Abstract
The study whose results are presented here aimed at identifying free-living protozoa (FLP) and conditions favoring the growth of these organisms and cultivable Legionella spp. in drinking water supplies in a tropical region. Treated and distributed water (±30°C) of the water supplies of three Caribbean islands were sampled and investigated with molecular techniques, based on the 18S rRNA gene. The protozoan host Hartmannella vermiformis and cultivable Legionella pneumophila were observed in all three supplies. Operational taxonomic units (OTUs) with the highest similarity to the potential or candidate hosts Acanthamoeba spp., Echinamoeba exundans, E. thermarum, and an Neoparamoeba sp. were detected as well. In total, 59 OTUs of FLP were identified. The estimated protozoan richness did not differ significantly between the three supplies. In supply CA-1, the concentration of H. vermiformis correlated with the concentration of Legionella spp. and clones related to Amoebozoa predominated (82%) in the protozoan community. These observations, the low turbidity (<0.2 nephelometric turbidity units [NTU]), and the varying ATP concentrations (1 to 12 ng liter−1) suggest that biofilms promoted protozoan growth in this supply. Ciliophora represented 25% of the protozoan OTUs in supply CA-2 with elevated ATP concentrations (maximum, 55 ng liter−1) correlating with turbidity (maximum, 62 NTU) caused by corroding iron pipes. Cercozoan types represented 70% of the protozoan clones in supply CA-3 with ATP concentrations of <1 ng liter−1 and turbidity of <0.5 NTU in most samples of distributed water. The absence of H. vermiformis in most samples from supply CA-3 suggests that growth of this protozoan is limited at ATP concentrations of <1 ng liter−1.
INTRODUCTION
In tropical regions, the water temperature in drinking water distribution system is permanently about 30°C (4). In these regions, free-living protozoa (FLP), serving as hosts for pathogenic bacteria, including Acanthamoeba spp. (1, 38), Hartmannella spp. (37), and Naegleria spp. (38, 49), have been observed in surface water, wastewater, cooling towers, and drinking water (3, 5, 19, 45). Certain FLP with pathogenic properties, viz. Acanthamoeba spp. (9, 21), Balamuthia mandrillaris (54), and Naegleria fowleri (59), can proliferate in drinking water-related biofilms at elevated temperatures (36). In addition, Legionella pneumophila, the main etiologic agent of Legionnaires' disease (12), which proliferates in freshwater at temperatures above 25°C (57), is frequently observed in these environments (14, 31, 39).
FLP in aquatic environments feed on bacteria, fungi, other protozoa, and organic detritus in biofilms and sediments or in the planktonic phase (32). The abundance of prey organisms and detritus depends on the water composition and the hydraulic conditions in distribution systems, which therefore also affect both the FLP abundance and community composition (52, 55). Most information on community composition and abundance of FLP in freshwater environments has been obtained by using cultivation methods and microscopy. Recently, however, the presence and identities of such organisms in drinking water supplies in temperate regions have been studied by using molecular methods for detection and identification (33, 51). In two groundwater supplies in the Netherlands a total of 127 operational taxonomic units (OTUs) of FLP were identified based on their 18S rRNA gene sequences. FLP, mostly pathogens, have been characterized in only a few studies in tropical regions (3, 5, 19). In recent reviews it was concluded that more research is needed to determine which factors favor the growth of these organisms in water supplies (47, 48).
Cases of Legionnaires' disease have been reported in relation to the presence of L. pneumophila in drinking water supplies in the Caribbean (8, 39, 41), but information about water quality characteristics was not provided. In this study, the occurrence and identity of FLP and other small eukaryotes in treated and distributed water of drinking water supplies on three islands in the Caribbean region were investigated with molecular techniques. In these supplies, drinking water is produced from seawater by using distillation and/or reverse osmosis (RO) for desalination. The objectives of this study were (i) to determine concentrations of the protozoa Acanthamoeba spp. and H. vermiformis and cultivable Legionella spp. in treated and distributed water of three different water supplies, (ii) to identify the predominant FLP in these supplies, and (iii) to identify conditions favoring the growth of FLP and Legionella spp. by comparing the characteristics of water quality and distribution systems.
(This research has been published as part of the Ph.D. dissertation of Rinske Valster.)
MATERIALS AND METHODS
Drinking water supplies.
The free-living protozoan communities in the drinking water supplies of three Caribbean islands (latitude and longitude, 12°6′ to 12°30′N and 68°19′ to 69°58′W) belonging to the Leeward Antilles were investigated. Water treatment plant CA-1 has a daily production of 4.4 × 104 m3, 18% of which is produced by RO and 82% by distillation. Posttreatment with dolomite filtration to increase the hardness of the water and by the addition of corrosion inhibitors (pyrophosphate, 1.5 ppm; zinc orthophosphate, 2.5 ppm) is followed by storage in steel tanks and UV disinfection (35.99 mJ cm−2) prior to distribution. Mains of copper (42%) and cement-lined cast iron (39%) lead the treated water to seven steel service reservoirs in the supply area. Supply CA-2 includes two treatment facilities with a total daily production of 5.8 × 104 m3 drinking water. The distribution systems of both plants are interconnected. Desalination at plant CA-2a is done with RO and at plant CA-2b with RO (80%) and distillation (20%). Posttreatment of RO filtrate and distillate includes calcium hypochlorite dosage (0.3 mg liter−1), addition of carbon dioxide, limestone and granular activated carbon (GAC) filtration, addition of fluoride (0.3 to 0.7 mg liter−1), and disinfection with UV radiation (CA-2a, 15.4 mJ cm−2; CA-2b, 7.5 to 12.2 mJ cm−2). Pipes of high-density polyethylene, copper, and galvanized iron comprise about 70% of the distribution system, with seven service reservoirs. The main pipes (26%) and the transportation pipes consist of cast iron, with and without cement lining. In supply CA-3, with a daily production of 3.8 × 103 m3, seawater is treated with RO, followed by limestone filtration and GAC filtration in parallel, addition of chlorine (0.2 mg liter−1), and storage in two steel tanks. Treated water with a chlorine residual of <0.05 ppm is transported to five cast iron service reservoirs. The distribution system consists of pipes of cast iron (63%), polyethylene (11%), and polyvinyl chloride (9%). Maximum residence times in the distribution systems of the supplies, including the service tanks, range from 48 to about 96 h.
Sample collection.
From supply CA-1, two sample series were collected, one in November 2007 and one in November 2009. Both series included four samples at different treatment stages and seven samples from the distribution system after each reservoir (6 to 12 km from the plant). From supply CA-2, samples were collected in May 2008 and in January 2009. Both series included one sample before UV from both treatment plants. In addition, 15 samples were collected from the distribution system (5 to 15 km from the plant) in 2008 and 7 samples in 2009. Treated water of supply CA-3 was sampled at the plant, and 13 samples were collected from the distribution system (5 to 10 km from the plant). All samples, contained in sterile 1-liter PE flasks, were stored on ice and processed within 24 to 72 h. The flasks for the samples of supply CA-3 contained 1 ml of sterile sodium thiosulfate (0.12 M) to neutralize the chlorine residual.
Analytical methods.
Total concentrations of ATP, representing active biomass, were measured in all water samples as described earlier (27). Buffered charcoal-yeast extract (BCYE) agar, incubated at 37°C for 7 days, was used to detect cultivable cells of Legionella spp. in the water samples (11, 29). Subsequently, the fraction of colonies related to L. pneumophila was determined by an agglutination test (Legionella latex test; Oxoid, United Kingdom).
Duplicate water samples of 500 ml were filtered using an RTTP Isopore membrane (Millipore, Molsheim, France) with a 1.2-μm pore size and a 55-mm diameter. Of the samples taken from supply CA-1 in 2007, volumes of 1.75 liter were filtered. Subsequently, DNA of the organisms retained on the membrane filter was isolated as previously described (51). Concentrations of H. vermiformis and Acanthamoeba spp. in the water samples were determined using quantitative PCR (qPCR) as described earlier (23, 35). In brief, quantification of H. vermiformis and Acanthamoeba spp. was based on calibration curves which were constructed by preparing 10-fold dilutions of DNA extracted from suspensions with counted numbers of cells of H. vermiformis (ATCC 50237) and Acanthamoeba castellanii (CCAP 1501) (23). All primers were produced at Biolegio (Malden, the Netherlands). All qPCR assays were performed in duplicate, using undiluted and 10-fold-diluted DNA extracts as templates in 96-well plates in a C1000 thermal cycler (Bio-Rad, Veenendaal, the Netherlands).
The richness and composition of the eukaryotic communities in the water samples were determined by terminal restriction fragment length polymorphism (T-RFLP) analysis and cloning followed by sequence analysis of 18S rRNA gene fragments (±550 bp) as described earlier (51). Clone libraries were constructed of 2 treated water samples from plant CA-1 and 5, 10, and 6 samples of distributed water from supplies CA-1, CA-2, and CA-3, respectively. These samples were selected based on high concentrations of cultivable Legionella spp., low concentrations of H. vermiformis, and preferably a location on the periphery of the distribution systems. Approximately 45 clones per sample were analyzed, resulting in a total of 991 partial 18S rRNA gene sequences.
The 18S rRNA gene sequences obtained were divided into operational taxonomic units (OTUs) using a threshold of 99% sequence similarity. Subsequently, these sequences were compared to sequences in the National Center for Biotechnology (NCBI) database by BLAST search and imported and aligned into the SSU Ref SILVA98 database released in March 2009 using the ARB software package (26, 34), as previously described (51). The estimated OTU richness was determined with the ChaoI estimator from randomized data (18). The obtained eukaryotic sequences were divided into higher taxa based on the classification system of Cavalier-Smith (6) and the structure in the SILVA database (34).
Statistical analyses.
The Kruskal-Wallis test was applied to determine differences in the concentrations of selected water quality parameters between the three supplies. In case of a statistical significant test result for a parameter, subsequent pairwise comparisons were used to determine which supplies differed. All tests were performed with 95% confidence and for the multiple comparisons the Bonferroni correction was applied. Linear regression analysis using log transformed concentrations was used to assess possible relationships between physical-chemical and microbiological parameters in the distributed water of the three supplies.
Nucleotide sequence accession numbers.
All partial 18S rRNA gene sequences determined in this study have been deposited in GenBank under accession numbers HQ998878 to HQ999868.
RESULTS
Quality characteristics of treated and distributed drinking water.
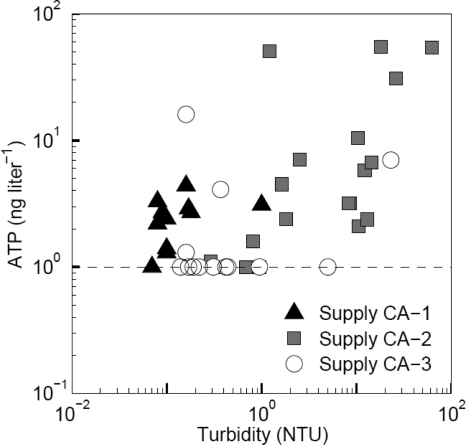
The water temperature at the treatment facilities and in the distribution systems of the three supplies ranged from about 28 to 34°C (Tables 1 and 2). The turbidity of treated water was low (≤0.3 nephelometric turbidity units [NTU]) at all facilities and remained low in the distribution system of supply CA-1. Turbidity exceeded 5 NTU at 10 locations in supply CA-2 and at two locations in supply CA-3 (Fig. 1). Similarly, the concentration of iron in treated water was low (≤0.04 mg Fe liter−1) and remained low (<0.01 mg Fe liter−1) in the distribution system of supply CA-1 but locally exceeded 1 mg Fe liter−1 in supply CA-2 (nine samples) and in supply CA-3 (one sample). The turbidity of the water in distribution systems CA-2 and CA-3 correlated significantly with the iron concentration, indicating that iron is the main cause of turbidity in these supplies (Table 3). These relationships yielded average iron-to-turbidity ratios of 0.24 ± 0.12 mg Fe liter−1/NTU−1 (supply CA-2) and 0.22 ± 0.13 mg Fe liter−1/NTU−1 (supply CA-3).
Table 1.
Average values of chemical and physical water quality characteristics of treated water of supplies CA-1, CA-2, and CA-3a
| Quality characteristic | Value for: |
|||
|---|---|---|---|---|
| Treated water CA-1 | Treated water CA-2a | Treated water CA-2b | Treated water CA-3 | |
| Temp (°C) | 30 | 28 | 31.6 | 28.7 |
| pH | 9.3 | 8.2 | 8.3 | 8.5 |
| Conductivity (μS cm−1) | 25 | 132 | 125 | 420 |
| Total hardness (CaCO3 mg liter−1) | 9.9 | 48.2 | 42.2 | 66.0 |
| Turbidity (NTU)b | 0.16 | 0.20 | 0.30 | 0.26 |
| Cl concn (mg liter−1) | 0.6 | 6.7 | 10.2 | 74 |
| Cu concn (mg liter−1) | 0.01 | 5 | 15 | 0.01 |
| Fe concn (mg liter−1) | 0.01 | 0.01 | 0.01 | 0.04 |
| NPOCc concn (mg C liter−1) | <0.05 | <0.05 | <0.05 | <0.05 |
Average values, based on routine monitoring over a period of 1 year.
NTU, nephelometric turbidity units.
NPOC, nonpurgeable organic carbon.
Table 2.
Quality characteristics of water sampled from the distributions systems of supplies CA-1, CA-2, and CA-3
| Parameter | Value for: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Supply CA-1 |
Supply CA-2 |
Supply CA-3 |
|||||||
| Median | Minimum | Maximum | Median | Minimum | Maximum | Median | Minimum | Maximum | |
| Temp (°C) | 32.7 | 31.5 | 34.4 | 31.9 | 27.4 | 32.9 | 29.1 | 27.8 | 31.4 |
| Turbidity (NTU) | 0.1 | 0.07 | 0.2 | 5.4 | 0.2 | 62.1 | 0.3 | 0.1 | 23.0 |
| Fe concn (mg liter−1) | <0.01 | <0.01 | <0.01 | 0.7 | 0.03 | 9.5 | 0.03 | <0.01 | 1.5 |
| ATP concn (ng liter−1) | 2.6 | 1.0 | 12.2 | 3.2 | <1.0 | 55.2 | <1.0 | <1.0 | 16.1 |
| H. vermiformis concn (cells liter−1) | 18 | 3 | 245 | 4 | <2 | 1670 | <2 | <2 | 4 |
| Acanthamoeba spp. concn (cells liter−1) | <2 | <2 | <2 | <2 | <2 | 56 | <2 | <2 | <2 |
| Cultivated Legionella spp. concn (CFU liter−1) | 1.3 × 104 | 3.0 × 102 | 2.5 × 105 | 7.1 × 103 | <2.5 × 102 | 1.0 × 105 | 7.5 × 102 | <2.5 × 102 | 6.5 × 104 |
Fig. 1.
Concentrations of turbidity and active biomass, measured as ATP, in the distributed water of supplies CA-1 (n = 14), CA-2 (n = 17), and CA-3 (n = 13). The detection limit for active biomass was 1 ng ATP liter−1.
Table 3.
Statistical analyses of differences in median concentrations (log transformed values) of selected water quality parameters of three drinking water supplies and correlations between the parameters in these suppliesa
| Parameter | Comparison between supplies |
||
|---|---|---|---|
| Supply CA-1 | Supply CA-2 | Supply CA-3 | |
| Turbidity (NTU) | <CA-2 | >CA-1, >CA-3 | <CA-2 |
| Iron concn (mg liter−1) | <CA-2 | >CA-1, >CA-3 | <CA-2 |
| ATP concn (ng liter−1) | NSb | >CA-3 | <CA-2 |
| H. vermiformis concn (cells liter−1) | >CA-2, >CA-3 | <CA-1 | <CA-1 |
| Legionella spp. concn (CFU liter−1) | >CA-3 | >CA-3 | <CA-1, <CA-2 |
| Correlation between parameters | |||
| Turbidity vs. iron concn | NS | R = 0.943 (P = 4.2 × 10−10) | R = 0.874 (P = 2.0 × 10−4) |
| Turbidity vs. ATP concn | NS | R = 0.705 (P = 5.2 × 10−4) | NS |
| Turbidity vs. Legionella spp. concn | NS | R = 0.821 (P = 9.1 × 10−6) | R = 0.834 (P = 7.4 × 10−4) |
| ATP concn vs. Legionella spp. concn | NS | R = 0.755 (P = 4.9 × 10−6) | NS |
| ATP concn vs. H. vermiformis concn | NS | NS | NS |
| H. vermiformis concn vs. Legionella spp. concn | R = 0.637 (P = 0.014) | R = 0.479 (P = 0.024) | NS |
Only results of parameters which are significantly different (P < 0.05) between two or three supplies are presented.
NS: not significant, P > 0.05.
In treated water of supply CA-1, a higher ATP concentration (3.7 ng ATP liter−1) was observed than in treated water of supplies CA-2 and CA-3, where the concentration was below the detection limit (<1 ng ATP liter−1). The elevated ATP concentration in treated water of supply CA-1 is due to an increase from <1 to 9 ng ATP liter−1 in the storage tanks at the plant. The ATP concentration did not increase during distribution in supply CA-1. ATP concentrations in distributed water of supply CA-2 (median, 3.2 ng ATP liter−1) were significantly higher than in supply CA-3 (median <1 ng ATP liter−1) and correlated with turbidity (Fig. 1 and Table 3).
H. vermiformis, Acanthamoeba spp., and cultivable Legionella spp. in treated and distributed water.
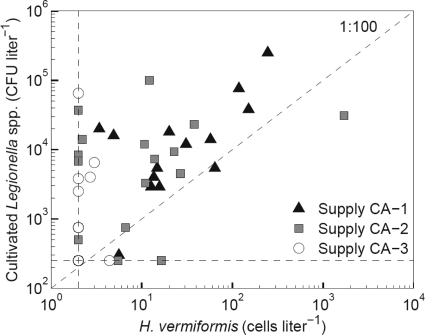
H. vermiformis (about 3 cells liter−1) and cultivable cells of Legionella spp. (1.0 × 102 CFU liter−1) were detected in the treated water of supply CA-1 after UV treatment. These microorganisms had grown in the storage tanks at the treatment plant where the concentration of H. vermiformis increased from <2 to 18 cells liter−1 and the concentration of Legionella spp. increased from <1 × 102 to 1.5 × 104 CFU liter−1. H. vermiformis was detected in all 14 samples collected from the distribution system of supply CA-1 (median value, 18 cells liter−1), in 12 of 20 samples of supply CA-2 (median value, 4 cells liter−1) (Fig. 2 and Table 2), and in 4 of the 13 samples from supply CA-3 at concentrations of about 3 cells liter−1. The concentrations of H. vermiformis in the distributed water of supply CA-1 were significantly higher than in the distributed water of supplies CA-2 and CA-3 (Table 3). Acanthamoeba spp. were observed in 5 samples of distributed water of supply CA-2 at concentrations ranging from 2 to 56 cells liter−1 (median, 8.0 cells liter−1) (Table 2). H. vermiformis was also detected in these samples.
Fig. 2.
Concentrations of H. vermiformis and cultivated Legionella spp. in distributed water of supplies CA-1, CA-2, and CA-3. Detection limits for H. vermiformis (2 cells liter−1) and for cultivated Legionella spp. (250 CFU liter−1) are shown as dotted lines.
Legionella spp. were cultured from 41 of the 49 samples of distributed water and from 28 of these 41 samples containing H. vermiformis (Fig. 2). In supplies CA-1 and CA-2, L. pneumophila represented 80 to 100% of the cultured Legionella colonies and 40 to 100% in supply CA-3. The colony counts of Legionella spp. in the distributed water of supplies CA-1 and CA-2 were significantly higher than those in supply CA-3 (Table 3). The concentration of Legionella spp. correlated significantly with turbidity in the distributed water of supplies CA-2 and CA-3, with the concentrations of ATP in supply CA-2 and with concentrations of H. vermiformis in supplies CA-1 and CA-2. In 13 of 41 distributed water samples with cultivated Legionella spp., the concentrations of H. vermiformis and Acanthamoeba spp. were below the detection limit of 2 cells liter−1.
Richness and identity of free-living protozoa.
T-RFLP analyses using 18S rRNA gene-targeting primers revealed that the eukaryotic richness in the distributed water was higher than in the treated water at the four plants (data not shown). A total of 225 (25%) of the 908 partial 18S rRNA gene sequences in the clone libraries of the three types of distributed water clustered within FLP and represented 59 (30%) of the 195 OTUs (≥99% sequence similarity) (Table 4). Up to 11 OTUs of FLP were obtained from 0.5 liter of distributed water of supplies CA-1, CA-2, and CA-3. The highest protozoan richness was observed in supply CA-2, but the estimated total OTU richness for FLP did not differ significantly between the three supplies (Table 5).
Table 4.
Classification of eukaryotic clones with >75% similarity to sequences in the SSU Ref SILVA98 database obtained from distributed water of supplies CA-1, CA-2, and CA-3a
| Kingdom, subkingdom, or group | Distributed water CA-1 |
Distributed water CA-2 |
Distributed water CA-3 |
All analyzed samples |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of OTUsb | % of OTUs | % of clones in libraries | No. of OTUsb | % of OTUs | % of clones in libraries | No. of OTUsb | % of OTUs | % of clones in libraries | No. of OTUsb | % of OTUs | % of clones in libraries | |
| Free-living protozoa | 23 | 36.5 | 36.0 | 36 | 33.6 | 25.5 | 13 | 25.0 | 14.6 | 59 | 30.3 | 24.8 |
| Fungi | 33 | 52.4 | 53.3 | 41 | 38.3 | 35.1 | 17 | 32.7 | 15.0 | 83 | 42.6 | 33.5 |
| Metazoa | 1 | 1.6 | 3.3 | 22 | 20.6 | 34.2 | 9 | 17.3 | 47.9 | 29 | 14.9 | 30.9 |
| Cryptophyta and Viridiplantae | 2 | 3.2 | 2.3 | 7 | 6.5 | 4.7 | 12 | 23.1 | 21.7 | 18 | 9.2 | 9.1 |
| Sequences with <75% similarity | 4 | 6.3 | 5.1 | 1 | 0.9 | 0.5 | 1 | 1.9 | 0.7 | 6 | 3.1 | 1.7 |
| Total | 63 | 100 | 100 | 107 | 100 | 100 | 52 | 100 | 100 | 195 | 100 | 100 |
Data are totals for all analyzed samples of distributed water of the indicated supply.
OTUs obtained from more than one sample are included only once. Each OTU contains 18S rRNA gene sequences with a minimum of 99% similarity.
Table 5.
Numbers of retrieved clones and OTUs and estimated richness of OTUs (sequence similarity of ≥99%) in distributed water of supplies CA-1, CA-2, and CA-3d
| Type and source of organism | No. of clones | No.a of OTUs identified | Coverage indexb | Total estimated OTU richness (Chao1)c |
||
|---|---|---|---|---|---|---|
| Mean | Lower limit | Upper limit | ||||
| Eukaryotes in clone libraries | ||||||
| Distributed water CA-1 | 214 | 63 | 30 | 129 | 89 | 236 |
| Distributed water CA-2 | 427 | 107 | 25 | 222 | 165 | 337 |
| Distributed water CA-3 | 267 | 52 | 19 | 118 | 81 | 209 |
| Total | 908 | 195 | 21 | 452 | 347 | 633 |
| Free-living protozoa in clone libraries | ||||||
| Distributed water CA-1 | 77 | 23 | 30 | 42 | 27 | 95 |
| Distributed water CA-2 | 109 | 36 | 33 | 59 | 43 | 110 |
| Distributed water CA-3 | 39 | 13 | 33 | 22 | 15 | 58 |
| Total | 225 | 59 | 26 | 111 | 79 | 190 |
OTUs obtained from more than one sample are included only once; therefore, the sum of the OTUs in the three supplies gives excess values.
Number of OTUs/number of sequences × 100%.
The Chao1 index (7) was calculated with DOTUR (40). Chao1 estimation is based on the total of the clones; therefore, the sums of the estimated values per supply give other values.
Data are totals for all analyzed samples of distributed water of the indicated water supply.
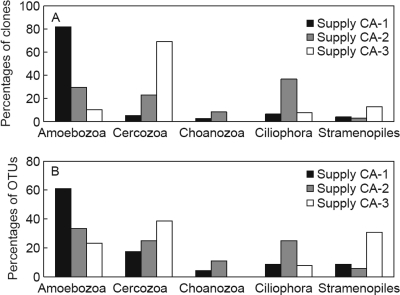
OTUs of Amoebozoa represented a large proportion of the clones retrieved from the distributed water of supply CA-1 (Fig. 3; see also Table S1 in the supplemental material). Eukaryotic clone libraries were also constructed from the two samples of treated water before UV treatment at plant CA-1, because Legionella spp. were detected in the water before and after UV treatment. OTUs which clustered with Amoebozoa also predominated (>90%) in these clone libraries (see Table S2 in the supplemental material). A large proportion (39%) of the protozoan-related OTUs in the clone libraries of distributed water of supply CA-3 clustered within the phylum of the Cercozoa, whereas a large and even distribution of Amoebozoa (33%), Ciliophora (25%), and Cercozoa (25%) was observed in the clone libraries of distributed water of supply CA-2. A few OTUs clustering within the Choanozoa phylum were obtained from supplies CA-1 and CA-2 (Fig. 3). The OTUs related to the Stramenopiles clustered with flagellates and algae observed in freshwater or soils, although many Stramenopiles types occur in marine environments (3, 37).
Fig. 3.
(A) Taxonomic distribution of free-living protozoa, based on 18S rRNA gene clones retrieved from distributed water of supplies CA-1, CA-2, and CA-3. (B) Taxonomic distribution of OTUs with highest similarity to free-living protozoa retrieved from distributed water of supplies CA-1, CA-2, and CA-3. Data are totals for all analyzed samples of distributed water of the indicated supply.
Of the 59 OTUs of FLP which showed the highest similarity to H. vermiformis and Hemiophrys procera, 2 (3%) were obtained from all three supplies (see Table S1 in the supplemental material for details). Of these 59 OTUs, 9 (15%) were obtained from the clone libraries of two supplies. Approximately 30% of the OTUs of supply CA-1 were observed in more than one sample and about 20% of the OTUs in supplies CA-2 and CA-3 were observed at more than one location in the distribution system. Hence, the protozoan communities in the three supplies differed from each other and between locations within one supply. H. vermiformis predominated in the clone libraries of distributed water in four of five samples from supply CA-1, in two of nine samples from supply CA-2, and in one of six samples from supply CA-3. OTUs with the highest similarity to Acanthamoeba spp. and to candidate hosts for L. pneumophila, viz., Echinamoeba exundans, Echinamoeba thermarum, and Neoparamoeba spp., were observed in supplies CA-1 and CA-2.
Fungi and other small eukaryotes.
A total of 83 OTUs (43%) showed the highest similarity to fungi, 46 of which clustered within the Ascomycota, the predominating fungus (≥49%) in all three supplies (Table 4; see also Table S3 in the supplemental material). One OTU with >99% similarity to the potential pathogen Mucor racemosus was obtained from treated water of supply CA-1, and two OTUs with >99% similarity to M. racemosus and the potential pathogen Malassezia restricta were obtained from distributed water of supply CA-2.
The metazoa were represented by 29 OTUs, 19 (66%) of which showed the highest similarity to species of nematodes (Table 4; see also Table S4 in the supplemental material). From supply CA-1 only one metazoan OTU, which clustered with Rotifera, was retrieved, and the estimated average OTU richness for metazoa in this supply (1 OTU) was significantly lower than in the supplies CA-2 (40 OTUs) and CA-3 (34 OTUs).
Ten OTUs, obtained from all three supplies, clustered within the Cryptophyta phylum and one of these OTUs, with 93% similarity to Chroomonas sp., was obtained from all three supplies (Table 4; see also Table S5 in the supplemental material). A total of eight OTUs, obtained from all three distributed water types, showed the highest similarity to viridiplantae. Six (3%) of the 195 obtained OTUs had similarities below 75% for described sequences in the SILVA database and remained unidentified, as described before (51).
These observations show that the eukaryotic communities and the concentrations of cultivable Legionella spp. differed in the investigated supplies all using seawater as a source.
DISCUSSION
Detection of free-living protozoa with PCR-based methods.
In the present study, molecular techniques targeting the 18S rRNA gene were instrumental for the detection and identification of a large variety of small eukaryotes, including potential protozoan hosts for Legionella spp., in three drinking water supplies in the Caribbean at water temperatures of about 30°C. The relative abundances of different sequences in the clone libraries may not represent the community composition because different eukaryotic species can largely differ in 18S rRNA gene copy numbers, in particular in metazoa (25). For obtaining quantitative information, specific qPCR assays were used for the detection of two groups of FLP serving as environmental hosts for L. pneumophila, viz., H. vermiformis and Acanthamoeba spp.
H. vermiformis was observed with the specific qPCR (23) in all samples from which clones with ≥99% similarity to this organism were retrieved. However, such clones were not obtained from a few samples of supplies CA-2 and CA-3 which were positive with the qPCR for H. vermiformis. Obviously, the eukaryotic communities in these samples were predominantly populated by other organisms. Clones with 82 to 86% similarity to Acanthamoeba spp. were retrieved from several samples of supplies CA-2 and CA-3, but the specific qPCR for Acanthamoeba spp. (35) was negative in these samples. These 18S rRNA genes were not amplified with the Acanthamoeba genus-specific primers, suggesting that these sequences did not represent Acanthamoeba spp. These observations confirm the utility of qPCR methods for detecting specific FLP.
Conditions affecting microbial growth and protozoan richness in the three supplies.
The water quality of the three supplies, using seawater as a source, is influenced by the treatment processes, e.g., type of desalination, filtration processes, softening, addition of corrosion inhibitors or chlorine, and the conditions in the distribution system, e.g., pipe materials, hydraulics, and residence time. Treated water from the examined treatment plants, all using seawater as a source, contained a very low concentration of natural organic matter (NOM, <0.1 mg C liter−1) and had a low turbidity (Table 1). Still, the three supplies differed in concentrations of ATP, H. vermiformis, colony-forming Legionella spp., and compositions of communities of free-living protozoa and other eukaryotes in the distributed water (Tables 3 and 5). These parameters also varied between the different locations within one supply area, demonstrating the complexity of the interactions with environmental conditions. Comparison of the observations in the three Caribbean supplies with those in a similar study of two water supplies in the Netherlands (51) and the typical behavior of certain identified organisms enable the determination of several conditions affecting the microbial communities.
The water temperature in the Caribbean supplies was 10 to 15°C above the temperature in temperate regions. Temperature can affect the identity of the FLP in drinking water supplies, but in both regions, clones clustered within Amoebozoa, Cercozoa, Choanozoa, Ciliophora, and Stramenopiles (Fig. 3) (33, 50, 51). However, sequences related to Euglenozoa and Myzozoa were observed only in the temperate region, although certain euglenozoan types have been found in a volcanic area at a temperature above 30°C (44). The clear differences between the taxonomic distributions within the FLP communities in the three Caribbean supplies (Fig. 3) demonstrate that certain environmental conditions other than temperature affect the abundance and community composition of FLP in water supplies.
The varying ATP concentrations, combined with the low turbidity and the low iron concentration in distributed water of supply CA-1, suggest that microbial growth mainly occurs in biofilms on the walls of reservoirs and pipes of this supply (Table 2). This suggestion is supported by the relatively high concentrations of H. vermiformis and the large proportion and high richness of Amoebozoa in this supply, because amoebae feed much more effectively on microorganisms in a biofilm than on suspended prey (32). Furthermore, the absence of metazoa in most samples of supply CA-1 indicates that concentrations of sediments, which are needed for their growth, were low (53).
The high turbidities in supply CA-2 correlate with iron concentrations, indicating that sediments originate from corroding cast iron pipes (Tables 2 and 3). The correlations between turbidity and ATP and between turbidity and cultivable Legionella spp. demonstrate that these sediments support microbial growth. In comparison with supplies of CA-1 and CA-3, sequences related to Ciliophora constituted a relatively high proportion (25%) of OTUs of the FLP in supply CA-2 (Fig. 3). Ciliates feed effectively on suspended bacteria, and their relatively large cell size enables these organisms to consume a large variety of prey types, such as algae, flagellates, and other ciliates (32). In addition, a large number of metazoan OTUs, namely, 22, were observed in supply CA-2 (Table 4). Ciliates and metazoa also constituted significant proportions of the eukaryotic community in the distribution system of a groundwater supply in the Netherlands, with elevated concentrations of ATP (10 ng liter−1) and NOM (8 mg C liter−1) (51). Obviously, these conditions and accumulation of sediments promote the growth of metazoa, ciliates, and also cultivable Legionella spp.
At most locations in supply CA-3, low turbidities (<0.5 NTU) and low concentrations of iron (<0.05 mg liter−1), ATP (<1 ng liter−1), H. vermiformis (<2 cells liter−1), and cultivable Legionella spp. (<1 × 103 CFU liter−1) were observed (Tables 2 and 3). Elevated ATP concentrations (>4 ng liter−1) at three locations, indicating local accumulation of biomass, did not all correspond with elevated turbidity. Small flagellated cercozoan types, mainly Cercomonas spp., predominated (69% of clones) in the free-living protozoan communities in the clone libraries of supply CA-3 (Fig. 3). These flagellates can produce pseudopodia which attach to surfaces, but they preferentially feed on suspended prey (28, 32). In an experimental distribution system, flagellates predominated in the drinking water but were absent in the related biofilm (43). Cercozoan types also predominated in the biofilm in a groundwater supply in the Netherlands with low concentrations of ATP (<1 ng liter−1) and NOM (<0.5 mg C liter−1), but they were a minor fraction in a groundwater supply with elevated concentrations of ATP and NOM (51).
No significant correlation was observed between the concentrations of ATP and H. vermiformis in the water samples collected from the three Caribbean supplies (Table 3). In supply CA-3, the H. vermiformis concentration was below the detection level (<2 cell liter−1) in all but one of the samples with an ATP concentration below 1 ng liter−1. Also, this organism was not detected in water and biofilms in the distribution system of a groundwater supply in the Netherlands with ATP concentrations of <1 ng liter−1 and low biofilm concentrations (51). H. vermiformis was observed at concentrations up to 815 cells liter−1 in the summer in the Netherlands in distributed water with elevated concentrations of NOM and ATP. These observations suggest that growth of H. vermiformis in drinking water distribution systems is limited at ATP concentrations of <1 ng liter−1.
Host protozoa, pathogenic free-living protozoa, and fungi.
The detection of H. vermiformis, Acanthamoeba spp., and L. pneumophila in the investigated supplies is consistent with results of other studies on drinking water systems in tropical regions (3, 5, 31, 39, 45). The current study confirmed that H. vermiformis is a much more common amoeba in drinking water than Acanthamoeba spp. (50). This difference may in part be explained by the higher yield of H. vermiformis than Acanthamoeba spp. when feeding on prey bacteria (58).
The colony counts of Legionella spp. in supplies CA-1 and CA-2 correlated significantly with the concentration of H. vermiformis (Fig. 2 and Table 3). The log value of the ratios between the concentrations of Legionella spp. and H. vermiformis in distributed water samples containing both organisms ranged from 1.2 to 3.9. These values are below the upper tolerance limit of 4.5 as determined in biofilm batch tests using different types of freshwater inoculated with L. pneumophila and H. vermiformis and incubated at 37°C (50). Above this upper tolerance limit, protozoan hosts for growth of L. pneumophila other than H. vermiformis were observed in these tests. The observations of the present study thus confirm the prominent position of H. vermiformis as a host for L. pneumophila in freshwater environments (13, 24, 50, 56). In all three supplies, OTUs related to the described hosts E. exundans (13) and Acanthamoeba spp. (1, 38) were detected in samples with H. vermiformis, indicating that more than one protozoan species may have served as a host for Legionella spp. at these locations. Also the candidate hosts Neoparamoeba sp. and E. thermarum (50) were observed in the present study, but none of the other protozoa were identified as hosts by using in vitro experiments (13, 22, 42). However, certain protists belonging to the genera Naegleria and Vahlkampfia, which include hosts for L. pneumophila (37, 49) and/or human pathogens (59), were not amplified with the primers used. Acanthamoeba spp. have been identified as opportunistic human pathogens (9, 21), but it is unclear whether the sequences related to such species represent organisms with pathogenic characteristics.
Fungi, metazoa, viridiplantae, and Chryptophyta species are relatively common in drinking water distribution systems (15, 16, 20, 51). Clones with >99% similarity to the pathogenic fungi Mucor racemosus and Malassezia restricta (17, 46) were obtained from supplies CA-1 and CA-2. OTUs clustering with the genera Basidiobolus, Candida, Pichia, and Penicillium, which include pathogenic species (2, 10, 30, 60), were also obtained from the distributed water of all three supplies. However, the public health significance of the presence of fungi related to pathogenic species is not clear (16, 51).
In conclusion, highly diverse communities of free-living protozoa and other small eukaryotes were observed in the three investigated supplies. The growth of these organisms and Legionella spp. is enhanced by biofilms and corrosion-related sediments. An ATP concentration of <1 ng liter−1 in drinking water indicates growth-limiting conditions for H. vermiformis. Limiting the multiplication of Legionella spp. therefore implies reduction of the growth potential of the water and prevention of sediment accumulation.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Caribbean Water Association (CWA), Delft Cluster (project CT 06.10) and the water supply companies in the Netherlands in the framework of the Joint Research Program.
The assistance of the CWA representatives in providing information about the water supplies and selection of sampling locations and in facilitating sampling and chemical analysis is gratefully acknowledged. The statistical support of Paul Baggelaar (Icastat) is greatly appreciated. We thank Hauke Smidt (Wageningen University) and Wim Hoogenboezem (Het Water Laboratorium, Haarlem) for valuable discussions and critical reading of the manuscript and the staff of the Laboratory for Microbiology of KWR Watercycle Research Institute for skillful technical assistance.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 26 August 2011.
REFERENCES
- 1. Anand C. M., Skinner A. R., Malic A., Kurtz J. B. 1983. Interaction of L. pneumophilia and a free living amoeba (Acanthamoeba palestinensis). J. Hyg. (Lond.) 91:167–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bergman M. M., Gagnon D., Doern G. V. 1998. Pichia ohmeri fungaemia.. Diagn. Microbiol. Infect. Dis. 30:229–231 [DOI] [PubMed] [Google Scholar]
- 3. Bonilla-Lemus P., et al. 2010. Acanthamoeba spp. in domestic tap water in houses of contact lens wearers in the metropolitan area of Mexico City. Exp. Parasitol. 126:54–58 [DOI] [PubMed] [Google Scholar]
- 4. Bonnélye V., et al. 2007. Curacao, Netherlands Antilles: a successful example of boron removal on a seawater desalination plant. Desalination 205:200–205 [Google Scholar]
- 5. Carlesso A. M., Simonetti A. B., Artuso G. L., Rott M. B. 2007. Isolation and identification of potentially pathogenic free-living amoebae in samples from environments in a public hospital in the city of Porto Alegre, Rio Grande do Sul. Rev. Soc. Bras. Med. Trop. 40:316–320 (In Portuguese) [DOI] [PubMed] [Google Scholar]
- 6. Cavalier-Smith T. 2002. The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa. Int. J. Syst. Evol. Microbiol. 52:297–354 [DOI] [PubMed] [Google Scholar]
- 7. Chao A. 1987. Estimating the population size for capture-recapture data with unequal catchability. Biometrics 43:783–791 [PubMed] [Google Scholar]
- 8. Cowgill K. D., et al. 2005. Recurrence of legionnaires disease at a hotel in the United States Virgin Islands over a 20-year period. Clin. Infect. Dis. 40:1205–1207 [DOI] [PubMed] [Google Scholar]
- 9. Culbertson C. G. 1961. Pathogenic Acanthamoeba (Hartmanella). Am. J. Clin. Pathol. 35:195–202 [DOI] [PubMed] [Google Scholar]
- 10. Deng Z., Ribas J. L., Gibson D. W., Connor D. H. 1988. Infections caused by Penicillium marneffei in China and Southeast Asia: review of eighteen published cases and report of four more Chinese cases. Rev. Infect. Dis. 10:640–652 [DOI] [PubMed] [Google Scholar]
- 11. Edelstein P. H. 1981. Improved semiselective medium for isolation of Legionella pneumophila from contaminated clinical and environmental specimens. J. Clin. Microbiol. 14:298–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fields B. S., Benson R. F., Besser R. E. 2002. Legionella and Legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 15:506–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fields B. S., et al. 1989. Intracellular multiplication of Legionella pneumophila in amoebae isolated from hospital hot water tanks. Curr. Microbiol. 18:131–137 [Google Scholar]
- 14. Goh K. T., Ng D. L., Yap J., Ma S., Ooi E. E. 2005. Surveillance, prevention, and control of legionellosis in a tropical city-state. Am. J. Infect. Control 33:286–291 [DOI] [PubMed] [Google Scholar]
- 15. Hageskal G., Knutsen A. K., Gaustad P., de Hoog G. S., Skaar I. 2006. Diversity and significance of mold species in Norwegian drinking water. Appl. Environ. Microbiol. 72:7586–7593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hageskal G., Lima N., Skaar I. 2009. The study of fungi in drinking water. Mycol. Res. 113:165–172 [DOI] [PubMed] [Google Scholar]
- 17. Hata D. J., Buckwalter S. P., Pritt B. S., Roberts G. D., Wengenack N. L. 2008. Real-time PCR method for detection of zygomycetes. J. Clin. Microbiol. 46:2353–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hughes J. B., Hellmann J. J., Ricketts T. H., Bohannan B. J. 2001. Counting the uncountable: statistical approaches to estimating microbial diversity. Appl. Environ. Microbiol. 67:4399–4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Inglis T. J., et al. 2004. Preliminary report on the northern Australian melioidosis environmental surveillance project. Epidemiol. Infect. 132:813–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jjemba P. K., Weinrich L. A., Cheng W., Giraldo E., Lechevallier M. W. 2010. Regrowth of potential opportunistic pathogens and algae in reclaimed water distribution systems. Appl. Environ. Microbiol. 76:4169–4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones D. B., Visvesvara G. S., Robinson N. M. 1975. Acanthamoeba polyphaga keratitis and Acenthamoeba uveitis associated with fatal meningoencephalitis. Trans. Ophthalmol. Soc. U. K. 95:221–232 [PubMed] [Google Scholar]
- 22. Kikuhara H., Ogawa M., Miyamoto H., Nikaido Y., Yoshida S. 1994. Intracellular multiplication of Legionella pneumophila in Tetrahymena thermophila. J. UOEH 16:263–275 [DOI] [PubMed] [Google Scholar]
- 23. Kuiper M. W., et al. 2006. Quantitative detection of the free-living amoeba Hartmannella vermiformis in surface water by using real-time PCR. Appl. Environ. Microbiol. 72:5750–5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuiper M. W., Wullings B. A., Akkermans A. D., Beumer R. R., van der Kooij D. 2004. Intracellular proliferation of Legionella pneumophila in Hartmannella vermiformis in aquatic biofilms grown on plasticized polyvinyl chloride. Appl. Environ. Microbiol. 70:6826–6833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Long E. O., Dawid I. B. 1980. Repeated genes in eukaryotes. Annu. Rev. Biochem. 49:727–764 [DOI] [PubMed] [Google Scholar]
- 26. Ludwig W., et al. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Magic-Knezev A., van der Kooij D. 2004. Optimisation and significance of ATP analysis for measuring active biomass in granular activated carbon filters used in water treatment. Water Res. 38:3971–3979 [DOI] [PubMed] [Google Scholar]
- 28. Myl'nikov A. P., Karpov S. A. 2004. Review of diversity and taxonomy of cercomonads. Protistology 3:201–217 [Google Scholar]
- 29. Nedelands Normalisatie Instituut 1991. Bacteriological examination of water: examination on the presence and the number of colony forming units (CFU) of Legionella bacteria. NEN6265. Nederlands Normalisatie Instituut, Delft, the Netherlands [Google Scholar]
- 30. Odds F. C. 1987. Candida infections: an overview. Crit. Rev. Microbiol. 15:1–5 [DOI] [PubMed] [Google Scholar]
- 31. Ortiz-Roque C. M., Hazen T. C. 1987. Abundance and distribution of Legionellaceae in Puerto Rican waters. Appl. Environ. Microbiol. 53:2231–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parry J. D. 2004. Protozoan grazing of freshwater biofilms. Adv. Appl. Microbiol. 54:167–196 [DOI] [PubMed] [Google Scholar]
- 33. Poitelon J. B., et al. 2009. Identification and phylogeny of eukaryotic 18S rDNA phylotypes detected in chlorinated finished drinking water samples from three Parisian surface water treatment plants. Lett. Appl. Microbiol. 49:589–595 [DOI] [PubMed] [Google Scholar]
- 34. Pruesse E., et al. 2007. SILVA; a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 35:7188–7196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qvarnstrom Y., Visvesvara G. S., Sriram R., da Silva A. J. 2006. Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri. J. Clin. Microbiol. 44:3589–3595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rodriguez-Zaragoza S. 1994. Ecology of free-living amoebae. Crit. Rev. Microbiol. 20:225–241 [DOI] [PubMed] [Google Scholar]
- 37. Rowbotham T. J. 1986. Current views on the relationships between amoebae, legionellae and man. Isr. J. Med. Sci. 22:678–689 [PubMed] [Google Scholar]
- 38. Rowbotham T. J. 1980. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 33:1179–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schlech W. F., Gorman G. W., Payne M. C., Broome C. V. 1985. Legionnaires' disease in the Caribbean. An outbreak associated with a resort hotel. Arch. Intern. Med. 145:2076–2079 [PubMed] [Google Scholar]
- 40. Schloss P. D., Handelsman J. 2005. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl. Environ. Microbiol. 71:1501–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scrimini S., Junemann A., Luna C. M. 2007. Community acquired pneumonia in the tropics. Curr. Opin. Pulm. Med. 13:170–176 [DOI] [PubMed] [Google Scholar]
- 42. Shadrach W. S., et al. 2005. Balamuthia mandrillaris, free-living ameba and opportunistic agent of encephalitis, is a potential host for Legionella pneumophila bacteria. Appl. Environ. Microbiol. 71:2244–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sibille I., Sime-Ngando T., Mathieu L., Block J. C. 1998. Protozoan bacterivory and Escherichia coli survival in drinking water distribution systems. Appl. Environ. Microbiol. 64:197–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sittenfeld A., et al. 2002. Characterization of a photosynthetic Euglena strain isolated from an acidic hot mud pool of a volcanic area of Costa Rica. FEMS Microbiol. Ecol. 42:151–161 [DOI] [PubMed] [Google Scholar]
- 45. Storey M. V., Kaucner C. E., Angles M. L., Blackbeard J. R., Ashbolt N. J. 2008. Opportunistic pathogens in drinking and recycled water distribution systems. Water 35:38–45 [Google Scholar]
- 46. Sugita T., Tajima M., Amaya M., Tsuboi R., Nishikawa A. 2004. Genotype analysis of Malassezia restricta as the major cutaneous flora in patients with atopic dermatitis and healthy subjects. Microbiol. Immunol. 48:755–759 [DOI] [PubMed] [Google Scholar]
- 47. Thomas J. M., Ashbolt N. J. 2011. Do free-living amoebae in treated drinking water systems present an emerging health risk? Environ. Sci. Technol. 45:860–869 [DOI] [PubMed] [Google Scholar]
- 48. Thomas V., McDonnell G., Denyer S. P., Maillard J. Y. 2009. Free-living amoebae and their intracellular pathogenic microorganisms: risks for water quality. FEMS Microbiol. Rev. 34:231–259 [DOI] [PubMed] [Google Scholar]
- 49. Tyndall R. L., Domingue E. L. 1982. Cocultivation of Legionella pneumophila and free-living amoebae. Appl. Environ. Microbiol. 44:954–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Valster R. M., Wullings B. A., van der Kooij D. 2010. Detection of protozoan hosts for Legionella pneumophila in engineered water systems by using a biofilm batch test. Appl. Environ. Microbiol. 76:7144–7153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Valster R. M., Wullings B. A., Bakker G., Smidt H., van der Kooij D. 2009. Free-living protozoa in two unchlorinated drinking water supplies identified by phylogenetic analysis of 18S rRNA gene sequences. Appl. Environ. Microbiol. 75:4736–4746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Van der Kooij D. 1998. Potential for biofilm development in drinking water distribution systems. J. Appl. Microbiol. 85(Suppl.):39S–44S [DOI] [PubMed] [Google Scholar]
- 53. Van Lieverloo J. H. M., van der Kooij D., Hoogenboezem W. 2002. Invertebrates and protozoa (free-living) in drinking water distribution systems, p. 1718–1733 In Bitton G. (ed.), Encyclopedia of environmental microbiology. Wiley, New York, NY [Google Scholar]
- 54. Visvesvara G. S., Schuster F. L., Martinez A. J. 1993. Balamuthia mandrillaris, N. G., N. Sp., agent of amebic meningoencephalitis in humans and other animals. J. Eukaryot. Microbiol. 40:504–514 [DOI] [PubMed] [Google Scholar]
- 55. Volk C. J., LeChevallier M. W. 1999. Impacts of the reduction of nutrient levels on bacterial water quality in distribution systems. Appl. Environ. Microbiol. 65:4957–4966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wadowsky R. M., et al. 1988. Growth-supporting activity for Legionella pneumophila in tap water cultures and implication of hartmannellid amoebae as growth factors. Appl. Environ. Microbiol. 54:2677–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wadowsky R. M., Wolford R., McNamara A. M., Yee R. B. 1985. Effect of temperature, pH, and oxygen level on the multiplication of naturally occurring Legionella pneumophila in potable water. Appl. Environ. Microbiol. 49:1197–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weekers P. H., Bodelier P. L., Wijen J. P., Vogels G. D. 1993. Effects of grazing by the free-living soil amoebae Acanthamoeba castellanii, Acanthamoeba polyphaga, and Hartmannella vermiformis on various bacteria. Appl. Environ. Microbiol. 59:2317–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Willaert E. 1974. Primary amoebic meningo-encephalitis. A selected bibliography and tabular survey of cases. Ann. Soc. Belg. Med. Trop. 54:429–440 [PubMed] [Google Scholar]
- 60. Zavasky D. M., Samowitz W., Loftus T., Segal H., Carroll K. 1999. Gastrointestinal zygomycotic infection caused by Basidiobolus ranarum: case report and review. Clin. Infect. Dis. 28:1244–1248 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.