Abstract
The S213C, I33L, and I33L S213C variants of d-psicose 3-epimerase from Agrobacterium tumefaciens, which were obtained by random and site-directed mutagenesis, displayed increases of 2.5, 5, and 7.5°C in the temperature for maximal enzyme activity, increases of 3.3-, 7.2-, and 29.9-fold in the half-life at 50°C, and increases of 3.1, 4.3, and 7.6°C in apparent melting temperature, respectively, compared with the wild-type enzyme. Molecular modeling suggests that the improvement in thermostability in these variants may have resulted from increased putative hydrogen bonds and formation of new aromatic stacking interactions. The immobilized wild-type enzyme with and without borate maintained activity for 8 days at a conversion yield of 70% (350 g/liter psicose) and for 16 days at a conversion yield of 30% (150 g/liter psicose), respectively. After 8 or 16 days, the enzyme activity gradually decreased, and the conversion yields with and without borate were reduced to 22 and 9.6%, respectively, at 30 days. In contrast, the activities of the immobilized I33L S213C variant with and without borate did not decrease during the operation time of 30 days. These results suggest that the I33L S213C variant may be useful as an industrial producer of d-psicose.
INTRODUCTION
d-Psicose (d-allulose), a carbon-3 epimer of d-fructose, is present in small quantities as a nonfermentable constituent of cane molasses (1), as a sugar moiety of the nucleoside antibiotic psicofuranine (4), and as a free sugar in wheat (21) and Itea plants (5). This rare sugar provides suitable sweetness, smooth texture, favorable mouthfeel, and long-time storage stability in food products (23). Psicose is considered to be a potential reduced energy sweetener because the sugar suppresses hepatic lipogenic enzyme activity (18) and does not contribute to calorie production (19).
Biological production of psicose from fructose has been studied using d-psicose 3-epimerase from Agrobacterium tumefaciens (10, 13, 16) and d-tagatose 3-epimerases from Pseudomonas cichorii (7, 24) and Rhodobacter sphaeroides (26). d-Psicose 3-epimerase from A. tumefaciens has been more effective than the d-tagatose 3-epimerases for the production of psicose. However, d-psicose 3-epimerase is inefficient for the industrial production of psicose because of its short half-life of 63 min at 50°C (10). Thus, improvement in the thermostability of A. tumefaciens d-psicose 3-epimerase is essential for the industrial production of psicose.
Random mutagenesis and rational protein design are typical tools for improving enzyme thermostability in the protein engineering field. Random mutagenesis techniques such as error-prone PCR and DNA shuffling can be easily applied for the improvement of enzyme thermostability (8, 9, 12, 28) because these techniques do not require detailed structural information or accurate predictions at the substituted residues (14).
In the present study, thermostable variants of d-psicose 3-epimerase from A. tumefaciens were obtained by random and site-directed mutagenesis. The temperature for maximal activity, thermostability, apparent melting temperature (Tm), kinetic parameters, and operational stability in a bioreactor of the variants were determined and compared with those of the wild-type enzyme. Moreover, improvement in the thermostability of the variants was elucidated structurally by molecular modeling.
MATERIALS AND METHODS
Error-prone PCR and site-directed mutagenesis.
A mutant library of d-psicose 3-epimerase from A. tumefaciens ATCC 33970 was constructed by an error-prone PCR, which was carried out using a PCR mutagenesis kit (ClonTech Laboratories, Palo Alto, CA) with a mutation rate of 2 to 3 mutations per 1,000 bp using plasmid pTrc99A. Forward primer DPE-F (5′-AGGAAACAGACCATGGGTAAACACGGCA-3′) and reverse primer DPE-R (5′-GCTTGCATGCCTGCAGTCAGCCACCAAG-3′) were designed to introduce the NcoI and PstI restriction sites (underlined) and were synthesized by Bioneer (Daejon, South Korea). The PCR products, which were obtained by PCR with Taq polymerase (ClonTech Laboratories), were used for transformation in the Escherichia coli BL21 strain. Site-directed mutagenesis was performed using the QuikChange site-directed mutagenesis kit and protocol (Stratagene, Beverly, MA). DNA sequencing was performed at the DNA sequencing facility of Macrogen (Seoul, South Korea).
Screening for thermostable variants.
E. coli BL21 cells harboring pTrc99A plasmid containing a mutant gene coding for A. tumefaciens d-psicose 3-epimerase were cultivated in a 96-well plate with 200 μl of Luria-Bertani (LB) medium containing 50 μg/ml of ampicillin per well at 37°C with shaking at 500 rpm for 6 h in a plate shaker. Subsequently, 10 μl of culture broth was added to the wells of a new 96-well plate with 200 μl of LB medium containing 50 μg/ml of ampicillin and 0.1 mM isopropyl β-d-thiogalactopyranoside (IPTG) for induction of enzyme expression, followed by incubation at 37°C with shaking at 500 rpm for 6 h. The cultured mutants were then heated at 60°C for 5 min. After heating, 100 μl of culture broth and 50 μl fructose at a final concentration of 15 mM were added to the wells of another 96-well plate and incubated at 50°C for 30 min. The fructose concentration of the reaction mixture was determined using a fructose assay kit with d-fructose dehydrogenase, according to the manufacturer's instructions (Toyobo, Osaka, Japan). Mutants that exhibited fructose-converting activities greater than 1.2-fold that of wild-type cells were selected.
For the second round of selection, mutants were cultivated in 3 ml of LB broth containing 50 μg/ml of ampicillin in a 20 ml-test tube at 37°C with shaking at 200 rpm for 6 h. Then, IPTG was added to a final concentration of 0.1 mM to induce expression, and the culture was incubated at 37°C with shaking at 200 rpm for another 6 h. The cells were harvested and disrupted on ice using a sonicator. Cell debris was removed by centrifugation at 13,000 × g for 20 min at 4°C. The crude extracts were obtained as the supernatants were heated at 55°C for 5 min and then incubated at 50°C for 10 min in 50 mM N-(2-hydroxyethyl)piperazine-N′-(3-propanesulfonic acid) (EPPS) buffer (pH 8.0) containing 20 mM fructose. Mutants were selected by determining the enzyme activity for psicose production using high-performance liquid chromatography (HPLC).
After the second round of selection, the mutant genes coding for d-psicose 3-epimerase in pTrc99A of the selected mutants were transferred to pET-24a(+), and the recombinant plasmid was transformed into E. coli BL21. The recombinant cells were cultivated in a 2-liter flask with 500 ml of LB broth containing 50 μg/ml of kanamycin at 37°C with shaking at 200 rpm. When the optical density of bacteria reached 0.6 at an absorbance of 600 nm, IPTG was added to a final concentration of 0.1 mM, and the culture was incubated at 16°C with shaking at 150 rpm for 16 h. The purified enzymes were prepared as a described below. Based on the psicose-producing activities and half-lives of the purified enzymes, the variants were finally selected.
Enzyme purification.
The cells were harvested from culture broth by centrifugation at 6,000 × g for 30 min at 4°C, washed twice with 0.85% NaCl, and then resuspended in 50 mM phosphate buffer (pH 8.0) containing 300 mM KCl and 10 mM imidazole. The resuspended cells were disrupted on ice using a sonicator. The unbroken cells and cell debris were removed by centrifugation at 13,000 × g for 20 min at 4°C, and the supernatant was applied to an immobilized metal ion affinity chromatography (IMAC) cartridge (Bio-Rad, Hercules, CA) equilibrated with 50 mM phosphate buffer (pH 8.0). The cartridge was washed extensively with the same buffer, and the bound protein was eluted with a linear gradient between 10 to 500 mM imidazole with a flow rate of 1 ml/min. The eluent was collected and loaded immediately onto a Bio-Gel P-6 desalting cartridge (Bio-Rad), previously equilibrated with 50 mM EPPS buffer (pH 8.0). The loaded protein was eluted with 50 mM EPPS buffer (pH 8.0) at a flow rate of 1 ml/min, and the resultant solution was used as a purified enzyme. All purification steps using the cartridges were carried out in a cold room at 4°C with a Profinia protein purification system (Bio-Rad).
Enzyme assay.
To bind the d-psicose 3-epimerase with Mn2+ and to remove unbound Mn2+, the enzyme was incubated at 20°C with 1 mM Mn2+ for 4 h and dialyzed at 4°C for 16 h against 50 mM EPPS buffer (pH 8.0). Unless otherwise stated, the reactions were performed at 50°C for 10 min in 50 mM EPPS buffer (pH 8.0) containing 20 mM fructose and 0.21 U/ml of enzyme and stopped by the addition of HCl to achieve a final concentration of 200 mM. One unit of d-psicose 3-epimerase activity was defined as the amount of the enzyme required to produce 1 μmol of psicose per min at pH 8.0 and 50°C.
Effects of pH and temperature.
To evaluate the effects of pH and temperature on the activities of the wild-type and variant enzymes, pH value was varied between 6.5 and 8.5 using 50 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) buffer (pH 6.5 to 7.5) and 50 mM EPPS buffer (pH 7.5 to 8.5), and the temperature was varied from 40 to 70°C at pH 8.0. The experimental data for enzyme deactivation were fitted to a first-order curve, and the enzyme half-life was calculated using Sigma Plot 10 software (Systat Software, San Jose, CA).
Determination of apparent melting temperature.
The apparent melting temperatures (Tm) of the wild-type and variant enzymes were measured calorimetrically at temperatures ranging from 20 to 100°C at a scan rate of 90°C/h. A differential scanning calorimeter (VP-DSC; MicroCal, Piscataway, NJ) was employed using 150 μM enzyme in 50 mM potassium phosphate buffer (pH 8.0).
Molecular modeling.
Based on the three-dimensional structure of d-psicose 3-epimerase from A. tumefaciens (11), optimized three-dimensional models of the wild-type and variant enzymes were generated using molecular dynamics (MD) simulation, to elucidate the improvement in their thermostability. All of the structures generated were minimized using CHARMM (17), and all of the molecular parameters and atomic charges were obtained from the set of CHARMM force field parameters. After energy minimization, MD was performed at 300 K and 1 atom for 500 ps with 1 fs each step. The conformations of the I33L and S213C variants were constructed from the minimized structure of the wild-type enzyme by replacing each amino acid at the respective position. All simulation experiments were performed using an HP XW6200 Workstation with dual Intel Xeon 3.2-GHz processors.
Operation of packed-bed bioreactor.
Duolite A568 beads (10 g wet weight) carrying the wild-type or I33L S213C variant enzyme of d-psicose 3-epimerase (140 mg protein) were packed into a reactor (XK 16; Amersham Pharmacia Biotech, Uppsala, Sweden) and used for continuous production of psicose. The working and effective working volumes of the reactor (height, 50 mm; diameter, 16 mm) were 10 and 2.6 ml, respectively, and the total activity of the enzyme was 330 U. A solution of 104 g/liter borate buffer (pH 9.0) or 50 mM EPPS buffer (pH 8.0) containing 500 g/liter fructose in the feeding reservoir was fed continuously into the reactor, and the effluent flowed out of the reactor to the outside reservoir using a peristaltic pump. The temperature was maintained at 45°C using a water circulator. The reactor was washed by feeding the fructose solution with a flow rate of 10 ml/min until protein in the effluent was not detected. After removing unbound enzyme, long-term operation of the reactor was performed at a dilution rate of 1.62 liters/h with borate or at 4.15 liters/h without borate.
Analytical methods.
The concentrations of fructose and psicose were determined by an HPLC system (SCL-10A; Shimadzu, Kyoto, Japan) equipped with a Shimadzu RID-10A detector and a BP-100 Ca2+ carbohydrate column (Benson Polymeric Inc., Reno, NV). The column was eluted at 80°C with water at a flow rate of 0.5 ml/min.
RESULTS AND DISCUSSION
Screening of thermostable variants created by random mutagenesis.
A mutant library of cells containing mutant genes of A. tumefaciens d-psicose 3-epimerase was constructed by error-prone PCR. After heat treatment at 60°C for 5 min, 150 mutants exhibiting fructose-converting activity greater than 1.2-fold that of wild-type cells were selected from the 5,000 initially obtained mutants. These first-selected mutants were cultured in 20-ml test tubes, and after the crude extracts obtained from the culture broths were assayed, 23 variants were selected. The cells containing the second-selected mutant genes were cultured in a 2-liter flask, and the crude extracts obtained from the culture broths were used to purify the variants by IMAC and Bio-Gel P-6 desalting cartridges, giving each variant as a single band by SDS-PAGE (data not shown). The activities and half-lives of the purified variants were measured at 55°C. Based on the half-lives of the variants, the five best variants were selected. Although the half-lives of the five mutants followed the order G67C (132 min) > I33L (64 min) > S213C (28 min) > V96A (18 min) > S8T (15 min), the G67C variant showed remarkably decreased specific activity, and the V96A and S8T variants displayed 51 and 29% of the wild-type activity, respectively. Thus, the S213C and I33L variants were finally chosen.
Site-directed mutagenesis of the residues at positions 33 and 213.
To look for a d-psicose 3-epimerase that was more thermostable than the chosen S213C and I33L variants, site-directed mutagenesis of the residues at positions 213 and 33 was performed. The polar uncharged Ser residue at position 213 was substituted for with the polar uncharged residues Thr, Cys, and Met; the nonpolar residue Pro; the negatively charged residue Glu; and the positively charged residue Lys. The S213K variant showed no activity. The half-lives of the wild-type and variant enzymes at position 213 followed the order S213C (28 min) > wild-type enzyme (10 min) > S213P (6 min) > S213T (5 min) > S213M (3.2 min) > S213E (2.8 min). The nonpolar residue Ile at position 33 was substituted for with other typical amino acids, including the polar uncharged residue Cys; the nonpolar residues Val, Leu, and Pro; the negatively charged residue Glu; and the positively charged residue Lys. The I33P, I33K, and I33E variants showed no activity. The half-lives of the wild-type and variant enzymes at position 33 followed the order I33L (63 min) > I33C (24 min) > I33V (12 min) > wild-type enzyme (10 min). These results indicated that Ser at position 213 and Leu at position 33 were the most suitable residues for enhancing thermostability, and thus the I33L and S213C variants were selected.
A double-site variant was generated using the combination of the I33L and S213C mutations. The half-lives of the constructed double-site variant I33L S213C (265 min) were 26-, 9-, and 4-fold the half-lives of the wild-type, S213C, and I33L variant enzymes, respectively. The thermostability of the double-site variant was improved synergistically by the mutations of I33L and S213C.
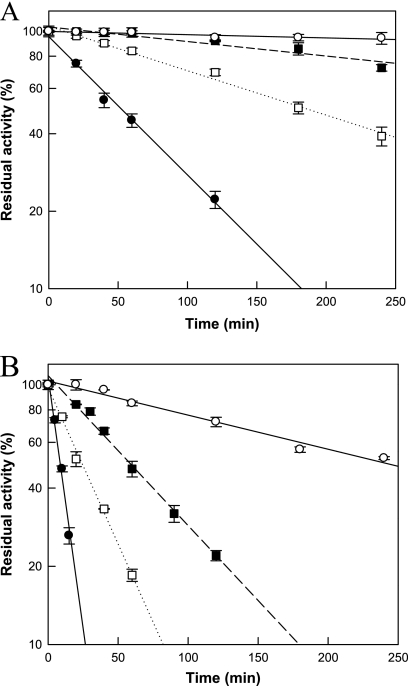
Characterization of the wild-type and variant enzymes.
The optimal pH values of the wild-type and three variant enzymes were the same (pH 8.0) (data not shown). However, the maximal activities for the wild-type and S213C, I33L, and I33L S213C variant enzymes were observed at 50, 52.5, 55, and 57.5°C, respectively. The thermal stabilities at 50 and 55°C were investigated for the four enzymes. The wild-type, S213C, I33L, and I33L S213C variant enzymes had half-lives of 62, 202, 445, and 1,853 min at 50°C, respectively (Fig. 1A), and 10, 28, 64, and 265 min at 55°C, respectively (Fig. 1B). The half-lives of the S213C, I33L, and I33L S213C variants were 3.3-, 7.2-, and 29.9-fold the half-life of the wild-type enzyme at 50°C, respectively, and 2.8-, 6.4-, and 26.5-fold the half-life of the wild-type enzyme at 55°C, respectively. The apparent melting temperatures (Tm) of the wild-type, S213C, I33L, and I33L S213C variant enzymes were 60.5, 63.6, 64.8, and 68.1°C, respectively, reflecting increases of 3.1, 4.3, and 7.6°C for the S213C, I33L, and I33L S213C variants, respectively, compared with the Tm of the wild-type enzyme. The two residue substitutions appeared to contribute independently to the increase in Tm, as the sum of the Tm increases of the single-site variants S213C and I33L was similar to the increase of the double-site variant S213C I33L.
Fig. 1.
Thermal inactivation for the activities of the wild-type (•), S213C (□), I33L (▪), and I33L S213C (○) variant enzymes of d-psicose 3-epimerase from A. tumefaciens at 50°C (A) and 55°C (B). A sample was withdrawn at each time interval, and the relative activity was determined under identical conditions in 50 mM EPPS buffer (pH 8.0) containing 20 mM fructose and 0.21 U/ml of enzyme at 50°C for 10 min. Data represent the means of three experiments, and error bars represent standard deviations.
The kinetic parameters of the wild-type, S213C, I33L, and I33L S213C variant enzymes were determined (Table 1). The kinetic parameters of the single-site variants I33L and S213C were almost similar to those of the wild-type enzyme. However, the kcat/Km of the double-site variant I33L S213C was approximately 1.4-fold that of the wild-type enzyme because of its approximately 1.4-fold-lower Km.
Table 1.
Kinetic parameters of the wild-type and S213C, I33L, and I33L S213C mutant enzymes of d-psicose 3-epimerase from A. tumefaciens for fructosea
| Enzyme | Km (mM) | kcat (min−1) | kcat/Km (min−1 mM−1) |
|---|---|---|---|
| Wild type | 44 ± 0.4 | 4,300 ± 6 | 99 ± 0.9 |
| Mutant variant | |||
| S213C | 42 ± 1.0 | 4,200 ± 63 | 101 ± 3.0 |
| I33L | 40 ± 0.7 | 4,200 ± 65 | 105 ± 2.5 |
| I33L S213C | 31 ± 0.1 | 4,100 ± 99 | 134 ± 3.2 |
The reactions of the wild-type and variant enzymes of d-psicose 3-epimerase from A. tumefaciens were performed under identical conditions in 50 mM EPPS buffer (pH 8.0) containing 20 mM fructose and 0.21 U/ml of enzyme for 10 min. Data represent the means and standard deviations from three separate experiments.
Molecular modeling of the wild-type and variant enzymes.
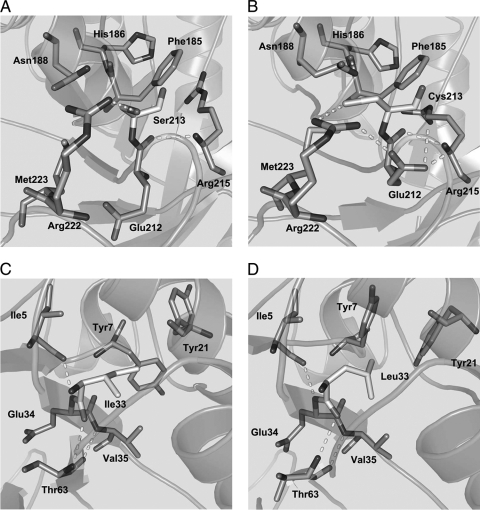
Molecular modeling demonstrated that the region neighboring Ser213 in the wild-type enzyme contained two putative hydrogen bonds, one between Ser213 and Arg222 and one between Glu212 and Arg215 (Fig. 2A). In contrast, the region neighboring Cys213 in the S213C variant involved six putative hydrogen bonds: one each between Phe185 and Asn188, Cys213 and Arg222, and Glu212 and Arg222, and three between Glu212 and Asn215 (Fig. 2B). In general, an increase in the number of hydrogen bonds between side chains of residues improves protein thermostability (22, 25). This suggests that the higher number in putative hydrogen bonds in the S213C variant enzyme may contribute to its increased thermostability.
Fig. 2.
Molecular modeling of the wild-type, S213C, I33L, and I33L S213C variant enzymes of d-psicose 3-epimerase from A. tumefaciens. Shown are models for the putative hydrogen bonds of the region neighboring the residue at position 213 for the wild-type (A) and S213C variant (B) enzymes and models for the putative hydrogen bonds and aromatic ring configurations of the region neighboring the residue at position 33 for the wild-type (C) and I33L variant (D) enzymes. The residue and putative hydrogen bond are represented as stick models and dashed lines, respectively.
In the molecular models, the wild-type enzyme showed no aromatic stacking interactions (Fig. 2C), whereas the I33L variant exhibited aromatic stacking interactions between Tyr7 and Tyr21 (Fig. 2D). The formation of aromatic stacking interactions via strong Van der Waals binding has been reported to increase the thermostability of a protein (6, 20). Thus, the aromatic stacking interactions in the I33L variant may improve its thermostability.
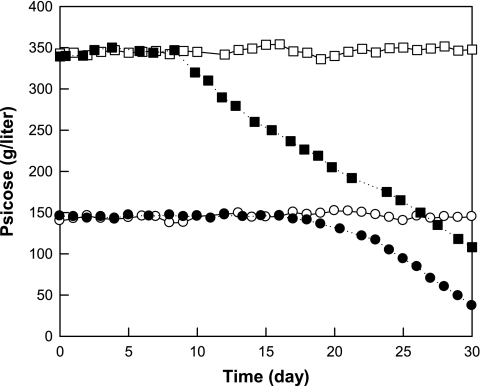
Continuous psicose production by the immobilized wild-type and variant enzymes with and without borate.
Borate forms complexes with carbohydrates, interacts with enzyme systems, and changes the equilibrium of reactions involving cis-diol carbohydrates based on the difference in binding affinities for sugars (3). Complex formation of l-ribulose, d-tagatose, or d-psicose with borate results in high conversion yields of these sugars (13, 15, 27). Thus, continuous production of psicose by immobilized wild-type and I33L S213C variant enzymes in a packed-bed reactor was evaluated with and without borate for 30 days (Fig. 3). The optimal dilution rates with and without borate using the immobilized wild-type enzyme were determined in previous studies (16). The immobilized wild-type enzyme with and without borate maintained activity for 8 days at a conversion yield of 70% (350 g/liter psicose) and for 16 days at a conversion yield of 30% (150 g/liter psicose), respectively. After 8 or 16 days, the enzyme activity gradually decreased, and the conversion yields with and without borate were reduced to 22 and 9.6% at 30 days, respectively. In contrast, the activity of the immobilized I33L S213C variant with and without borate did not decrease during the operation time of 30 days. These results indicate that the addition of borate was effective for enhanced production of psicose from fructose by d-psicose 3-epimerase and that the immobilized I33L S213C variant was suitable for long-term production of psicose in a bioreactor because of its improved thermostability.
Fig. 3.
Continuous production of psicose for the immobilized wild-type and I33L S213C variant enzymes of d-psicose 3-epimerase from A. tumefaciens in a packed-bed reactor. Psicose production by the wild-type (•) and I33L S213C (○) variant enzymes at dilution rate of 4.15 liters/h without borate and by the wild-type (▪) and I33L S213C (□) variant enzymes at 1.62 1iters/h with borate.
In summary, the I33L S213C double-site variant of d-psicose-3-epimerase obtained from random and site-directed mutagenesis showed increases of 7.5°C, 29.9-fold, and 7.6°C in optimal temperature, half-life at 50°C, and melting temperature, respectively, compared with the wild-type enzyme. Psicose was continuously produced from fructose in the packed-bed reactor without the decrease of activity during the operation time of 30 days because of the improved stability of the I33L S213C variant. Thus, the double-site variant may be useful for the commercial manufacture of psicose using an enzymatic process.
ACKNOWLEDGMENTS
This study was supported by the National Research of Korea (NRF) through the National Research Laboratory Program, Ministry of Education, Science and Technology (R0A-2007-000-20015-0) and by the Next-Generation BioGreen 21 Program grant, Rural Development Administration, Republic of Korea.
Footnotes
Published ahead of print on 26 August 2011.
REFERENCES
- 1. Binkley W. W. 1963. The fate of cane juice simple sugars during molasses formation. IV. Probable conversion of D-fructose to D-psicose. Int. Sugar J. 65:105–106 [Google Scholar]
- 2.Reference deleted.
- 3. De Muynck C., Beauprez J., Soetaert W., Vandamme E. J. 2006. Boric acid as a mobile phase additive for high performance liquid chromatography separation of ribose, arabinose and ribulose. J. Chromatogr. A 1101:115–121 [DOI] [PubMed] [Google Scholar]
- 4. Eble T. E., Hoeksema H., Boyack G. A., Savage G. M. 1959. Psicofuranine. I. Discovery, isolation, and properties. Antibiot. Chemother. 6:419–420 [PubMed] [Google Scholar]
- 5. Hough L., Stacey B. E. 1963. The occurrence of D-ribohexulose in Itea ilicifolia, Itea virginica, and Itea yunnanensis. Phytochemistry 2:315–320 [Google Scholar]
- 6. Hunter C. A., Lawson K. R., Perkins J., Urch C. J. 2001. Aromatic interactions. J. Chem. Soc. Perkin Trans. 2:651–669 [Google Scholar]
- 7. Itoh H., Sato T., Izumori K. 1995. Preparation of D-psicose from D-fructose by immobilized D-tagatose 3-epimerase. J. Ferment. Bioeng. 80:101–103 [Google Scholar]
- 8. Jang M. K., et al. 2010. Enhancement of the thermostability of a recombinant beta-agarase, AgaB, from Zobellia galactanivorans by random mutagenesis. Biotechnol. Lett. 32:943–949 [DOI] [PubMed] [Google Scholar]
- 9. Khan M. I., et al. 2005. Molecular properties and enhancement of thermostability by random mutagenesis of glutamate dehydrogenase from Bacillus subtilis. Biosci. Biotechnol. Biochem. 69:1861–1870 [DOI] [PubMed] [Google Scholar]
- 10. Kim H. J., Hyun E. K., Kim Y. S., Lee Y. J., Oh D. K. 2006. Characterization of an Agrobacterium tumefaciens D-psicose 3-epimerase that converts D-fructose to D-psicose. Appl. Environ. Microbiol. 72:981–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim K., Kim H. J., Oh D. K., Cha S. S., Rhee S. 2006. Crystal structure of D-psicose 3-epimerase from Agrobacterium tumefaciens and its complex with true substrate D-fructose: a pivotal role of metal in catalysis, an active site for the non-phosphorylated substrate, and its conformational changes. J. Mol. Biol. 361:920–931 [DOI] [PubMed] [Google Scholar]
- 12. Kim M. S., Lei X. G. 2008. Enhancing thermostability of Escherichia coli phytase AppA2 by error-prone PCR. Appl. Microbiol. Biotechnol. 79:69–75 [DOI] [PubMed] [Google Scholar]
- 13. Kim N. H., et al. 2008. Conversion shift of D-fructose to D-psicose for enzyme-catalyzed epimerization by addition of borate. Appl. Environ. Microbiol. 74:3008–3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim Y. W., et al. 2003. Directed evolution of Thermus maltogenic amylase toward enhanced thermal resistance. Appl. Environ. Microbiol. 69:4866–4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lim B. C., Kim H. J., Oh D. K. 2007. High production of D-tagatose by the addition of boric acid. Biotechnol. Prog. 23:824–828 [DOI] [PubMed] [Google Scholar]
- 16. Lim B. C., Kim H. J., Oh D. K. 2009. A stable immobilized D-psicose 3-epimerase for the production of D-psicose in the presence of borate. Process Biochem. 44:822–828 [Google Scholar]
- 17. MacKerell A. D., Jr., et al. 1998. CHARMM: the energy function and its parameterization with an overview of the program, p. 271–277.In Schleyer P. von Rague, et al. (ed.), The encyclopedia of computational chemistry, vol. 1 John Wiley & Sons, Chichester, United Kingdom [Google Scholar]
- 18. Matsuo T., et al. 2001. Dietary D-psicose, a C-3 epimer of D-fructose, suppresses the activity of hepatic lipogenic enzymes in rats. Asia Pac. Clin. Nutr. 10:233–237 [DOI] [PubMed] [Google Scholar]
- 19. Matsuo T., Suzuki H., Hashiguchi M., Izumori K. 2002. D-Psicose is a rare sugar that provides no energy to growing rats. J. Nutr. Sci. Vitaminol. (Tokyo) 48:77–80 [DOI] [PubMed] [Google Scholar]
- 20. McGaughey G. B., Gagne M., Rappe A. K. 1998. π-stacking interactions. Alive and well in proteins. J. Biol. Chem. 273:15458–15463 [DOI] [PubMed] [Google Scholar]
- 21. Miller B. S., Swain T. 1960. Chromatographic analyses of the free amino acids, organic acids and sugars in wheat plant extracts. J. Sci. Food Agric. 11:344–348 [Google Scholar]
- 22. Pei X. Q., Yi Z. L., Tang C. G., Wu Z. L. 2011. Three amino acid changes contribute markedly to the thermostability of beta-glucosidase BglC from Thermobifida fusca. Bioresour. Technol. 102:3337–3342 [DOI] [PubMed] [Google Scholar]
- 23. Sun Y., Hayakawa S., Ogawa M., Fukada K., Izumori K. 2008. Influence of a rare sugar, D-psicose, on the physicochemical and functional properties of an aerated food system containing egg albumen. J. Agric. Food Chem. 56:4789–4796 [DOI] [PubMed] [Google Scholar]
- 24. Takeshita K., Suga A., Takada G., Izumori K. 2000. Mass production of D-psicose from D-fructose by a continuous bioreactor system using immobilized D-tagatose 3-epimerase. J. Biosci. Bioeng. 90:453–455 [DOI] [PubMed] [Google Scholar]
- 25. Vieira D. S., Degrève L. 2009. An insight into the thermostability of a pair of xylanases: the role of hydrogen bonds. Mol. Phys. 107:59–69 [Google Scholar]
- 26. Zhang L., Mu W., Jiang B., Zhang T. 2009. Characterization of D-tagatose-3-epimerase from Rhodobacter sphaeroides that converts D-fructose into D-psicose. Biotechnol. Lett. 31:857–862 [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y. W., Jeya M., Lee J. K. 2010. L-Ribulose production by an Escherichia coli harboring L-arabinose isomerase from Bacillus licheniformis. Appl. Microbiol. Biotechnol. 87:1993–1999 [DOI] [PubMed] [Google Scholar]
- 28. Zhang Z. G., Yi Z. L., Pei X. Q., Wu Z. L. 2010. Improving the thermostability of Geobacillus stearothermophilus xylanase XT6 by directed evolution and site-directed mutagenesis. Bioresour. Technol. 101:9272–9278 [DOI] [PubMed] [Google Scholar]