples stored in air and in vacuum packaging (Tables 3 and 5). High levels of acetoin were detected in samples inoculated with strain 2M, 9P, or H173 and stored in air (Table 3) and in samples inoculated with strain I174 or Ic73 and stored in vacuum packs (Table 5). High levels of 3-octanone were recorded for meat samples inoculated with C. maltaromaticum strain 2M or I174 and stored in air (Table 3). Butanoic acid was observed in most of the inoculated meat samples under both storage conditions. Strains 2M and Ic73 were the strongest producers of butanoic acid in air storage and vacuum packaging, respectively (Tables 3 and 5). Finally, the levels of alcohols detected were also higher in air-stored meat (Tables 4 and 5). The highest quantities of 1-octen-3-ol and 3-methyl-1-butanol were found in samples stored in air and were present in samples contaminated by most strains (Table 4). In vacuum-packed meat, 1-octen-3-ol was the most abundant alcohol produced (Table 5).
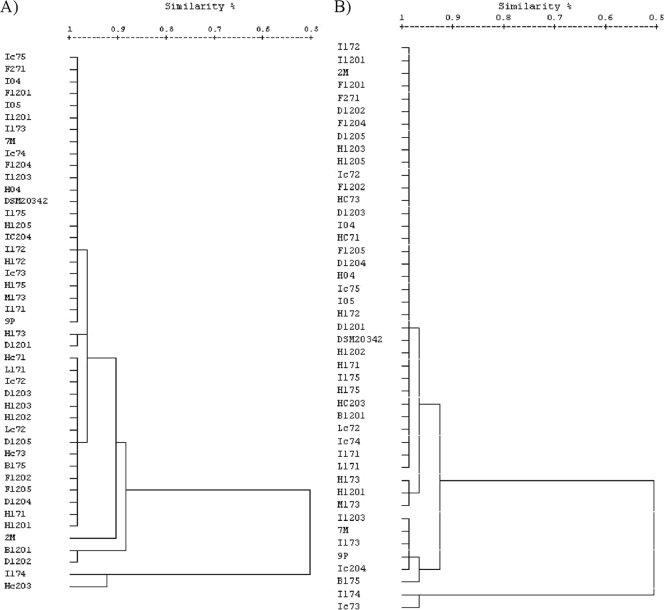
The similarity dendrograms based on quantitative data showed that, within the same storage conditions, a very high level of similarity was found between the 45 strains used (Fig. 3).
Fig. 3.
Similarity dendrograms based on quantitative data for VOCs detected in meat inoculated with 45 strains of C. maltaromaticum. (A) C. maltaromaticum strains after 7 days of storage in air at 4°C. (B) C. maltaromaticum strains after 7 days of storage in vacuum packs.
Sensory analysis.
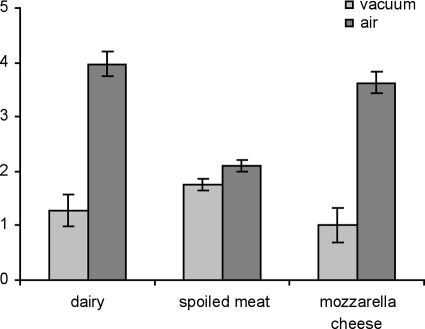
ANOVA results showed no significant strain effect (P > 0.05) for any of the sensory odors; thus, no sensory differences were found between inoculated and control samples. However, the storage conditions significantly (P < 0.05) affected the intensity of dairy, spoiled-meat, and mozzarella cheese odors (Table 6). In particular, dairy, spoiled-meat, and mozzarella cheese odors were perceived as more intense in samples stored in air than in those stored in vacuum packs (Fig. 4). In all cases, the odor intensity was lower than the average intensity (5.0) in the evaluation scale (from 0.0 to 10.0). The perceived differences in intensity were more evident for dairy and mozzarella cheese odors than for the spoiled-meat odor, for which mean values ranged from 1.7 to 2.1 (Fig. 4).
Table 6.
Effects of the strain inoculateda and the storage condition on the meat odor profile as determined by ANOVA
| Odor |
P |
|
|---|---|---|
| Strain | Storage condition | |
| Salami | 0.96 | 0.66 |
| Dairy | 0.29 | <0.001 |
| Pungent | 0.98 | 0.21 |
| Mushroom | 0.88 | 0.17 |
| Spoiled meat | 0.68 | 0.03 |
| Putrid | 0.67 | 0.11 |
| Mozzarella cheese | 0.94 | <0.001 |
| Meat broth | 0.53 | 0.43 |
| Fruity | 0.90 | 0.91 |
| Yeast | 0.77 | 0.49 |
| Ammoniac | 0.71 | 0.45 |
| Alcohol | 0.92 | 0.98 |
| Canned legumes | 0.28 | 0.06 |
n = 10 (9 inoculated samples and 1 control sample).
Fig. 4.
Mean odor intensities (± standard errors) of samples stored in air and in vacuum packs. Both the means and the standard errors were calculated across all the samples (n = 10).
DISCUSSION
Enterobacteriaceae, B. thermosphacta, Pseudomonas spp., and LAB are frequently found in meat and are reported to be important players in meat spoilage dynamics (13, 33, 38). C. divergens and C. maltaromaticum strains were dominant in vacuum-packaged meat (19) and have been found to possess extensive potential for meat spoilage (12, 25). Our isolates were identified by 16S rRNA gene sequencing and were biotyped by RAPD-PCR. Almost all the strains were able to grow at 4, 10, and 20°C, while only 10 strains grew at 40°C. Generally, the growth curve parameters were affected by pH and salinity. Our results are in agreement with those of other authors who found that a C. divergens strain was able to grow between 0 and 37°C, in 0 to 10% (wt/vol) NaCl, and at pH 5 to 10 (37), and also with those of another study where Carnobacterium spp. were able to grow between 0 and 30°C, in 0 to 6% (wt/vol) NaCl, and at pH 5.5 to 9.1 (20). The results obtained in this study showed that 89% of the strains could be regarded as psychrotrophic bacteria, while 100% of the strains grew at pH 6 to 9 and in the presence of 2.5% NaCl. Acidity and salt concentrations negatively affected the growth of C. maltaromaticum. Hammes and Hertel (17) emphasized that the nonaciduric behavior of C. maltaromaticum is the reason why it has rarely been isolated from fermented sausages.
The roles of 45 C. maltaromaticum strains in the development of spoilage-related molecules during meat storage were evaluated. A higher diversity of VOCs was found in samples analyzed after storage in air than in samples stored under a vacuum. Since the 45 C. maltaromaticum strains analyzed could grow both in air and in vacuum packs, it was the packaging conditions that induced changes in the metabolic activities of Carnobacterium spp., as previously reported (28). The strains that induced high frequencies of certain molecules when meat was stored in air did not necessarily determine high quantities of the same compounds during storage in vacuum packs. The compounds most frequently found under both storage conditions were acetoin, 1-octen-3-ol, and butanoic acid. Acetoin was produced by more than 80% of the strains under both storage conditions.
C. divergens and C. maltaromaticum are able to use ribose and gluconic acid as substrates for growth (4, 10) and may also produce acetoin, diacetyl, and 2,3-butanediol upon citrate consumption (3, 16). Both species have been shown to produce acetate, acetoin, CO2, ethanol, formate, lactate, and many alcohols from glucose in laboratory media (6). High concentrations of acetoin from C. maltaromaticum have also been found in a sausage mince (24). Moreover, high concentrations of diacetyl have been found in batches of shrimp inoculated with different mixtures of C. maltaromaticum and C. divergens (25).
Acetoin is considered an important component of flavor. Its presence is related to a creamy dairy odor (40, 41), and Dainty et al. (9) have reported that the accumulation of acetoin was not unpleasant and caused the meat to be regarded not as spoiled but rather as “not fresh.” Another important odor-active volatile compound detected in this study was 1-octen-3-ol, a common oxidized product from lipids, which was also found in meat and shrimp inoculated with strains of C. maltaromaticum and C. divergens (12, 26). Butanoic acid was the main carboxylic acid produced in all the samples analyzed. This compound is commonly associated with meat spoilage (19) and can derive from microbial consumption of free amino acids via the Stickland reaction (29). There was considerable production of alcohols and aldehydes in all meat samples inoculated with different C. maltaromaticum strains and stored in air at 4°C. 3-Methyl-1-butanol and 2-ethyl-1-hexanol were the most frequently detected alcohols after 1-octen-3-ol. High concentrations of 3-methyl-1-butanol were found in shrimp inoculated with C. maltaromaticum (26). Several aldehydes and alcohols known to derive from lipid oxidation in meat are absent from samples stored under a vacuum. 3-Methyl-1-butanol from leucine catabolism was detected only in samples stored in air, while phenylethyl alcohol and benzaldehyde derived from phenylalanine catabolism occurred only at low frequencies under a vacuum in comparison with storage in air (14, 40). Some ketones, such as 6-methyl-2-heptanone and 3-octanone, were also detected only in air. These results clearly indicate a metabolic shift of the C. maltaromaticum strains during growth in meat stored in vacuum packs.
On the basis of the occurrence and concentrations of 3-methyl-1-butanol, 1-octen-3-ol, butanoic acid, and acetoin under both storage conditions, these could be regarded as the main spoilage molecules produced by Carnobacterium spp. 1-Octen-3-ol is reported to be a key odorant in most cheeses and is a main odor-active compound of soft cheese (8). 3-Methyl-1-butanol with acetoin/diacetyl is associated with cheesy odors of meat (5), while butanoic acid, with a rancid cheese-like odor, plays an important role in the flavor of many cheeses (8) and is commonly associated with meat spoilage (19).
In order to investigate the effects of the different strains and storage conditions and the synergistic effect of VOCs on the sensory impact of meat, sensory evaluation of the contaminated meat samples was performed. Sensory analysis showed that the panelists could distinguish between the samples on the basis of the storage conditions, but the strains did not produce different sensory profiles. Accordingly, similarity dendrograms of quantitative GC-MS data indicated that, within the same storage conditions, the 45 different C. maltaromaticum strains displayed a very high level of similarity, suggesting that within this species there is weak strain variability in the metabolic activities performed in meat. The dairy and mozzarella cheese odors enabled a clear differentiation of the storage conditions, since the samples stored in air smelled much more of these odors than meat stored in vacuum packs. This result is probably due to the occurrence and concentrations of the dominant molecules and is also supported by quantitative data on VOCs. The strains involved in the sensory evaluation produced high concentrations of 1-octen-3-ol and 3-methyl-1-butanol during storage in air, which could result in the dairy and mozzarella cheese off-odors in meat. As reported above, 1-octen-3-ol can be a determinant in the flavor of some cheeses (8), while 3-methyl-1-butanol, found only in air, is also identified as a compound that confers a pleasant aroma of fresh mozzarella (31). In similar research, the same panel used in this study evaluated the sensory impact of Pseudomonas fragi strains in meat (11). The sensory scores for the different descriptors were higher in P. fragi-inoculated meat (11) than in the meat samples studied here, which can be considered a further confirmation of the low sensory impact of Carnobacterium spp. in meat. This is certainly a favorable finding for the possible employment of specific strains as protective cultures.
In conclusion, different molecular types of C. maltaromaticum can grow efficiently in meat stored at low temperatures both in air and in vacuum packs, producing volatile molecules with potential odor impacts. The release of such molecules is more evident during storage in air than under a vacuum, even though these molecules do not necessarily affect the sensory quality of meat. Although C. maltaromaticum is recognized as one of the dominant LAB occurring during meat storage, the contribution of cultures of carnobacteria to meat spoilage in the absence of other microorganisms is negligible at bacterial levels reaching log 7 CFU g−1.
Supplementary Material
ACKNOWLEDGMENTS
This study was partly supported by an EU project (SYMBIOSIS-EU) within the 7th Framework Programme (grant agreement 211638).
The information in this article reflects only the authors' views, and the Community is not liable for any use that may be made of the information contained herein.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 22 July 2011.
REFERENCES
- 1. Afzal M. I., et al. 2010. Carnobacterium maltaromaticum: identification, isolation tools, ecology and technological aspects in dairy products. Food Microbiol. 27:573–579 [DOI] [PubMed] [Google Scholar]
- 2. Altschul S. F., et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Axelsson L. 2008. Lactic acid bacteria: classification and physiology, p. 19–66 In Salminen S., von Wright A., Ouwehand A. (ed.), Lactic acid bacteria: microbiological and functional aspects. Marcel Dekker, New York, NY [Google Scholar]
- 4. Borch E., Molin G. 1989. The aerobic growth and product formation of Lactobacillus, Leuconostoc, Brochothrix, and Carnobacterium in batch cultures. Appl. Microbiol. Biotechnol. 30:81–88 [Google Scholar]
- 5. Borch E., Kant-Muermans M. L., Blixt Y. 1996. Bacterial spoilage of meat and cured meat products. Int. J. Food Microbiol. 33:103–120 [DOI] [PubMed] [Google Scholar]
- 6. Brillet A., Pilet M. F., Prevost H., Cardinal M., Leroi F. 2005. Effect of inoculation of Carnobacterium divergens V41, a biopreservative strain against Listeria monocytogenes risk, on the microbiological, chemical and sensory quality of cold-smoked salmon. Int. J. Food Microbiol. 104:309–324 [DOI] [PubMed] [Google Scholar]
- 7. Chenoll E., Macian M. C., Elizaquıvel P., Aznar R. 2007. Lactic acid bacteria associated with vacuum-packed cooked meat product spoilage: population analysis by rDNA-based methods. J. Appl. Microbiol. 102:498–508 [DOI] [PubMed] [Google Scholar]
- 8. Curioni P. M. G., Bosset J. O. 2002. Key odorants in various cheese types as determined by gas chromatography-olfactometry. Int. Dairy J. 12:959–984 [Google Scholar]
- 9. Dainty R. H., Edwards R. A., Hibbard C. M., Marnewick J. J. 1989. Volatile compounds associated with microbial growth on normal and high pH beef stored at chill temperatures. J. Appl. Bacteriol. 66:281–289 [DOI] [PubMed] [Google Scholar]
- 10. De Bruyn I., Holzapfel W. H., Wisser L., Louw A. I. 1988. Glucose metabolism by Lactobacillus divergens. J. Gen. Microbiol. 134:2103–2109 [DOI] [PubMed] [Google Scholar]
- 11. Ercolini D., et al. 2010. Different biotypes of Pseudomonas fragi have the same overall potential as spoilage agents of meat. Int. J. Food Microbiol. 142:120–131 [DOI] [PubMed] [Google Scholar]
- 12. Ercolini D., Russo F., Nasi A., Ferranti P., Villani F. 2009. Mesophilic and psychrotrophic bacteria from meat and their spoilage potential in vitro and in beef. Appl. Environ. Microbiol. 75:1990–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ercolini D., Russo F., Torrieri E., Masi P., Villani F. 2006. Changes in the spoilage-related microbiota of beef during refrigerated storage under different packaging conditions. Appl. Environ. Microbiol. 72:4663–4671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gänzle G., Vermeulen N., Vogel R. F. 2007. Carbohydrate, peptide and lipid metabolism of lactic acid bacteria in sourdough. Food Microbiol. 24:128–138 [DOI] [PubMed] [Google Scholar]
- 15. Gonzalez C. J., Encinas J. P., Garcıa-Lopez M. L., Otero A. 2000. Characterization and identification of lactic acid bacteria from freshwater fishes. Food Microbiol. 17:383–391 [Google Scholar]
- 16. Güzel-Seydim Z. B., Seydim A. C., Greene A. K., Bodine A. B. 2000. Determination of organic acids and volatile flavor substances in kefir during fermentation. J. Food Composition Anal. 13:35–43 [Google Scholar]
- 17. Hammes W. P., Hertel C. 15 December 2003, posting date. The genera Lactobacillus and Carnobacterium, p. 320–403 In Dworkin M. (ed.), The prokaryotes: an evolving electronic resource for the microbiological community. Springer-Verlag, New York, NY [Google Scholar]
- 18. Immanuel G., Palanichamy E., Jebadhas A., Iyapparaj P., Palavesam A. 2008. Investigation of lipase production by milk isolate Serratia rubidaea. Food Technol. Biotechnol. 46:60–65 [Google Scholar]
- 19. Jones R. J. 2004. Observations on the succession dynamics of lactic acid bacteria populations in chill-stored vacuum packaged beef. Int. J. Food Microbiol. 90:273–282 [DOI] [PubMed] [Google Scholar]
- 20. Kim D. H., Austin B. 2008. Characterization of probiotic carnobacteria isolated from rainbow trout (Oncorhynchus mykiss) intestine. Lett. Appl. Microbiol. 47:141–147 [DOI] [PubMed] [Google Scholar]
- 21. Kim M. S., Roh S. W., Nam Y. D., Yoon J. H., Bae J. W. 2009. Carnobacterium jeotgali sp. nov, isolated from traditional fermented food in Korea. Int. J. Syst. Evol. Microbiol. 59:3168–3171 [DOI] [PubMed] [Google Scholar]
- 22. Larrouture C., Ardaillon V., Pepin M., Montel M. C. 2000. Ability of meat starter culture to catabolize leucine and evaluation of the degradation products by using an HPLC method. Food Microbiol. 17:563–570 [Google Scholar]
- 23. Larrouture-Thiveyrat C., Montel M. C. 2003. Effects of environmental factors on leucine catabolism by Carnobacterium piscicola. Int. J. Food Microbiol. 81:177–184 [DOI] [PubMed] [Google Scholar]
- 24. Larrouture-Thiveyrat C., Pepin M., Leroy-Sétrin S., Montel M. C. 2003. Effect of Carnobacterium piscicola on aroma formation in sausage mince. Meat Sci. 63:423–426 [DOI] [PubMed] [Google Scholar]
- 25. Laursen B. G., et al. 2005. Carnobacterium divergens and Carnobacterium maltaromaticum as spoilers or protective cultures in meat and seafood: phenotypic and genotypic characterization. Syst. Appl. Microbiol. 28:151–164 [DOI] [PubMed] [Google Scholar]
- 26. Laursen B. G., Leisner J. J., Dalgaard P. 2006. Carnobacterium species: effect of metabolic activity and interaction with Brochothrix thermosphacta on sensory characteristics of modified atmosphere packed shrimp. J. Agric. Food Chem. 54:3604–3611 [DOI] [PubMed] [Google Scholar]
- 27. Leisner J. J., Laursen B. G., Prevost H., Drider D., Dalgaard P. 2007. Carnobacterium: positive and negative effects in the environment and in foods. FEMS Microbiol. Rev. 31:592–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leisner J. J., Greer G. G., Dilts B. D., Stiles M. E. 1995. Effect of growth of selected lactic acid bacteria on storage life of beef stored under vacuum and in air. Int. J. Food Microbiol. 26:231–243 [DOI] [PubMed] [Google Scholar]
- 29. Martín A., et al. 2010. Characterization by volatile compounds of microbial deep spoilage in Iberian dry-cured ham. J. Food Sci. 75:360–366 [DOI] [PubMed] [Google Scholar]
- 30. Mauriello G., Casaburi A., Villani F. 2002. Proteolytic activity of Staphylococcus xylosus strains on pork myofibrillar and sarcoplasmic proteins and use of selected strains in the production of “Naples type” salami. J. Appl. Microbiol. 92:482–490 [DOI] [PubMed] [Google Scholar]
- 31. Moio L., Langlois D., Etilevant P. X., Addeo F. 1993. Powerful odorants in water buffalo and bovine Mozzarella cheese by use of extract dilution sniffing analysis. Italian J. Food Sci. 3:227–237 [Google Scholar]
- 32. Moschetti G., Blaiotta G., Villani F., Coppola S. 2000. Specific detection of Leuconostoc mesenteroides subsp. mesenteroides with DNA primers identified by randomly amplified polymorphic DNA analysis. Appl. Environ. Microbiol. 66:422–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nychas G. J. E., Skandamis P. N., Tassou C. C., Koutsoumanis K. P. 2008. Meat spoilage during distribution. Meat Sci. 78:77–89 [DOI] [PubMed] [Google Scholar]
- 34. Pennacchia C., Ercolini D., Villani F. 2011. Spoilage-related microbiota associated with chilled beef stored in air or vacuum pack. Food Microbiol. 28:84–93 [DOI] [PubMed] [Google Scholar]
- 35. Ringø E., Olsen R. E. 1999. The effect of diet on aerobic bacterial flora associated with intestine of Arctic charr (Salvelinus alpinus L.). J. Appl. Microbiol. 86:22–28 [DOI] [PubMed] [Google Scholar]
- 36. Ringø E., Holzapfel W. 2000. Identification and characterization of carnobacteria associated with the gills of Atlantic salmon (Salmo salar L.). Syst. Appl. Microbiol. 23:523–527 [DOI] [PubMed] [Google Scholar]
- 37. Ringø E., et al. 2002. Characterization of Carnobacterium divergens strain 6251 isolated from intestine of Arctic charr (Salvelinus alpinus L.). Syst. Appl. Microbiol. 25:120–129 [DOI] [PubMed] [Google Scholar]
- 38. Russo F., Ercolini D., Mauriello G., Villani F. 2006. Behaviour of Brochothrix thermosphacta in presence of other meat spoilage microbial groups. Food Microbiol. 23:797–802 [DOI] [PubMed] [Google Scholar]
- 39. Sakala R. M., et al. 2002. Change in the composition of the microflora on vacuum-packaged beef during chiller storage. Int. J. Food Microbiol. 74:87–99 [DOI] [PubMed] [Google Scholar]
- 40. Smit G., Smit B. A., Engels W. J. M. 2005. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol. Rev. 29:591–610 [DOI] [PubMed] [Google Scholar]
- 41. Soncin S., Chiesa L. M., Cantoni C., Biondi P. A. 2007. Preliminary study of the volatile fraction in the raw meat of pork, duck and goose. J. Food Composition Anal. 20:436–439 [Google Scholar]
- 42. Susiluoto T., Korkeala H., Bjorkroth K. J. 2003. Leuconostoc gasicomitatum is the dominating lactic acid bacterium in retail modified-atmosphere-packaged marinated broiler meat strips on sell-by-day. Int. J. Food Microbiol. 80:89–97 [DOI] [PubMed] [Google Scholar]
- 43. Vauterin L., Vauterin P. 1992. Computer-aided objective comparison of electrophoretic patterns for grouping and identification of microorganisms. Eur. Microbiol. 1:37–47 [Google Scholar]
- 44. Vihavainen E., et al. 2007. Role of broiler carcasses and processing plant air in contamination of modified-atmosphere-packaged broiler products with psychrotrophic lactic acid bacteria. Appl. Environ. Microbiol. 73:1136–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Villani F., et al. 2007. Study of the microbial ecology of the “Soppressata of Vallo di Diano,” a traditional dry fermented sausage from Southern Italy, and in vitro and in situ selection of autochthonous starter cultures. Appl. Environ. Microbiol. 73:5453–5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wasney M. A., Holley R. A., Jayas D. S. 2001. Cresol red thallium acetate sucrose inulin (CTSI) agar for the selective recovery of Carnobacterium spp. Int. J. Food Microbiol. 64:167–174 [DOI] [PubMed] [Google Scholar]
- 47. Weisburg W. G., Barns S. M., Pelletier D. A., Lane D. J. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zwietering M. H., Jongerburger I., Rombouts F. M., van 't Riet K. 1990. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 56:1875–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.