Abstract
A tetracycline-resistant (Tetr) dairy Enterococcus faecium isolate designated M7M2 was found to carry both tet(M) and tet(L) genes on a 19.6-kb plasmid. After consecutive transfer in the absence of tetracycline, the resistance-encoding plasmid persisted in 99% of the progenies. DNA sequence analysis revealed that the 19.6-kb plasmid contained 28 open reading frames (ORFs), including a tet(M)-tet(L)-mob gene cluster, as well as a 10.6-kb backbone highly homologous (99.9%) to the reported plasmid pRE25, but without an identified toxin-antitoxin (TA) plasmid stabilization system. The derived backbone plasmid without the Tetr determinants exhibited a 100% retention rate in the presence of acridine orange, suggesting the presence of a TA-independent plasmid stabilization mechanism, with its impact on the persistence of a broad spectrum of resistance-encoding traits still to be elucidated. The tet(M)-tet(L) gene cluster from M7M2 was functional and transmissible and led to acquired resistance in Enterococcus faecalis OG1RF by electroporation and in Streptococcus mutans UA159 by natural transformation. Southern hybridization showed that both the tet(M) and tet(L) genes were integrated into the chromosome of S. mutans UA159, while the whole plasmid was transferred to and retained in E. faecalis OG1RF. Quantitative real-time reverse transcription-PCR (RT-PCR) indicated tetracycline-induced transcription of both the tet(M) and tet(L) genes of pM7M2. The results indicated that multiple mechanisms might have contributed to the persistence of antibiotic resistance-encoding genes and that the plasmids pM7M2, pIP816, and pRE25 are likely correlated evolutionarily.
INTRODUCTION
Enterococci are opportunistic pathogens able to survive under a wide range of environmental conditions (15). They are commonly associated with mammalian gastrointestinal (GI) tracts and are considered indicators of fecal contamination from humans and animals. Enterococci are prone to horizontal gene transfer (HGT) mechanisms, a feature that empowered this group of bacteria to evolve quickly by rapid acquisition and dissemination of beneficial trait-encoding elements, including antibiotic resistance (AR) genes, from the surroundings and to flourish in both host and natural environments (14). In fact, Enterococcus faecalis and Enterococcus faecium are among the leading causes of nosocomial infections by Gram-positive bacteria (20). In particular, the emergence of vancomycin-resistant enterococci (VRE) and their potential involvement in the dissemination of resistance-encoding traits to other pathogens, such as Staphylococcus spp., have become a major public health concern (7, 51). To date, various resistance traits (conferring resistance to glycopeptides, aminoglycosides, quinupristin-dalfopristin, tetracycline, chloramphenicol, erythromycin, β-lactams, and others) have been identified in enterococci, and many are encoded by mobile elements, such as plasmids (33, 42, 45, 46), transposons (17, 36–38, 44), and integrons (4). However, many mobile genetic elements in enterococci, especially large plasmids with multiple insertion sequence (IS) elements, remain cryptic (19).
Enterococci have also been associated with various food products, especially meat, dairy, and produce items (16, 29, 42). In addition to fecal or environmental contaminants on raw food materials, certain enterococcal strains have also been used historically as fermentation starter cultures and probiotics (15). However, food-borne enterococci can also acquire genes encoding resistance, virulence, and other traits through HGT events. For example, Eaton and Gasson (12) demonstrated that food-borne enterococcal strains were able to acquire virulence factors from clinical isolates through conjugation. Cocconcelli et al. (5) further illustrated that AR can be transferred in food matrices during processing, with the highest frequency among the conditions examined observed for sausage fermentation. In the past few years, various antibiotic-resistant (ART) commensal enterococcal strains have been isolated from food products (9, 24, 29, 34, 42), suggesting that daily food intake might be an important avenue for the influx of ART enterococci and AR-encoding genes impacting the human gut microflora.
It is well documented that applications of antibiotics in clinical therapy, as well as in food animal, agriculture, and aquaculture production, have played an important role in the selection and maintenance of the ART bacterial population (28). However, recent data from food animal studies showed that limiting the use of antibiotics in food animal production resulted in modest reductions in the prevalence of resistance, instead of elimination of resistance, in certain bacteria (23, 47). Furthermore, results from human infant studies revealed that ART bacteria established, amplified, and persisted in the human GI tract even in the absence of corresponding antibiotic exposure (27, 35, 54). In addition to incorporation into the chromosomal DNA, plasmid-borne genes can be retained in bacterial hosts by a number of stabilization mechanisms, such as active partitioning systems (52), the resolution system for plasmid multimers (48, 52), and toxin-antitoxin (TA) systems (49). TA systems contain a toxin protein and an antitoxin component. Plasmid-encoded TA elements are important for plasmid maintenance, as daughter cells without the plasmid-encoded labile antitoxins are consequently killed by the stable and potent toxins (18). To date, various TA systems, such as ω-ε-ζ, axe-txe, and tasA-tasB, have been reported for bacteria (13, 18, 32), including that of the multidrug resistance-encoding plasmid pRE25, originally isolated from an E. faecium isolate from fermented sausage (42). A recent report further found that pRE25-, pRUM-, pIP501-, and pHTbeta-related replicons associated with glycopeptide resistance and their stabilizing TA systems are widely distributed in E. faecium (39). The persistence of AR genes in microbial ecosystems even without antibiotic selective pressure presents a serious challenge for effective mitigation, and revealing the molecular mechanisms involved is essential for the development of targeted counterstrategies.
During our study of the ART bacteria from food products, we isolated a tetracycline-resistant (Tetr) isolate, designated M7M2, from a cheese sample made at the Ohio State University (OSU) pilot plant. The Tetr trait was found to be quite stable after consecutive transfer of the strain in bacterial culture medium without the corresponding antibiotic. Therefore, the purpose of this study was to reveal the genetic characteristics of the Tetr-encoding element to understand the potential persistence mechanism.
MATERIALS AND METHODS
Strain cultivation.
The isolated Tetr strain, M7M2, was cultured using brain heart infusion (BHI; Becton Dickinson and Company, Sparks, MD) broth or BHI agar plates with 16 μg/ml tetracycline (Sigma-Aldrich, St. Louis, MO) and was incubated at 37°C for 18 h. Streptococcus mutans UA159 was cultured with BHI medium, and E. faecalis OG1RF was cultured using BHI broth or BHI agar plates with 25 μg/ml rifampin and was incubated at 37°C for 18 h. All cultures were incubated under aerobic conditions, except for S. mutans UA159 on BHI agar plates, which was incubated anaerobically. SR plates (10 g tryptone, 5 g yeast extract, 200 g sucrose, 10 g glucose, 25 g gelatin, and 15 g agar per liter, 2.5 mM MgCl2, 2.5 mM CaCl2, pH 6.8) (22) supplemented with 8 μg/ml tetracycline and 25 μg/ml rifampin were used to select Tetr transformants of E. faecalis OG1RF after electroporation.
DNA extraction, AR gene screening, and strain identification.
Plasmid DNA or total DNA was isolated following the method of Anderson and McKay (2) or Yu and Morrison (53). The DNA extract from a 5-ml culture was treated with 20 μg RNase (USB, Santa Clara, CA). Plasmid DNA from a 25-ml culture was further purified using a plasmid minikit (Qiagen), with slight modifications. Instead of using culture, 300 μl each of buffers P1, P2, and P3 was mixed in a 1.5-ml Eppendorf tube and centrifuged at 16,100 × g for 10 min, and then the supernatant was mixed with the crude plasmid DNA extract before loading onto the column for further purification following the manufacturer's procedures.
Conventional PCR was used to detect the presence of tetracycline resistance genes as reported previously (29), with modifications. Primer TetMFP595 (5′-GAACTCGAACAAGAGGAAA-3′) was used instead of TetMFP600, and the sequence for TetSFP160 was changed, giving TetSFP160(2) (5′-GAACGCCAGAGAGGTATTAC-3′). Bacterial isolates were identified by 16S rRNA gene partial DNA sequence analysis following published procedures (50). Enterococcal species-specific primers Fea1 (5′-CCAAGGCTTCTTAGAGA-3′) and Fea2 (5′-CATCGTGTAAGCTAACTAACTTC-3′), modified from the work of Dutka-Malen et al. (11) and targeting the ddl gene of E. faecium, were used to confirm E. faecium isolates.
AR stability assessment.
An overnight culture of the ART strain was obtained by inoculating 5 ml of BHI broth with a single colony from an overnight culture plate and incubating it at 37°C for 18 h. The culture was then inoculated consecutively at 1:1,000 or 1:100,000 into fresh BHI, with or without 10 μg/ml acridine orange (Sigma-Aldrich) and in the absence of tetracycline, for more than 500 generations (>30 days). Serially diluted cultures were spread plated on BHI agar plates and incubated at 37°C for 24 h. The resistance retention rate was determined by randomly picking at least 100 colonies from the BHI plates, spotting them onto new BHI plates with and without tetracycline (16 μg/ml), and calculating the ratio of resistant to total colonies. Both the resistant and susceptible colonies were further assessed for the presence of AR genes and the identities of the plasmid backbone and species by PCR and were subjected to confirmation by plasmid extraction and electrophoresis following standard procedures.
Southern blotting.
The purified PCR amplicons of the tet(L) and tet(M) genes were labeled using the hybridization probe from a DIG DNA labeling and detection kit (Roche, Indianapolis, IN). Hybridization and color detection were conducted following the manufacturer's procedures.
AR gene functionality and mobility assessments.
Purified DNA of pM7M2 was transferred to S. mutans UA159 by natural transformation following previously described procedures (50). Briefly, the recipient strain after 18 h of culture was diluted 1:20 to 1:80 in fresh medium and grown to the exponential growth phase (optical density at 600 nm [OD600] = 0.1 to 0.4). Plasmid extract was added directly to the culture and further incubated for 1 h before the mixture was spread on agar plates with antibiotic for the selection of transformants. The Tetr transformants were screened on BHI plates supplemented with 16 μg/ml tetracycline. Plates were incubated anaerobically at 37°C for 48 h. Purified pM7M2 was also electroporated into E. faecalis OG1RF, as described by Cruz-Rodz and Gilmore (6). The Tetr transformants were screened on SR plates supplemented with 8 μg/ml tetracycline and 25 μg/ml rifampin and incubated aerobically at 37°C for 24 h. The locations of the resistance genes in transformants were examined by Southern hybridization.
MICs.
MIC values for the strains and derivatives were determined using 96-well microtiter plates, with wells containing 0.25 to 512 μg/ml tetracycline in BHI broth as described previously (50).
Cloning and sequencing of plasmid pM7M2.
Purified plasmid DNA was digested with ATP-dependent DNase (Epicentre Biotechnologies, Madison, WI) to eliminate residues of chromosomal DNA. To determine the sequence of pM7M2, the purified plasmid was digested with HindIII and XbaI (Invitrogen, Carlsbad, CA) and ligated by use of T4 ligase to pBluescript II KS(+) digested by the same set of enzymes (Invitrogen). The ligation product was transformed into competent Escherichia coli DH5α prepared by CaCl2 treatment following a standard protocol (3, 41). Transformants carrying recombinant plasmids with different pM7M2 fragments were subjected to DNA sequence analysis at the Plant Genomic Analysis Facility at the Ohio State University, using an ABI Prism 3700 DNA analyzer (Applied Biosystems). To close the gaps, primers were designed according to identified sequences within the fragments and used to PCR amplify the flanking products containing the gaps. Both the PCR products and purified pM7M2 were further used as templates for direct sequencing. The whole plasmid sequence was covered by the combination of the above methods at least twice. The DNA sequences were compared with those deposited in the NCBI database by BLASTN searches. The whole plasmid sequence was compiled using DNASTAR software (DNASTAR Inc., Madison, WI). The putative open reading frames (ORFs) were analyzed and identified by GeneMark.hmm for Prokaryotes, version 2.8 (31; http://exon.biology.gatech.edu/gmhmm2_prok.cgi), and by BLASTX (http://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastx&BLAST_PROGRAMS=blastx&PAGE_TYPE=BlastSearch&SHOW_DEFAULTS=on&LINK_LOC=blasthome).
Tetracycline-induced transcription of AR genes.
For RNA preparation, 1% of a fresh M7M2 culture after 18 h of incubation was inoculated into BHI medium containing 0, 8, 16, or 32 μg/ml tetracycline and then incubated at 37°C to an OD600 of 0.25 to 0.9. Total RNA was extracted from approximately 3 × 108 to 5 × 108 exponentially growing cells by use of an RNeasy minikit (Qiagen) and was further treated with 2 U DNase I (Invitrogen) at 37°C for 30 min. The chromosome-borne d-alanine:d-alanine ligase (ddl) gene was used as an internal standard for data normalization. In addition to a previously described specific primer pair and probe for tet(M) (26), tet(L)- and ddl-specific primers [tet(L)-RT-FP, 5′-CGTCTCATTACCTGATATTGC-3′; tet(L)-RT-RP, 5′-AGGAGTAACCTTTTGATGCC-3′; ddl-rt-fp, 5′-CTTTAGCAACAGCCTATCAG-3′; and ddl-rt-rp, 5′-ACGTCTTTTACGACTTCACC-3′] and fluorescence-labeled probes [5′-FAM-AACCACCTGCGAGTACAAACTGG-BHQ-3′ for tet(L) and 5′-FAM-TCGTTGAACAAGGAATTGAAGC-BHQ-3′ for ddl (FAM, 6-carboxyfluorescein)] were used for quantitative real-time reverse transcriptase PCR (real-time RT-PCR) assessment of the transcription of the tet(M) and tet(L) genes and the internal control (ddl). Reaction mixtures (25 μl) contained 12.5 μl of real-time RT-PCR reaction mix (iScript one-step PCR kit for probes; Bio-Rad Laboratories), 0.012 nmol of each primer, 0.006 nmol of probe, 0.5 μl iScript Moloney murine leukemia virus reverse transcriptase from the iScript one-step PCR kit, 1 μl DNase I-treated RNA extract, and H2O. A sample without reverse transcriptase was always included for each sample as a control for genomic DNA contamination. Real-time RT-PCR was performed with an iCycler iQ real-time PCR detection system (Bio-Rad Laboratories). The PCR mixture was held at 10 min for 50°C for reverse transcription and at 95°C for 5 min for inactivation of the reverse transcriptase, with the following PCR cycling conditions: 95°C for 30 s, 35 cycles of 95°C for 30 s, 55°C for 30 s, and 68°C for 20 s, and 68°C for 5 min. The transcription ratios for 8, 16, and 32 μg/ml tetracycline compared to 0 μg/ml tetracycline were calculated by using the threshold cycle (ΔΔCT) method (30). Data reported represent the means for three RNA preparations from different days, and at least three technical replicates were performed for each RNA sample. Comparisons of expression levels with different tetracycline treatments were analyzed using one-way analysis of variance (ANOVA) following Tukey's post hoc comparisons, using PASW Statistics 18 software (SPSS Inc., Chicago, IL).
Nucleotide sequence accession number.
The nucleotide sequence of the 19.6-kb plasmid pM7M2 has been deposited in GenBank under accession no. JF800907.
RESULTS
Strain identification and Tetr determinants.
Strain M7M2 was identified as Enterococcus faecium by 16S rRNA gene partial DNA sequence analysis. The strain was found to contain both tet(M) and tet(L) genes by PCR. Plasmid gel electrophoresis revealed the presence of a 19.6-kb plasmid (data not shown). Southern blot analysis showed that both resistance-encoding genes were located on the plasmid.
Nucleotide sequence of the 19.6-kb pM7M2 plasmid.
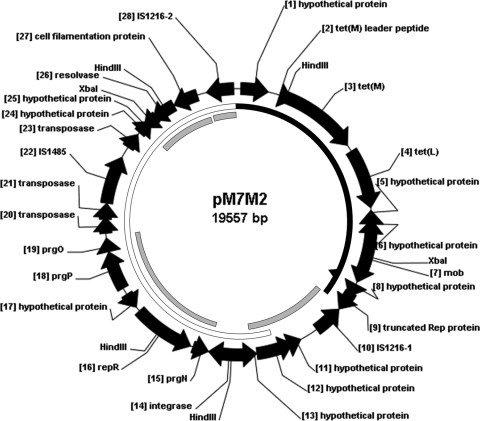
The 19,557-bp plasmid pM7M2 (GenBank accession no. JF800907) contains 28 ORFs, initiated by 22 ATG, 3 TTG, and 3 GTG start codons, with a total G+C content of 35.1%. As illustrated in Fig. 1 and Table 1, pM7M2 contains an 8.6-kb fragment including an IS1216 element at each end and harbors both the tet(M) and tet(L) genes, as well as the plasmid mobilization gene mob. Within the 8.6-kb region, the resistance gene tet(M) and the tet(M) leader peptide, as well as a 982-bp fragment upstream of the tet(M) leader peptide, share 95.4% and 98.7% nucleotide sequence identity, respectively, with the corresponding sequences of the conjugative transposon Tn916 (GenBank accession no. U09422.1), while sharing 100% and 99.9% nucleotide sequence identity, respectively, with the corresponding sequences of the genome of Streptococcus gallolyticus UCN34 (GenBank accession no. FN597254.1). The tet(L) gene was found downstream of the tet(M) gene, with a 193-bp spacer between the stop codon of tet(M) and the start codon of tet(L). The first 90 bp of the spacer is identical to the tet(M) sequence downstream from Tn916, and the rest of the spacer, which includes a 63-bp fragment encoding a tet(L) leader peptide of 20 amino acids, and the tet(L) coding region share 99.9% nucleotide sequence identity with the tet(L) upstream and coding sequences from Bacillus cereus (GenBank accession no. X51366.1). However, a mutation from T to A changed the coding sequence of the third amino acid of the tet(L) leader peptide in pM7M2 from Cys to a stop codon. The rest of pM7M2 includes an 11-kb fragment containing ORF11 to ORF27. A fragment with part of ORF9 to part of ORF13 has 99.9% sequence identity with a fragment of pIP816 (46). Starting from part of ORF12 through ORF28, the 10.6-kb fragment of pM7M2 has 99.9% sequence identity with a fragment sequence in pRE25 (42) and with E. faecium plasmid p5753cB (GenBank accession no. GQ900487.1). ORF15 to ORF19, part of ORF24 to ORF27, and ORF28 have at least 99.9% nucleotide sequence identity with the corresponding fragments of pIP816. Other plasmids which share partial high homology with certain regions of pM7M2 include E. faecium plasmids pVEF3, pVEF2, pVEF1, and pEF1.
Fig. 1.
Circular map of plasmid pM7M2 (see Table 1 for further details). The putative open reading frames are numbered. The coding regions are represented by arrows indicating the direction of transcription, and putative proteins encoded by them correspond to the proteins from the database with the highest amino acid homology. Black solid ring, 99.5% nucleotide sequence identity (positions 1 to 7097) with S. gallolyticus UCN34 (GenBank accession no. FN597254.1) genome, which has an additional 32 bp behind position 6493 of pM7M2, as indicated by the black solid triangle; gray solid ring, 99% to 100% nucleotide sequence identity (positions 7098 to 9438, 10500 to 14353, 17014 to 18706, and 18749 to 19557) with E. faecium plasmid pIP816 (GenBank accession no. AM932524.1); open ring, 99.9% nucleotide sequence identity (positions 8744 to 19557) with E. faecalis plasmid pRE25 (GenBank accession no. X92945.2) and E. faecium plasmid p5753cB (GenBank accession no. GQ900487.1).
Table 1.
Amino acid and nucleotide sequence identities of putative proteins encoded by pM7M2 (GenBank accession no. JF800907)
| ORF | Locus position | Gene length (nt) | Product; identification(s) (GenBank accession no. for sequence with highest homology)a | % aa identity | % nt identity |
|---|---|---|---|---|---|
| 1 | 91–708 | 618 | Hypothetical protein, truncated transposon protein; part of Staphylococcus rostri transposon Tn916 (FN550102.1); E. faecalis plasmid pCF10 (part of Tn916-like transposon Tn925 [AY855841]); 99.0% nt identity with Dialister microaerophilus UPII 345-E (hypothetical protein [NZ_AENT01000012.1]); 99.8% nt identity with S. gallolyticus UCN34 (FN597254.1); 99.7% nt identity with S. suis BM407 [another fragment connected with tet(M)-tet(L)-mob gene cluster shares 99.5% nt identity (FM252032.1)] | 100 | 100 |
| 2 | 983–1069 | 87 | tet(M) leader peptide; S. gallolyticus UCN34 (FN597254.1), Streptococcus suis BM407 (FM252032.1), E. faecalis (CP002491.1), Lactobacillus sakei (EF605269.1), E. coli (DQ534550.1) | 100 | 100 |
| 3 | 1085–3004 | 1,920 | Tetracycline resistance protein TetM; S. gallolyticus UCN34 (FN597254.1), E. faecium plasmid pYA409-1 (DQ223244.1), 99.5% nt identity with S. suis BM407 (FM252032.1) | 100 | 100 |
| 4 | 3198–4574 | 1,377 | Tetracycline resistance protein TetL; S. gallolyticus UCN34 (FN597254.1), Staphylococcus aureus plasmid pKKS627 (FN390948.1), E. faecalis (AF503772.1), B. cereus plasmid pJHI (AY129652.1), 99.9% nt identity with S. suis BM407 (FM252032.1) | 100 | 100 |
| 5 | 4623–4823 | 201 | Hypothetical protein; E. faecalis T2 plasmid (NZ_GG692852), S. gallolyticus UCN34 (FN597254.1), S. suis BM407 (FM252032.1), S. gallolyticus subsp. gallolyticus ATCC BAA-2069 plasmid pSGG1 (NC_015219.1), E. faecalis T3 plasmid (NZ_GG670370.1) | 100 | 100 |
| 6 | 4858–5031 | 174 | Hypothetical protein; E. faecalis T2 plasmid (NZ_GG692852), S. gallolyticus UCN34 (FN597254.1), S. suis BM407 (FM252032.1), S. aureus A9719 (NZ_ACKJ01000060), E. faecalis T3 plasmid (NZ_GG670370.1) | 100 | 100 |
| 7 | 5138–6400 | 1263 | Putative plasmid recombination/mobilization protein (Mob); S. gallolyticus UCN34 (FN597254.1), S. suis BM407 (FM252032.1), S. aureus plasmid pKKS627 (FN390948.1), E. faecalis plasmid pAMalpha1 (AF503772.1) | 100 | 100 |
| 8 | 6423–6599 | 177 | Hypothetical protein; E. faecalis T2 plasmid (NZ_GG692852), S. aureus A9719 (NZ_ACKJ01000060), E. faecium TC 6 (NZ_GG703588.1), S. gallolyticus UCN34 (FN597254.1), S. suis BM407 (FM252032.1) | 100 | 100 |
| 9 | 6645–7118 | 474 | Truncated replication protein (Rep); E. faecalis TX0855 (NZ_AEBV01000023.1), first 453 bp (positions 6645 to 7097) have 100% nt identity with E. faecalis TX0855(NZ_AEBV01000023.1), Streptococcus pasteurianus ATCC 43144 (AP012054.1), S. aureus subsp. aureus ECT-R 2 (FR714927.1), S. gallolyticus UCN34 (FN597254.1), last 21 bp (positions 7098 to 7118) have 100% nt identity with E. faecalis TX0855 (not continuously with the first 453 bp [NZ_AEBV01000023.1]), pIP816 (positions 32988 to 33008) (AM932524.1) | 90.8 | 87.3 |
| 10 | 7152–7838 | 687 | IS1216-1; E. faecalis (AB247327.1), E. faecium plasmid pIP816 (CDS 21) (AM932524.1), E. faecium plasmid pVEF3 (AM931300), Lactococcus lactis plasmid 1 (CP000426.1) | 100 | 100 |
| 11 | 8265–8477 | 213 | Hypothetical protein; E. faecium DO (NZ_ACIY01000546), E. faecium plasmid pIP816 (positions 31841 to 31629) (AM932524.1), E. faecium plasmid pVEF3 (AM931300) | 100 | 100 |
| 12 | 8498–9349 | 852 | Hypothetical protein; E. faecium plasmid pIP816 (CDS 32) (AM932524.1), E. faecium plasmid pVEF3 (AM931300), positions 8744 to 9349 are 100% identical to E. faecalis plasmid pRE25 (positions 3727 to 3122) (X92945.2), E. faecalis plasmid p5753cB (positions 10002 to 10607) (GQ900487.1) | 100 | 100 |
| 13 | 9346–9465 | 120 | Hypothetical protein; E. faecalis plasmid p5753cB (GQ900487.1), E. faecium TX0082 (NZ_AEBU01000113.1), 99.2% nt identity with E. faecalis plasmid pRE25 (positions 3007 to 3125) (X92945.2), positions 9346 to 9438 are 100% identical to E. faecium plasmid pIP816 (positions 30668 to 30760) (AM932524.1) | 100 | 100 |
| 14 | 9532–10491 | 960 | Integrase, catalytic region; E. faecium plasmid p5753cB (GQ900487.1), E. faecium MV5 (HM921050.1), 99.9% nt identity with E. faecalis plasmid pRE25 (ORF3) (X92945.2) | 100 | 100 |
| 15 | 10515–10811 | 297 | Putative PrgN protein; E. faecalis plasmid pRE25 (ORF2) (X92945.2), E. faecalis plasmid p5753cB (hypothetical protein) (GQ900487.1), E. faecium plasmid pIP816 (CDS 31) (AM932524.1), E. faecium plasmid pVEF3 (AM931300) | 100 | 100 |
| 16 | 10946–12439 | 1,494 | Putative RepR protein; E. faecalis plasmid pRE25 (ORF1) (X92945.2), E. faecium plasmid p5753cB (plasmid replication protein) (GQ900487.1), RepE of E. faecium plasmid pIP816 (CDS 30) (AM932524.1), E. faecium plasmid pVEF3 (AM931300) | 100 | 100 |
| 17 | 12547–12681 | 135 | Hypothetical protein; E. faecalis T2 plasmid (NZ_GG692852.1), E. faecalis plasmid pRE25 (positions 50027 to 50161) (X92945.2), E. faecium plasmid p5753cB (positions 13805 to 13939) (GQ900487.1), E. faecium plasmid pVEF3 (AM931300), 99.3% nt identity with E. faecium plasmid pIP816 (positions 28624 to 28490) (AM932524.1) | 100 | 100 |
| 18 | 13051–14004 | 954 | Putative PrgP protein; E. faecalis plasmid pRE25 (ORF58) (X92945.2), E. faecium plasmid p5753cB (chromosome partitioning ATPase) (GQ900487.1), E. faecium plasmid pIP816 (CDS 29) (AM932524.1), E. faecium plasmid pVEF3 (AM931300) | 100 | 100 |
| 19 | 13976–14251 | 276 | Putative PrgO protein; E. faecalis plasmid pRE25 (ORF57) (X92945.2), E. faecalis plasmid p5753cB (PrgO-like protein for plasmid replication) (GQ900487.1), E. faecium plasmid pIP816 (CDS 28) (AM932524.1), E. faecium plasmid pVEF3 (AM931300) | 100 | 100 |
| 20 | 14444–14698 | 255 | Transposase; 99.6% nt identity with E. faecium plasmid p5753cB (GQ900487.1), 99.6% nt identity with E. faecalis plasmid pRE25 (positions 48264 to 48010) (X92945.2) | 98.8 | 99.6 |
| 21 | 14749–15063 | 315 | Transposase; E. faecium plasmid p5753cB (GQ900487.1), E. faecalis plasmid pRE25 (positions 47959 to 47645) (X92945.2) | 100 | 100 |
| 22 | 15104–16266 | 1,163 | IS1485; E. faecalis plasmid pRE25 (ORF55) (X92945.2), E. faecium plasmid p5753cB (positions 16362 to 17524, including transposase and integrase) (GQ900487.1) | 100 | 100 |
| 23 | 16517–16939 | 423 | Transposase; E. faecium plasmid p5753cB (GQ900487.1), E. faecalis plasmid pRE25 (positions 46191 to 45769) (X92945.2) | 100 | 100 |
| 24 | 16999–17160 | 162 | Hypothetical protein; E. faecalis (NZ_GG692631), E. faecalis plasmid pRE25 (positions 45719 to 45548) (X92945.2), E. faecium plasmid p5753cB (positions 18257 to 18418) (GQ900487.1), partial (positions 17014 to 17160) nt identity with E. faecium plasmid pIP816 (positions 22413 to 22559) (AM932524.1) | 100 | 100 |
| 25 | 17140–17292 | 153 | Hypothetical protein; E. faecalis TX0104 (NZ_ACGL01000088.1), E. faecium TX0133a01 (NZ_AECJ01000161.1), E. faecalis plasmid pRE25 (positions 45568 to 45416) (X92945.2), E. faecium plasmid p5753cB (positions 18398 to 18550) (GQ900487.1), E. faecium plasmid pIP816 (positions 22539 to 22691) (AM932524.1) | 100 | 100 |
| 26 | 17412–17984 | 573 | Resolvase; E. faecalis plasmid pRE25 (ORF53) (X92945.2), E. faecium plasmid pIP816 (CDS 23) (AM932524.1), E. faecium plasmid p5753cB (site-specific recombinase) (GQ900487.1) | 100 | 100 |
| 27 | 18000–18605 | 606 | Cell filamentation protein; E. faecalis plasmid pRE25 (ORF52) (X92945.2), E. faecium plasmid pIP816 (CDS 24) (AM932524.1), 99.8% nt identity with E. faecium plasmid p5753cB (positions 19258 to 19862) (GQ900487.1) | 100 | 100 |
| 28 | 18803–19489 | 687 | IS1216-2; E. faecalis plasmid pRE25 (ORF51) (X92945.2), E. faecium plasmid p5753cB (positions 20060 to 20746, including transposase) (GQ900487.1), E. faecium plasmid pIP816 (CDS 27) (AM932524.1), L. lactis plasmid 1 (CP000426.1), Listeria innocua plasmid pLI100 (AL592102.1) | 100 | 100 |
CDS numbers for pIP816 are from reference 46.
Tetracycline-induced transcription of tet(M) and tet(L).
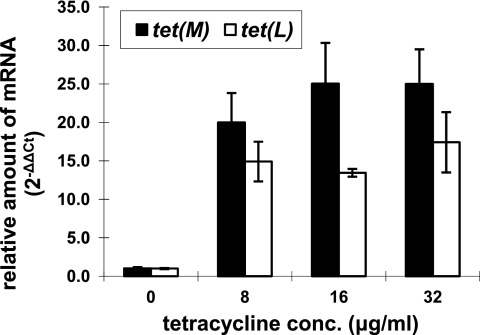
As shown in Fig. 2, quantitative real-time RT-PCR indicated that both the tet(M) and tet(L) genes were significantly induced in the presence of 8 to 32 μg/ml tetracycline (P < 0.05). However, there was no significant difference (P > 0.05) in the induced transcription levels of both genes within the range of tetracycline concentrations (8 to 32 μg/ml) tested in this study.
Fig. 2.
Relative quantification of tet(M) and tet(L) transcripts with 8, 16, and 32 μg/ml tetracycline compared to 0 μg/ml tetracycline. Bars represent means ± standard errors of the means for three independent biological replicates.
Stability of AR.
The persistence of AR genes in E. faecium M7M2 was assessed by the stability test. Resistance genes were highly stable in the absence of the plasmid curing agent acridine orange and the corresponding antibiotic tetracycline, with a retention rate of 99.04% ± 0.79% (mean ± standard deviation [SD]; n = 4). In the presence of acridine orange, the retention rate was lowered to 85.10% ± 15.51% (mean ± SD; n = 4). However, plasmid isolation of selective progenies which lost resistance after consecutive transfer showed that they still carried an 11.7-kb plasmid, designated pM7M2D, in contrast to the original pM7M2 plasmid. PCR mapping, restriction enzyme digestion, and partial DNA sequence analysis showed that a recombination event likely led to the elimination of the fragment flanked by the two identical insertion sequences of IS1216, including the tetracycline resistance genes.
Two E. faecium derivatives, M7M2D1 and M7M2D2, containing the small backbone plasmid pM7M2D, were further subjected to consecutive transfer in BHI broth with acridine orange. After more than 600 generations, 100% (52/52 progenies) of the progenies assessed retained the plasmid.
Functionality and mobility of AR genes.
Natural transformation and electroporation were used to examine the potential involvement of pM7M2 in HGT events. Tetr transformants were obtained by natural transformation of pM7M2 into S. mutans UA159 and by electroporation of the plasmid into E. faecalis OG1RF. Southern blot assessment of the transformants with tet(M) and tet(L) probes revealed that both genes were integrated into the chromosome in the S. mutans UA159 transformants, but the 19.6-kb plasmid was retained in the E. faecalis OG1RF progenies. The MIC test showed that transformants of S. mutans UA159 and E. faecalis OG1RF both had significantly increased resistance to tetracycline (>64 μg/ml) compared to the recipient strains, S. mutans UA159 (1 μg/ml) and E. faecalis OG1RF (0.5 μg/ml), and the resistance level was close to that of the donor strain, E. faecium M7M2 (>128 μg/ml). Both the MIC and Southern hybridization data suggested that the Tetr determinants were functional and transmissible and led to acquired resistance in human residential and pathogenic bacteria in laboratory settings.
DISCUSSION
Undoubtedly, the prudent use of antibiotics is essential to reduce the prevalence and slow down the emergence of ART bacteria, including ART pathogens. However, emerging data on the origins of certain AR-encoding genes, including the detection of immunity genes in antibiotic-producing organisms (1), the prevalence of ART bacteria in ready-to-consume foods (10, 29, 50), the colonization, persistence, and amplification of ART bacteria in host GI tracts in the absence of antibiotic exposure (27, 35, 54), and various AR gene stabilization, coselection, and niche fitness mechanisms (49), illustrate the complexities of the AR issue. A comprehensive understanding of the major pathways and mechanisms involved in AR emergence, amplification, persistence, and dissemination is greatly needed to achieve effective mitigation.
This study examined the genetic characteristics of a persistent AR gene carrier isolated from a dairy product. DNA sequence analysis data on pM7M2 showed that it carries a tet(M)-tet(L)-mob gene cluster. Not only did the tet(M), tet(L), and mob genes have high homology with previously identified ones associated with various mobile elements across a broad spectrum of bacteria, but the tandem tet(M)-tet(L)-mob gene cluster organization represents the first of its kind on a plasmid. The only other cases were found in the recently sequenced genome of S. gallolyticus UCN34 (100% nucleotide sequence identity), isolated from a colon cancer patient in France (40), and in the zoonotic pathogen Streptococcus suis BM407 (99.8% nucleotide sequence identity), isolated from a meningitis patient in Vietnam (21), where the tet(L) and mob genes were inserted behind the tet(M) gene in Tn916. It is worth noting that a mutation in the tet(L) leader peptide changed the 3rd amino acid from Cys to a stop codon in pM7M2. The same exact mutation was observed in the gene cluster in S. gallolyticus UCN34 and S. suis BM407. Schwarz et al. (43) reported that the tet(L) leader peptide is involved in modulating the expression of tet(L) at the translational level. However, Kehrenberg et al. (25) reported that a tet(L) gene missing the entire leader peptide in plasmid pCCK3259 from Mannheimia still conferred resistance to tetracycline. In this study, the real-time RT-PCR results illustrated induced transcription of both tet(M) and tet(L) in pM7M2. However, the exact impact of this point mutation leading to a truncated tet(L) leader peptide on the translational expression of tet(L), and therefore the overall resistance to tetracycline, has yet to be determined. The evolutionary significance of the tet(M)-tet(L) gene cluster also remains to be elucidated. In this study, we have also illustrated the functionality and mobility of pM7M2 within and across genera, and the AR genes from pM7M2 were incorporated into the chromosome of the human oral pathogen S. mutans UA159 by natural gene transformation in laboratory settings. Although we were unable to establish a physical connection among E. faecium M7M2, S. gallolyticus UCN34, and S. suis BM407 isolates from distant geographic locations, the high homology of the Tetr determinants found in the strains [>99% nucleotide sequence identity with the tet(M)-tet(L)-mob gene cluster, including the single mutation at the tet(L) leader peptide region, as well as >98% identity with the 982-bp fragment upstream of the tet(M) leader peptide] suggested the possibility of an HGT event(s), although the strains also could have gone through independent evolutionary events. The food-borne nature of M7M2 suggested that the microbiota associated with animal and human GI tracts and with animal food products and wastes could possibly be involved in the evolution, dissemination, and circulation of such AR-encoding genetic elements. Control interventions are thus essential to interrupt this amplified circulation and evolution of AR-encoding elements in microbial ecosystems.
Data from recent studies showed that many AR-encoding plasmids are very stable in the absence of corresponding antibiotic pressure because of specific maintenance mechanisms, such as the TA systems. Representative TA systems, such as ω-ε-ζ and axe-txe, were found to be ubiquitous in Vanr-encoding plasmids (32, 39), as well as in other plasmids conferring multidrug resistance (13, 42). The persistent pM7M2 plasmid has a backbone structure highly homologous to that of pIP816 and pRE25, but without the previously illustrated ω-ε-ζ plasmid-stabilizing TA system. However, all three plasmids carry a common fragment that includes the prgO, prgP, and prgN genes. After consecutive transfer in E. faecium in the absence of tetracycline but the presence of the plasmid curing agent, the Tetr trait was lost in a small population of the progenies, likely due to a recombination event(s) eliminating the IS1216-flanked fragment, including the tet(M)-tet(L)-mob gene cluster. The persistence of the prgOPN gene cluster-containing backbone in plasmid derivative pM7M2D in the subsequent stability assessment, however, suggested the presence of a second plasmid stabilization system. In agreement, Derome et al. (8) examined the roles of prgP and prgO in plasmid segregation from pGENT, a Vanr-conferring plasmid from an enterococcal clinical isolate, and found that mutation of either prgP or prgO significantly decreased the retention rate of the associated plasmid. Therefore, the prgOPN gene cluster may have served as a TA-independent, though much less recognized, stabilization mechanism and may have contributed to the persistence and subsequent distribution of various AR-encoding plasmids, such as pM7M2 for tet(M) and tet(L), pRE25 for erm(B), aadE, cat, sat4, and aph3, and pIP816 as well as pVEF4 for van(A), in microbial populations in the absence of the corresponding antibiotic selective pressure. Further characterization of the AR persistence mechanism(s) is essential for the development of a targeted mitigation strategy.
ACKNOWLEDGMENTS
E. faecalis strain OG1RF was kindly provided by Gary Dunny (University of Minnesota), and S. mutans UA159 was provided by Robert Burne (University of Florida).
This project was sponsored by OARDC competitive research enhancement seed grant OHOA1084 and by Dairy Management Inc. grant OSURF60010225 to H. H. Wang.
Footnotes
Published ahead of print on 22 July 2011.
REFERENCES
- 1. Allen H. K., et al. 2010. Call of the wild: antibiotic resistance genes in natural environments. Nat. Rev. Microbiol. 8:251–259 [DOI] [PubMed] [Google Scholar]
- 2. Anderson D. G., McKay L. L. 1983. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl. Environ. Microbiol. 46:549–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ausubel F. M., et al. 1989. Current protocols in molecular biology, vol. 1 Wiley Press, New York, NY [Google Scholar]
- 4. Clark N. C., Olsvik O., Swenson J. M., Spiegel C. A., Tenover F. C. 1999. Detection of a streptomycin/spectinomycin adenylyltransferase gene (aadA) in Enterococcus faecalis. Antimicrob. Agents Chemother. 43:157–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cocconcelli P. S., Cattivelli D., Gazzola S. 2003. Gene transfer of vancomycin and tetracycline resistances among Enterococcus faecalis during cheese and sausage fermentations. Int. J. Food Microbiol. 88:315–323 [DOI] [PubMed] [Google Scholar]
- 6. Cruz-Rodz A. L., Gilmore M. S. 1990. High efficiency introduction of plasmid DNA into glycine treated Enterococcus faecalis by electroporation. Mol. Gen. Genet. 224:152–154 [DOI] [PubMed] [Google Scholar]
- 7. de Niederhausern S., et al. 2011. Vancomycin-resistance transferability from VanA enterococci to Staphylococcus aureus. Curr. Microbiol. 62:1363–1367 [DOI] [PubMed] [Google Scholar]
- 8. Derome A., et al. 2008. Centromere anatomy in the multidrug-resistant pathogen Enterococcus faecium. Proc. Natl. Acad. Sci. U. S. A. 105:2151–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Devirgiliis C., Barile S., Caravelli A., Coppola D., Perozzi G. 2010. Identification of tetracycline- and erythromycin-resistant Gram-positive cocci within the fermenting microflora of an Italian dairy food product. J. Appl. Microbiol. 109:313–323 [DOI] [PubMed] [Google Scholar]
- 10. Duran G. M., Marshall D. L. 2005. Ready-to-eat shrimp as an international vehicle of antibiotic-resistant bacteria. J. Food Prot. 68:2395–2401 [DOI] [PubMed] [Google Scholar]
- 11. Dutka-Malen S., Evers S., Courvalin P. 1995. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 33:24–27 (Erratum, 33:1434.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eaton T. J., Gasson M. J. 2001. Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Appl. Environ. Microbiol. 67:1628–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fico S., Mahillon J. 2006. tasA-tasB, a new putative toxin-antitoxin (TA) system from Bacillus thuringiensis pGI1 plasmid is a widely distributed composite mazE-doc TA system. BMC Genomics 7:259 doi:10.1186/1471-2164-7-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fisher K., Phillips C. 2009. The ecology, epidemiology and virulence of Enterococcus. Microbiology 155:1749–1757 [DOI] [PubMed] [Google Scholar]
- 15. Franz C. M. A. P., Holzapfel W. H., Stiles M. E. 1999. Enterococci at the crossroads of food safety? Int. J. Food Microbiol. 47:1–24 [DOI] [PubMed] [Google Scholar]
- 16. Franz C. M. A. P., et al. 2001. Incidence of virulence factors and antibiotic resistance among enterococci isolated from food. Appl. Environ. Microbiol. 67:4385–4389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Handwerger S., Skoble J. 1995. Identification of chromosomal mobile element conferring high-level vancomycin resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 39:2446–2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hayes F. 2003. Toxins-antitoxins: plasmid maintenance, programmed cell death, and cell cycle arrest. Science 301:1496–1499 [DOI] [PubMed] [Google Scholar]
- 19. Hegstad K., Mikalsen T., Coque T., Werner G., Sundsfjord A. 2010. Mobile genetic elements and their contribution to the emergence of antimicrobial resistant Enterococcus faecalis and Enterococcus faecium. Clin. Microbiol. Infect. 16:541–554 [DOI] [PubMed] [Google Scholar]
- 20. Hidron A. I., et al. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control Hosp. Epidemiol. 29:996–1011 [DOI] [PubMed] [Google Scholar]
- 21. Holden M. T., et al. 2009. Rapid evolution of virulence and drug resistance in the emerging zoonotic pathogen Streptococcus suis. PLoS One 4:e6072 doi:10.1371/journal.pone.0006072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Holo H., Nes I. F. 1989. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl. Environ. Microbiol. 55:3119–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnsen P. J., et al. 2005. Persistence of animal and human glycopeptide-resistant enterococci on two Norwegian poultry farms formerly exposed to avoparcin is associated with a widespread plasmid-mediated vanA element within a polyclonal Enterococcus faecium population. Appl. Environ. Microbiol. 71:159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnston L. M., Jaykus L. A. 2004. Antimicrobial resistance of Enterococcus species isolated from produce. Appl. Environ. Microbiol. 70:3133–3137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kehrenberg C., Catry B., Haesebrouck F., de Kruif A., Schwarz S. 2005. tet(L)-mediated tetracycline resistance in bovine Mannheimia and Pasteurella isolates. J. Antimicrob. Chemother. 56:403–406 [DOI] [PubMed] [Google Scholar]
- 26. Kinkelaar D. F. 2008. M.S. thesis The Ohio State University, Columbus, OH [Google Scholar]
- 27. Lancaster H., et al. 2003. Prevalence and identification of tetracycline-resistant oral bacteria in children not receiving antibiotic therapy. FEMS Microbiol. Lett. 228:99–104 [DOI] [PubMed] [Google Scholar]
- 28. Levy S. B., Marshall B. 2004. Antibacterial resistance worldwide: causes, challenges and responses. Nat. Med. 10:S122–S129 [DOI] [PubMed] [Google Scholar]
- 29. Li X., Wang H. H. 2010. Tetracycline resistance associated with commensal bacteria from representative ready-to-consume deli and restaurant foods. J. Food Prot. 73:1841–1848 [DOI] [PubMed] [Google Scholar]
- 30. Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 31. Lukashin A. V., Borodovsky M. 1998. GeneMark.hmm: new solutions for gene finding. Nucleic Acids Res. 26:1107–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moritz E. M., Hergenrother P. J. 2007. Toxin-antitoxin systems are ubiquitous and plasmid-encoded in vancomycin-resistant enterococci. Proc. Natl. Acad. Sci. U. S. A. 104:311–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murray B. E., Mederski-Samaroj B. 1983. Transferable beta-lactamase. A new mechanism for in vitro penicillin resistance in Streptococcus faecalis. J. Clin. Invest. 72:1168–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ozmen Togay S., Celebi Keskin A., Acik L., Temiz A. 2010. Virulence genes, antibiotic resistance and plasmid profiles of Enterococcus faecalis and Enterococcus faecium from naturally fermented Turkish foods. J. Appl. Microbiol. 190:1084–1092 [DOI] [PubMed] [Google Scholar]
- 35. Ready D., Bedi R., Spratt D. A., Mullany P., Wilson M. 2003. Prevalence, proportions, and identities of antibiotic-resistant bacteria in the oral microflora of healthy children. Microb. Drug Resist. 9:367–372 [DOI] [PubMed] [Google Scholar]
- 36. Rice L. B., Carias L. L. 1998. Transfer of Tn5385, a composite, multiresistance chromosomal element from Enterococcus faecalis. J. Bacteriol. 180:714–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rice L. B., Carias L. L., Rudin S., Hutton R. A., Marshall S. 2010. Multiple copies of functional, Tet(M)-encoding Tn916-like elements in a clinical Enterococcus faecium isolate. Plasmid 64:150–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roberts A. P., Davis I. J., Seville L., Villedieu A., Mullany P. 2006. Characterization of the ends and target site of a novel tetracycline resistance-encoding conjugative transposon from Enterococcus faecium 664.1H1. J. Bacteriol. 188:4356–4361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosvoll T. C., et al. 2010. PCR-based plasmid typing in Enterococcus faecium strains reveals widely distributed pRE25-, pRUM-, pIP501- and pHTbeta-related replicons associated with glycopeptide resistance and stabilizing toxin-antitoxin systems. FEMS Immunol. Med. Microbiol. 58:254–268 [DOI] [PubMed] [Google Scholar]
- 40. Rusniok C., et al. 2010. Genome sequence of Streptococcus gallolyticus: insights into its adaptation to the bovine rumen and its ability to cause endocarditis. J. Bacteriol. 192:2266–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sambrook J., Russell D. W. 2000. Preparation and transformation of competent E. coli using calcium chloride, p. 1.116—1.118 In Molecular cloning: a laboratory manual, 3rd ed., vol. 1 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 42. Schwarz F. V., Perreten V., Teuber M. 2001. Sequence of the 50-kb conjugative multiresistance plasmid pRE25 from Enterococcus faecalis RE25. Plasmid 46:170–187 [DOI] [PubMed] [Google Scholar]
- 43. Schwarz S., Cardoso M., Wegener H. C. 1992. Nucleotide sequence and phylogeny of the tet(L) tetracycline resistance determinant encoded by plasmid pSTE1 from Staphylococcus hyicus. Antimicrob. Agents Chemother. 36:580–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Simjee S., Manzoor S. E., Fraise A. P., Gill M. J. 2000. Nature of transposon-mediated high-level gentamicin resistance in Enterococcus faecalis isolated in the United Kingdom. J. Antimicrob. Chemother. 45:565–575 [DOI] [PubMed] [Google Scholar]
- 45. Simjee S., et al. 2006. Heterogeneity of vat(E)-carrying plasmids in Enterococcus faecium recovered from human and animal sources. Int. J. Antimicrob. Agents 28:200–205 [DOI] [PubMed] [Google Scholar]
- 46. Sletvold H., et al. 2010. Tn1546 is part of a larger plasmid-encoded genetic unit horizontally disseminated among clonal Enterococcus faecium lineages. J. Antimicrob. Chemother. 65:1894–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sorum M., et al. 2006. Prevalence, persistence, and molecular characterization of glycopeptide-resistant enterococci in Norwegian poultry and poultry farmers 3 to 8 years after the ban on avoparcin. Appl. Environ. Microbiol. 72:516–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Summers D. K., Sherratt D. J. 1984. Multimerization of high copy number plasmids causes instability: CoIE1 encodes a determinant essential for plasmid monomerization and stability. Cell 36:1097–1103 [DOI] [PubMed] [Google Scholar]
- 49. Wang H. H. 2009. Commensal bacteria, microbial ecosystems and horizontal gene transmission: adjusting our focus for strategic breakthroughs against antibiotic resistance, p. 267–281 In Jaykus L., Wang H. H., Schlesinger L. S. (ed.), Foodborne microbes: shaping the host ecosystems. ASM Press, Washington, DC [Google Scholar]
- 50. Wang H. H., et al. 2006. Food commensal microbes as a potentially important avenue in transmitting antibiotic resistance genes. FEMS Microbiol. Lett. 254:226–231 [DOI] [PubMed] [Google Scholar]
- 51. Willems R. J., Bonten M. J. 2007. Glycopeptide-resistant enterococci: deciphering virulence, resistance and epidemicity. Curr. Opin. Infect. Dis. 20:384–390 [DOI] [PubMed] [Google Scholar]
- 52. Williams D. R., Thomas C. M. 1992. Active partitioning of bacterial plasmids. J. Gen. Microbiol. 138:1–16 [DOI] [PubMed] [Google Scholar]
- 53. Yu Z., Morrison M. 2004. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 36:808–812 [DOI] [PubMed] [Google Scholar]
- 54. Zhang L., et al. 2011. Acquired antibiotic resistance: are we born with it? Appl. Environ. Microbiol. 77:7134.–7141 [DOI] [PMC free article] [PubMed] [Google Scholar]