Abstract
The first step of nitrification, the oxidation of ammonia to nitrite, is important for reducing eutrophication in freshwater environments when coupled with anammox (anaerobic ammonium oxidation) or denitrification. We analyzed active formerly biofilm-associated aerobic ammonia-oxidizing communities originating from Ammerbach (AS) and Leutra South (LS) stream water (683 ± 550 [mean ± standard deviation] and 16 ± 7 μM NH4+, respectively) that were developed in a flow-channel experiment and incubated under three temperature regimens. By stable-isotope probing using 13CO2, we found that members of the Bacteria and not Archaea were the functionally dominant autotrophic ammonia oxidizers at all temperatures under relatively high ammonium loads. The copy numbers of bacterial amoA genes in 13C-labeled DNA were lower at 30°C than at 13°C in both stream enrichment cultures. However, the community composition of the ammonia-oxidizing bacteria (AOB) in the 13C-labeled DNA responded differently to temperature manipulation at two ammonium concentrations. In LS enrichments incubated at the in situ temperature (13°C), Nitrosomonas oligotropha-like sequences were retrieved with sequences from Nitrosospira AmoA cluster 4, while the proportion of Nitrosospira sequences increased at higher temperatures. In AS enrichments incubated at 13°C and 20°C, AmoA cluster 4 sequences were dominant; Nitrosomonas nitrosa-like sequences dominated at 30°C. Biofilm-associated AOB communities were affected differentially by temperature at two relatively high ammonium concentrations, implicating them in a potential role in governing contaminated freshwater AOB distributions.
INTRODUCTION
As in terrestrial systems, aquatic ecosystems are commonly nitrogen limited (22, 73). However, nitrogen loading of terrestrial ecosystems is increasing worldwide (72), and the saturation of terrestrial ecosystems with nitrogen (1) causes a transfer of excess nitrogen with surface runoff and groundwater flow to headstreams, which have an important role in linking nutrient transport from terrestrial ecosystems to various aquatic ecosystems (12, 20). Thus, runoff events, as well as wastewater effluent, could overcome the limitation of primary productivity in these environments (22) and result in dissolved oxygen depletion, stimulation of algal growth, and toxicity to aquatic life (56). Microbial activity in headstreams is of great importance for preventing the accumulation of ammonium (4, 14, 56).
The oxidation of ammonia to nitrite is the first and rate-limiting step of inorganic nitrogen transformation and, thus, a central process in the global nitrogen cycle (37). The key enzyme, the ammonia monooxygenase (AMO), which catalyzes the oxidation of ammonia to hydroxylamine, is found in two monophyletic groups within the Betaproteobacteria and Gammaproteobacteria (2, 5). Metagenomic studies have shown that members of the kingdom Crenarchaeota (or Thaumarchaeota, as recently proposed [13, 63]) within the domain Archaea also contain the amo genes (69, 70), and subsequent studies have demonstrated the existence of ammonia-oxidizing archaea (AOA) (58). The numerical dominance of amoA-containing Archaea in soils (43) and in aquatic environments (30, 47), their distribution worldwide (24), and the capacity of enrichments of Archaea and isolates to oxidize ammonia (17, 28, 34) raise questions regarding their relevance to ammonia oxidation in freshwater environments and, specifically, within epilithic stream biofilms.
Temperature was found to be a selective factor for the community structure of soil ammonia-oxidizing bacteria (AOB) (8, 9, 23), as well as for soil AOA (67) and for both AOB and AOA in estuary sediments (61). Ammonium concentration is an additional selective factor for the community structure of soil AOB (37), and a previous study indicated that both the community structure and abundance of AOB were altered by interactions among temperature and ammonium concentration in soil incubations (6). Recent studies have suggested that AOA enrichments and pure cultures belonging to two taxonomic groups exhibited variation in their response to different ammonium concentrations (28, 46). Furthermore, community analysis of ammonia-oxidizing prokaryotes in freshwater sediments and soils indicated a numerical predominance of AOA at low ammonium concentrations (18, 29) and a positive effect of ammonium availability on the population size of AOB (53). Similar effects have been observed in natural and simulated small creek ecosystems with different ammonium concentrations (30). However, it is uncertain how temperature will affect the AOB dominance observed at relatively high ammonium availability, as well as the AOB and AOA community structures.
The failure to detect responses of AOB to manipulation of environmental conditions after 4 to 6 weeks of incubation in previous laboratory studies (10, 11, 66) raised the question whether it was a result of using a method detecting the resting (i.e., nonactive) AOB communities or a short-term survival strategy, as suggested previously (7). By using 13CO2 and stable-isotope probing (SIP) of nucleic acids (26), the active autotrophic ammonia oxidizers can be determined, as was done previously for enrichment cultures of AOB from a lake sediment (75), for AOB in estuary sediments (25), and more recently, for both AOB and AOA in soil microcosms (32, 77). Thus, we applied the SIP method to ammonia-oxidizing enrichments that had been obtained from biofilms grown in simulated creek ecosystems at two ammonium concentrations (30) to elucidate under specific stable conditions the active autotrophic community response to three different temperatures at relatively high levels of ammonium availability that are representative for contaminated streams.
MATERIALS AND METHODS
Biofilm starting material grown under different ammonium concentrations.
Biofilm starting material was obtained from simulated small-creek ecosystems as reported previously (30) and used for 3- and 6-week incubations with 13C-labeled bicarbonate for DNA-SIP analysis. Briefly, plexiglass flow channels of 160 cm by 10 cm by 18 cm were filled with 10 liters of creek water and circulated at a flow velocity of 0.3 m s−1 in a climate room at 13°C in the dark. Biofilms (∼5 mm thick, i.e., with minimal oxygen limitation) were developed on the surface of 60 clay tiles (each 4.7 by 4.7 cm). Water was obtained from two shell limestone creeks, Ammerbach (AS) and Leutra South (LS), located in Thuringia, Germany, which differed in their nutrient concentrations. The average concentrations of ammonium, phosphate, nitrate, and oxygen in the creek water during the period of this study (i.e., the first 9 weeks of incubation of the flow channels from 8 August to 28 September 2006) (Table 1) were determined. The temperature and pH were similar in the two creeks (Table 1). The ammonium concentrations and pH of the flow channel waters were adjusted twice a week to a pH of 8.0 and to values measured in the creek at the first two samplings (i.e., 350 and 50 μM ammonium for AS and LS, respectively). The water was replaced after 2, 4, and 7 weeks of incubation with fresh creek water. The potential nitrification activity (PNA) of the biofilms was measured once a week in the flow channel study, as previously reported (30), and biofilm samples were taken for establishing the stable-isotope probing (SIP) setup when PNA reached a steady state at 9 weeks of incubation.
Table 1.
Chemical and physical characterization of the creeks in this studya
| Parameter | Leutra South stream | Ammerbach stream |
|---|---|---|
| pH | 8.4 ± 0.3 | 7.9 ± 0.3 |
| Temp (°C) | 12.3 ± 0.6 | 13.7 ± 1.8 |
| Dissolved oxygen (mg/liter) | 11.7 ± 0.8 | 6.3 ± 0.9 |
| NH4+ (μM) | 15.9 ± 6.9 | 683 ± 550 |
| PO43− (μM) | 0.6 ± 0.5 | 29.0 ± 3.0 |
| NO3− (μM) | 799 ± 90 | 1017 ± 244 |
The data are the means ± standard deviations from the 9 weeks of incubation prior to SIP labeling.
SIP of biofilm-associated autotrophic ammonia-oxidizing prokaryotes at different temperatures.
The temperatures chosen for SIP experiments were 13°C (in situ temperature), 20°C (intermediate), and 30°C (high temperature representing warmer regions). Duplicate SIP incubation bottles were set up for each simulated creek ecosystem at the three temperatures (Table 2). Biofilm material was scraped off of four clay tiles (i.e., two of each replicate) into 50-ml autoclaved stream water and placed into 1,000-ml bottles. The bottles were sealed with butyl rubber stoppers and flushed with N2, and pure oxygen was added to a final concentration of 21% to create a CO2-free environment. The headspace gases were measured with Hewlett Packard Co. 5980 series II gas chromatographs fitted with a thermal conductivity detector (41) and adjusted to replenish O2 every 2 to 3 days during the experiment. A 7-day preincubation (without sealing the bottles) was conducted in order to reduce CO2 emission, which was high during the first 48 h but not when repeating the flushing after 7 days. Labeled substrate, NaH13CO3 (99%; Cambridge Isotope Laboratories, Inc.), was then added to each bottle to a final aqueous concentration of 1,000 μg ml−1. Labeled substrate was fully replaced after reflushing the bottles once a week. The incubation was performed in the dark for 6 weeks. One additional replicate of each treatment was incubated for 3 weeks (Table 2) in order to test whether this relative short period was sufficient for labeling ammonia-oxidizing prokaryotes. Ammonium (NH4Cl) was added at the beginning of incubation and once a week to the bottles containing LS or AS biofilms to final concentrations of 100 and 1,000 μM, respectively. Phosphate (as KH2PO4) was added to a final concentration of 50 μM to LS bottles at the beginning of incubation and to bottles from both streams every 2 weeks. The pH was monitored by using phenol red, added to autoclaved stream water at a final concentration of 0.001%. The pH was adjusted with NaOH when the color of the medium turned yellow, representing a pH of less than ∼7, a rough indication of ammonia oxidation activity. The controls were incubated in a similar manner, excluding the addition of labeled NaHCO3. The 50-ml sample incubations were filtered through 0.2-μm pore-size cellulose acetate filters (Sartorius AG, Göttingen, Germany) after 3 and 6 weeks of incubation (Table 2). The filters were stored at −20°C until DNA extraction was performed.
Table 2.
Setup of DNA stable-isotope-probing experiment with biofilm material obtained from simulated creek ecosystemsa
| Time of incubation, labeling | No. of samples of biofilm starting material from: |
|||||
|---|---|---|---|---|---|---|
| Leutra South (100 μM NH4Cl) |
Ammerbach (1,000 μM NH4Cl) |
|||||
| 13°C | 20°C | 30°C | 13°C | 20°C | 30°C | |
| 3-Week incubationb | ||||||
| NaH13CO3 | Single sample | Single samplec | Single sample | Single sample | Single samplec | Single sample |
| 6-Week incubation | ||||||
| NaH13CO3 | Duplicate | Duplicate | Duplicate | Duplicate | Duplicate | Duplicate |
| Unlabeled control | Duplicatec | Duplicate | Duplicate | Duplicate | Duplicate | Duplicate |
Previously studied; see reference 30.
No control was included for these treatments.
Sample was lost during analysis.
Nucleic acid extraction and SIP gradient fractionation.
Total DNA from SIP incubations of the two stream biofilms was extracted as described previously (48), with slight modifications. In brief, each filter was incubated in 500 μl of lysozyme solution (0.15 M NaCl, 0.3 M Na2-EDTA, 15 mg of lysozyme per ml) for 3 h at 37°C. Then, 500 μl of lysis buffer (0.1 M NaCl, 0.5 M Tris-HCl, pH 8.0, 10% sodium dodecyl sulfate) was added, and the suspension was subjected to three cycles of freezing (liquid nitrogen for 5 min) and thawing (65°C water bath for 10 min). Finally, proteinase K was added to a final concentration of 50 μg ml−1 and the tubes were incubated at 30°C for 30 min. DNA was purified by three cycles of chloroform-isoamyl alcohol (24:1) extraction, precipitated with 3 M sodium acetate (pH 5) and two volumes of isopropanol, washed with 80% (vol/vol) ethanol, and suspended in water. DNA was further purified by using a Wizard DNA cleanup kit (Promega, Madison, WI).
Gradient fractionation of total DNA extract from each sample was performed with an initial CsCl gradient with a buoyant density of 1.725 g ml−1 that was subjected to centrifugation in a VTi65.2 rotor (Beckman Coulter) at 177,000 × g for 36 h at 20°C (50). The density gradient was fractionated into 12 aliquots (∼400 μl each) containing nucleic acids, which were precipitated using a polyethylene glycol solution (25) and dissolved in 20 μl of TE (Tris-EDTA) buffer. Aliquots of 5 μl of DNA from each fraction were visualized on a 1% agarose gel after ethidium bromide staining.
Amplification of bacterial and archaeal amoA genes.
The heavy fractions (fractions 5 to 7) and light fractions (fractions 10 to 12) from each treatment (i.e., labeled and control biofilms of each stream incubated at three temperatures for 3 and 6 weeks) were used to amplify the archaeal amoA gene fragment by using the Arch-amoAF/Arch-amoAR assay (24), and the bacterial amoA gene fragments by using the amoA-1F* and amoA-2R primer set (60, 65) according to a protocol described previously by Avrahami et al. (10), with slight modifications. In brief, amplification was performed by using 1 μM each primer (Metabion, Martinsried, Germany) and 12.5 μl of Taq RED Mix (Genesee Scientific, San Diego, CA) containing 150 mM Tris-HCl (pH 8.5), 40 mM (NH4)SO4, 3.0 mM MgCl2, 0.2% Tween 20, 400 μM each deoxynucleoside triphosphate, 0.05 units μl−1 Apex Taq RED DNA polymerase with inert red dye and stabilizer (Genesee Scientific, San Diego, CA). The MgCl2 concentration was adjusted to 2.5 mM, and DNA and water (Sigma-Aldrich, Deisenhofen, Germany) were added to a final volume of 25 μl. Amplifications were always started by placing PCR tubes into a preheated (94°C) TProfessional basic gradient thermocycler (Biometra, Goettingen, Germany). The thermal profile used for amplification was modified after that of Rotthauwe et al. (60) and included 5 min at 94°C, followed by 30 to 37 cycles of 45 s at 94°C, 60 s at 57°C, 1 min at 72°C, and 10 min at 72°C for the last cycle.
Real-time PCR of bacterial amoA gene.
The copy number of the bacterial amoA gene was measured in all fractions of each of the samples in this SIP experiment. The abundance of the bacterial amoA gene was estimated by using the amoA-1F and amoA-2R primer set (60), slightly modified from Horz et al. (31). In brief, a real-time PCR standard was generated by using a plasmid DNA mixture of bacterial amoA genes from eight operational taxonomic unit (OTU)-representative clones (32). Real-time PCR was standardized by using a dilution series of bacterial amoA standard over six orders of magnitude covering 25 to 2.5 × 107 copies of template per assay. The 25-μl reaction mixture contained 12.5 μl of SYBR green jump-start Taq ReadyMix, 0.5 μM each primer, 200 ng μl−1 bovine serum albumin (BSA), 3.0 mM MgCl2, and 1.0 μl template. The thermal cycling conditions were described previously (31), with plate readings at 86°C for data acquisition to avoid any possible primer dimer interference. PCR amplification efficiencies of 91.9 to 102.8% were obtained, with R2 values of 0.957 to 0.998. Specific amplification of bacterial amoA was confirmed by melting curve analysis and agarose gel electrophoresis. The real-time PCR reliability was verified by spiking samples with an amoA-containing clone as described previously (32). The copy numbers of bacterial amoA genes were also measured in the total genomic DNA extracted from the original biofilm samples obtained after 9 weeks of development in the flow channels and after 6 weeks of incubation in the SIP setup.
Cloning and sequencing of the 16S rRNA gene.
The heavy fractions of the labeled and control treatments and the light fractions of the labeled treatments (Fig. 1, horizontal and vertical arrows, respectively) were PCR amplified, targeting the bacterial 16S rRNA gene using 27f-1492r (42). Triplicate PCR products were pooled and purified from the gel with a QIAquick gel extraction kit (Qiagen, Hilden, Germany). The purified PCR products were cloned by using the pGEM T-easy vector and JM109 competent cells. The bacterial 16S rRNA gene clones were screened with the CTO primer set (38). Sequencing of CTO-positive clones was performed by the ADIS DNA core facility (Max Planck Institute for Plant Breeding Research, Cologne, Germany) on an ABI Prism 3700 sequencer (Applera, Foster City, CA), and analyzed using the DNAStar software package.
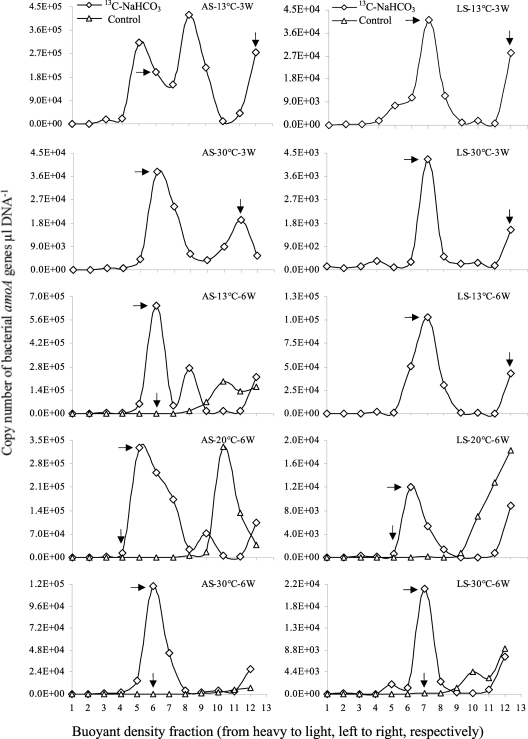
Fig. 1.
Copy numbers of bacterial amoA genes across DNA cesium chloride density gradient fractions from each treatment. The labeled treatments (diamonds) and control treatments (triangles) are shown for each stream biofilm incubated at 3 temperatures for 6 weeks. Leutra South (100 μM NH4Cl) and Ammerbach biofilm enrichments (1,000 μM NH4Cl) are denoted as LS and AS, respectively. The designation AS-13°C-3W indicates that Ammerbach biofilm enrichments were incubated at 13°C for 3 weeks. There were no control treatments for all 3-week or for 6-week incubations of LS at 13°C. The horizontal and vertical arrows indicate the DNA fractions used for clone library construction of active AOB and nonactive AOB 16S rRNA gene sequences, respectively, in Table 3.
Cloning, sequencing, and phylogenetic analysis of the bacterial amoA gene.
The heavy fractions of the labeled treatments incubated for 6 weeks (Fig. 1, horizontal arrow) were PCR amplified, targeting the bacterial amoA gene fragments by using the amoA-1F* and amoA-2R primer set (60, 65). PCR products were ligated into vectors of the CloneJET PCR cloning kit (Fermentas, St. Leon-Rot, Germany) according to the manufacturer's instructions. The vectors were then used to transform Escherichia coli DH5α competent cells. The clones were screened by amplification with the pJet1.2 forward and pJet1.2 reverse primer set (CloneJET PCR cloning kit, Fermentas, St. Leon-Rot, Germany) according to the manufacturer's instructions, and 50 clones in the expected size of each library were sequenced by Macrogen (Seoul, South Korea). The phylogenetic analysis of bacterial amoA sequences was performed using the ARB program package (45). The phylogenetic trees were inferred from the distance matrix using the Fitch algorithm with global rearrangement and randomized input order of sequences. The tree topology was compared to trees calculated by the neighbor-joining algorithm and the minimum evolution method (40).
Nucleotide sequence accession numbers.
The clone sequences were deposited in GenBank (accession no. HQ168204 to HQ168366).
RESULTS
SIP of CO2-assimilating autotrophic ammonia-oxidizing bacteria and archaea (amoA gene).
Incubations of 9-week-old biofilm starting materials (30) at three different temperatures (13, 20, and 30°C) (Table 2) in the presence of ammonium (1,000 and 100 μM in starting material from Ammerbach [AS] and Leutra South [LS], respectively) for 3 and 6 weeks were used for stable-isotope probing. Ultracentrifugation of the extracted DNA from these incubations resulted in 12 fractions with decreasing buoyant density from fraction 1 to fraction 12. In the 13C-labeled treatments, DNA could be visualized on an agarose gel in fractions 4 to 12, which represent the “heavy” and “light” fractions. In contrast, in the nonlabeled control treatments, the DNA was visible only in fractions 8 to 12, i.e., the light fractions (data not shown). In all cases, denaturing gradient gel electrophoresis (DGGE) fingerprinting of bacterial 16S rRNA genes demonstrated major differences in patterns between the heavy and light DNA fractions only when the biofilm material was incubated with [13C]bicarbonate and not in the unlabeled control incubations (see Fig. S1 in the supplemental material). These results provide evidence for successful labeling of the manipulated biofilms from the simulated stream system. The bacterial amoA gene could be amplified from the heavy DNA (fractions 5 to 7) of the 13C-labeled treatments and also from total DNA (before fractionation) and light DNA (fractions 10 to 12) of both the 13C-labeled and unlabeled treatments (data not shown), resulting in a PCR product of the expected size (∼490 bp). In contrast, archaeal amoA genes were only detected in the total DNA and in the light fractions (data not shown), indicating that autotrophic ammonia-oxidizing archaea (AOA) were not active in any treatment of either the LS or AS biofilm enrichment culture. Quantification of the bacterial amoA gene across the gradient fractions by quantitative real-time PCR (qPCR) showed that the highest copy numbers were detected in the heavy DNA fractions of the 13C-labeled treatments, while in the unlabeled control, the copy numbers were higher in the light DNA fractions (Fig. 1). This result provides strong evidence for successful labeling of biofilm-associated AOB microbial communities. DNA from heavy and light fractions (Fig. 1, horizontal and vertical arrows, respectively) was used to create clone libraries of nearly full-length bacterial 16S rRNA genes. Clones of bacterial 16S rRNA genes were screened with the CTO primer set. The frequency of clones identified as AOB was clearly higher in the heavy than in the light DNA fraction after 3 weeks of 13C labeling, both in AS and in LS biofilms (Table 3). After 6 weeks of incubation, the frequency of AOB clones was also higher in the heavy DNA of the 13C-labeled treatments than in the unlabeled control treatments (Table 3). This result provides strong evidence for greater enrichment of AOB in the heavy fractions of the labeled treatments than in the light fractions and control treatments. Due to the low number of positive bacterial 16S rRNA clones in general and their failure to detect all groups detected by the amoA primer set, we present here only the phylogenetic analysis of the amoA sequences.
Table 3.
Frequency of 16S rRNA genes sequences of ammonia-oxidizing bacteria in clone libraries constructed with DNA from the CsCl gradient fractionsa
| Length of incubation, labeling, CsCl gradient fraction | Frequencyb of sequences derived from starting material from: |
|||||
|---|---|---|---|---|---|---|
| Leutra South (100 μM NH4Cl) |
Ammerbach (1,000 μM NH4Cl) |
|||||
| 13°C | 20°C | 30°C | 13°C | 20°C | 30°C | |
| 3-Week incubation | ||||||
| NaH13CO3, heavy fraction | 6 (6/104) | NA | 17 (9/52) | 12 (13/108) | NA | 10 (5/52) |
| NaH13CO3, light fraction | 2 (1/52) | NA | 2 (1/52) | 6 (3/54) | NA | 4 (2/52) |
| 6-Week incubation | ||||||
| NaH13CO3, heavy fraction | 40 (21/52) | 12 (6/52) | 14 (7/52) | 10 (10/104) | 25 (13/52) | 12 (6/52) |
| Unlabeled control, heavy fraction | NAc (0/52) | 0 (0/52) | 0 (0/52) | 1 (2/156) | 6 (3/52) | 0 (0/52) |
See Fig. 1.
Values are percentages and, in parentheses, number of AOB-like sequences/total number of bacterial 16S rRNA gene clones screened. NA, not analyzed.
A clone library constructed from the light DNA fraction of the labeled treatments revealed no positive clones out of 52 clones tested.
Clone sequences and frequencies of bacterial amoA gene from heavy DNA.
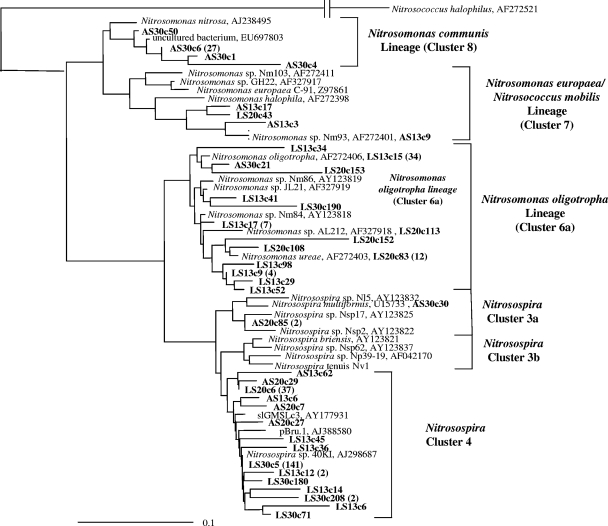
The bacterial amoA sequences retrieved from clone libraries of the heavy DNA fractions of AS and LS biofilm enrichments (Fig. 1, horizontal arrows) were closely related to known AOB (Fig. 2), and the majority of them were affiliated with three phylogenetic clades defined previously (10, 11, 36, 59): (i) the Nitrosomonas oligotropha lineage (Nitrosomonas cluster 6a), (ii) Nitrosospira AmoA cluster 4, corresponding to Nitrosospira 16S rRNA cluster 0, and (iii) the Nitrosomonas communis lineage (Nitrosomonas cluster 8 [Nitrosomonas nitrosa-like]). The relative frequencies of bacterial amoA gene clones related to different phylogenetic AOB groups in the labeled DNA after 6 weeks of incubation were influenced by temperature differently at the two ammonium concentrations (Table 4). In the heavy fraction of LS biofilm enrichments, incubated with 100 μM NH4Cl, the number of amoA clones affiliated to the N. oligotropha lineage decreased with increasing temperature (Table 4). The opposite trend was observed for the number of clones affiliated to Nitrosospira AmoA cluster 4 (Table 4). In AS biofilm enrichments, incubated with 1,000 μM NH4Cl, the majority of clones retrieved from the heavy fraction of samples incubated at 13 and 20°C were affiliated with Nitrosospira AmoA cluster 4 (Table 4). In contrast, N. nitrosa-like AOB were detected exclusively in AS biofilm enrichments incubated at 30°C and comprise 60% of clones identified in this sample. Assigning the 299 clones retrieved from these clone libraries to DGGE bands of the amoA gene from the heavy DNA fractions revealed that the relative proportions of the different phylogenetic groups agreed with the trend observed in the amoA clone library data (data not shown).
Fig. 2.
Fitch-Margoliash phylogenetic reconstruction (with global rearrangement and randomized input order; seven “jumbles”) of the relationship between AOB and the closest relatives retrieved from GenBank, based on partial bacterial AmoA sequences (150 amino acids) retrieved from the heavy fraction of the six treatments incubated for 6 weeks. Leutra South (100 μM NH4Cl) and Ammerbach biofilm enrichments (1,000 μM NH4Cl) are denoted as LS and AS, respectively. The clone designations give the biofilm enrichment and temperature of incubation followed by a random clone number; e.g., AS13c20 indicates a clone from the Ammerbach biofilm enrichment incubated at 13°C. The number of clones from each treatment and their phylogenetic affiliations are described in Table 4. Clones obtained from this experiment are highlighted in bold. The values in parentheses represent the total number of AmoA sequences with identities of >99% based on their amino acid sequences, with the group represented by a single clone sequence. GenBank accession numbers are shown. The scale bar indicates 10 changes per 100 nucleotide positions.
Table 4.
Percentage of amoA gene clones within each phylogenetic group in clone libraries constructed from 13C-enriched heavy DNA fractions from SIP experiments performed at different temperatures after 6 weeks of incubation
| Affiliation | % (no. of clones/total no. of clones in library)a of gene clones in starting material from: |
|||||
|---|---|---|---|---|---|---|
| Leutra South (100 μM NH4Cl) |
Ammerbach (1,000 μM NH4Cl) |
|||||
| 13°C | 20°C | 30°C | 13°C | 20°C | 30°C | |
| Nitrosospira cluster 4 | 44 (22/50) | 66 (33/50) | 78 (38/49) | 88 (44/50) | 94 (47/50) | 20 (10/50) |
| Nitrosomonas oligotropha, cluster 6a | 56 (28/50) | 32 (16/50) | 22 (11/49) | 6 (3/50) | 4 (2/50) | 16 (8/50) |
| Nitrosomonas communis (N. nitrosa-like), cluster 8 | ND | ND | ND | ND | ND | 60 (30/50) |
| Nitrosospira cluster 3a | ND | ND | ND | ND | 2 (1/50) | 4 (2/50) |
| Total abundance | 1.08E + 05 ± 6.6E + 04 | 1.80E + 04 ± 1.1E + 04 | 1.97E + 04 ± 6.2E + 03 | 6.11E + 05 ± 2.1E + 05 | 4.55E + 05 ± 4.2E + 05 | 1.39E + 05 ± 5.6E + 04 |
ND, not detected.
Total abundance of bacterial amoA genes.
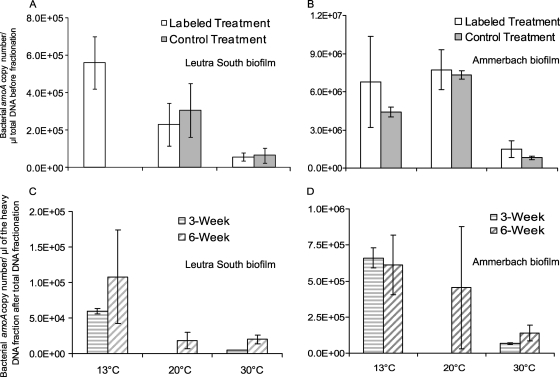
Bacterial amoA genes were quantified in DNA extracted from the flow channel biofilms incubated at 13°C before SIP incubation (“original samples”), in total DNA extracted after 6 weeks of SIP incubation at 13, 20, and 30°C, and in the heavy DNA fractions after 3 and 6 weeks of SIP incubation at 13, 20, and 30°C. The amoA copy numbers in the original samples were 1.8 × 103 ± 9.3 × 102 and 2.3 × 106 ± 7.7 × 105 μl−1 of total DNA for LS and AS biofilms, respectively, and were higher after SIP incubation. Furthermore, the amoA copy numbers in the week 6 samples were also equal to or higher than those in week 3 samples at both 13°C and 30°C, showing that AOB grew during the 6 weeks of SIP incubation (excluding AS biofilm enrichments at 30°C, where a 2-fold decline was observed) (Fig. 3). Quantification of the total DNA (i.e., nonfractionated DNA) of the labeled and control treatments indicated that the copy numbers of bacterial amoA genes were lower at 30°C than at 13°C (Fig. 3A and B). Similar results were obtained by quantification of active bacterial amoA genes in the presumably 13C-labeled fractions 5, 6, and 7 for both the 3- and 6-week incubations of labeled treatments (Fig. 3C and D).
Fig. 3.
Copy numbers of bacterial amoA genes in total DNA isolated from biofilm enrichments (A and B) and presumably labeled DNA after isopycnic centrifugation (C and D) of total DNA extracts. “Labeled” (NaH13CO3) and “Control” refer to the respective treatment of stream biofilms for a 6-week incubation. The 13C-associated bacterial amoA copy numbers in C and D are the sum of those in the heavy DNA—fractions 5, 6, and 7—of the labeled treatments incubated for 3 and 6 weeks in the experiment whose results are shown in Fig. 1.
DISCUSSION
Biofilms grown in simulated creek ecosystems, which varied in potential nitrification activity, with the Ammerbach (AS) biofilm having a 10-fold higher activity than the Leutra South (LS) biofilm (30), were chosen to study the potential impact of temperature on active ammonia-oxidizing prokaryotes at relatively high ammonium availability. By applying DNA-SIP to these biofilm starting materials incubated in bottles under two ammonium concentrations (100 and 1,000 μM, for LS and AS, respectively) and three temperatures (13, 20, and 30°C), we obtained direct evidence that AOB were the dominant autotrophic ammonia oxidizers in all treatments. These results agreed with the dominant role of AOB in nitrification processes with increasing incubation time and with increasing ammonium availability in the biofilms grown in the simulated creek ecosystems, despite the high abundance of AOA amoA gene copy numbers in the water phase of both creeks (30). Note that the current study is extended from this previous study (as stated in Materials and Methods). Bacterial rather than archaeal amoA genes were also dominant in estuarine systems with relatively high nutrient loads (49, 62) and soil microcosms amended with high ammonia concentrations (71). However, the opposite trend has also been reported, although it is unclear whether this discrepancy is due to variations in physical and chemical conditions in the estuaries studied or to methodological differences (15). Here, we demonstrated that temperature manipulation did not affect the dominance of AOB over AOA in our biofilm enrichments incubated at an ammonium concentration of at least 100 μM. Thus, the active AOB communities in these enrichments appear to be a product of the relatively high and continuous ammonium load and stable temperature regime applied for each of the three temperatures, which is not necessarily reflective of a natural ecosystem.
Effect of temperature on AOB communities at two ammonium concentrations.
Natural creeks are usually characterized by relatively low ammonium concentrations, similar to those measured in LS creek water (16 ± 7 μM), as ammonium is usually removed from the water within a few tens to hundreds of meters of the inlet (57). The majority of the active AOB community represented in the heavy DNA fractions of labeled treatments of LS biofilm enrichments incubated at in situ temperature (13°C) were affiliated with Nitrosomonas oligotropha-like AOB (56% of the clones). Our data are in agreement with those of previous studies demonstrating that pure cultures within this group are adapted to low ammonium concentrations (35). Sequencing analyses in other studies also revealed a dominance of N. oligotropha-like AOB in freshwater environments located in cold temperate European regions (16, 25, 64). In addition to N. oligotropha-like AOB, we found that AOB affiliated with Nitrosospira AmoA cluster 4 (44% of the clones) (Table 4) coexisted in LS biofilm enrichments incubated at 13°C. A previous study on LS creek water and LS water and biofilm of simulated flow channels revealed only Nitrosospira-like clones (30), suggesting that the presence of Nitrosospira cluster 4 in flow channels was not an artifact. However, we cannot rule out the possibility that the use of the nondegenerate primer amoA-2R-GG of bacterial amoA genes in the previous and not in this study may have led to some bias in favor of Nitrosospira clusters, as previously reported (11). In addition, enrichment bias cannot be ruled out both in the flow channel and our SIP experiment.
Nitrosospira AmoA cluster 4 (i.e., 16S rRNA cluster 0) was also detected in AS biofilm enrichments incubated at an in situ temperature (13°C). In this high-nutrient-load biofilm enrichment, Nitrosospira AmoA cluster 4 was the dominant active AOB group (88% of the clones). Because this AOB cluster has commonly been detected in soils (37), it is possible that agricultural runoff events (20) have introduced it into AS and LS stream biofilms. However, our data indicate that the ammonium concentration was the main factor that determined the relatively high frequency of Nitrosospira AmoA cluster 4 as active AOB in the biofilm enrichments.
The ammonium concentrations in Ammerbach creek were 242 μM in the summer sampling and reached a value of 1,458 μM in the winter sampling. The relatively high average concentrations in AS in comparison to those in LS creek water (683 ± 550 and 15.9 ± 6.9 μM, respectively) are probably a result of discharges of wastewater effluent into the AS stream during the period of the investigation or differences in the discharge regimen. AOB belonging to the Nitrosomonas europaea/Nitrosococcus mobilis lineage have frequently been detected in activated sludge and wastewater treatments (33, 74) and, recently, also in a desert ephemeral stream receiving untreated wastewater (3). However, only 6% of the clones affiliated with this lineage were detected in AS biofilm enrichments incubated at 13°C, in contrast to the creek and to the simulated creek ecosystems (30), where this group dominated. This difference might be a result of the high concentration of organic matter in wastewater and in the contaminated creek but not in the setup of our present study.
The natural temperatures of these two creeks do not typically exceed 15°C, but other riverine ecosystems in warmer regions might be subjected to higher temperatures. The majority of the active AOB community in the LS biofilm enrichments incubated at 20°C was affiliated with Nitrosospira AmoA cluster 4 (66%), indicating that this group had a competitive advantage at 20°C compared to the community composition at 13°C at low ammonium concentration. In contrast, the community structure of the active AOB in AS biofilm enrichments did not change at 20°C compared to that at 13°C, implying a potential interaction between ammonium concentrations and temperature in the way they affect the community structure of AOB. Here, insignificant differences in copy number were observed for bacterial amoA gene abundance at 13 and 20°C, suggesting that this increase of temperature at a relatively high ammonium concentration provided no advantage for the growth of Nitrosospira AmoA cluster 4. This study supports the high versatility of AmoA cluster 4 in ammonium concentrations at low and moderate temperatures observed in previous studies (9, 39).
The dominance of the active AOB communities identified in the two biofilm enrichments at 30°C was influenced by the ammonium concentration. In the heavy fraction of LS biofilm enrichments incubated at 30°C, the relative frequency of AOB affiliated with AmoA cluster 4 was higher than at lower temperatures. In contrast, in AS biofilm enrichments, their relative frequency was decreased (20%) at 30°C, similar to the results of previous studies, which could not detect this group in warm regions (8), and after a relatively long period of incubation at 30°C (16 to 20 weeks) (9, 11). In contrast to the results of previous laboratory studies (10, 11, 66), our data reveal changes in the AOB community after a relatively short period of 6 weeks and indicate that members of AmoA cluster 4 were able to assimilate CO2 and survive for a period of 6 weeks in LS biofilm enrichments at a temperature which was previously reported as not suitable for their survival for longer periods (9, 11).
AS biofilm enrichments incubated at 30°C were dominated by active AOB populations affiliated with Nitrosomonas nitrosa (cluster 8; 60% of the clones), which were exclusively detected in this treatment. A previous study suggested that N. nitrosa has an advantage in environments with fluctuating oxygen levels or during the absence of oxygen (44). This may be a reason why N. nitrosa has been retrieved not only from various wastewater treatment plants (21, 27, 55) but also from two soils located in warm regions and supplied with a relatively high input of nitrogen (51, 54). Strains of N. nitrosa that were commonly isolated from eutrophic freshwater exhibited relatively high ammonia Ks values (14 to 43 μM NH3) and, thus, seem to be better adapted to high ammonia concentrations than, for example, N. oligotropha (Ks = 1.9 to 4.2 μM NH3) (35). Together, these reports indicate adaptation of N. nitrosa to high nitrogen, oxygen limitation, and high temperature, similar to the results of this study; thus, it can be expected to be detected in warm regions with constant high nitrogen supply.
The response to increased temperature was observed in both the composition and abundance of the AOB community. An increase in temperature from 13°C to 30°C resulted in a decrease in copy numbers of bacterial amoA genes in nonfractionated DNA extracts of LS and AS biofilm enrichment cultures regardless of the labeling treatment (Fig. 3A and B). A similar observation has previously been made for soil AOB communities (7). Furthermore, the copy numbers of the amoA gene in the heavy fractions of the labeled treatments were one order of magnitude lower at 30°C than at 13°C, regardless of the incubation period (Fig. 3C and D), suggesting lower proliferation and survival of the active AOB at high temperatures.
Ammonia oxidation by Archaea.
Our results showed that CO2 assimilation by AOA could not be detected under the experimental conditions used (i.e., incubations at different constant temperatures over 3 and 6 weeks), presumably due to the relatively high stable ammonium concentrations applied, as was previously observed in two agricultural soils (19, 32), but in contrast to autotrophic growth of uncultivated AOA that has been observed in North Sea water during an in situ [13C]bicarbonate tracer experiment (76) and in a Scottish agricultural soil (52). AOA have also been detected in these creeks in a previous study (30) and exhibit higher abundance than AOB in LS creek water and equal abundance to AOB in AS creek water. However, increasing the incubation time in the simulated creek ecosystems resulted in a higher abundance of AOB than of AOA (30), similar to the observation in a soil microcosm (71). Thus, we conclude that the contribution of AOA to ammonia oxidation (which was not been measured in this study) in our incubation can probably be neglected; ammonia oxidation is expected to correlate with the abundance of bacterial amoA, as was observed in the simulated creek ecosystems (30). We assume that the AOA gene sequences detected in the total DNA and the light DNA fractions of this study (belonging to soil group I.1b; data not shown) were not active, since they were probably dependent on small amounts of organic compounds, as recently demonstrated for a new Thaumarchaeota soil isolate, Nitrososphaera viennensi (68). The marine AOA isolate N. maritimus (34) is affiliated with soil group 1.1a. However, most of the archaeal amoA sequences retrieved in this study belong to the “soil and soil/sediment” cluster (24), similar to those retrieved in a DNA-SIP study of a German agricultural soil (32), and are affiliated with soil group 1.1b. Both groups are now proposed to belong to the new phylum Thaumarchaeota (13, 63).
In summary, we demonstrated that AOB and not AOA were dominant and active in manipulative SIP incubations at anthropogenic ammonium-loading levels (100 to 1,000 μM NH4+). We furthermore showed that under these conditions, the ammonium concentration and the temperature affected the composition of the active AOB community, an observation that should be followed by future studies under field conditions.
Supplementary Material
ACKNOWLEDGMENTS
This study was financially supported by the European Commission Marie Curie Action for Transfer of Knowledge (TOK) (project no. 29983), S.A. was partly supported by the Center for Absorption in Science, Ministry of Immigration, State of Israel, Z.J. received a postdoctoral fellowship from the Max Planck Society, and J.D.N. is supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (NSERC).
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 2 September 2011.
REFERENCES
- 1. Aber J., et al. 1998. Nitrogen saturation in temperate forest ecosystems—hypotheses revisited. Bioscience 48:921–934 [Google Scholar]
- 2. Alzerreca J. J., Norton J. M., Klotz M. G. 1999. The amo operon in marine, ammonia-oxidizing gamma-proteobacteria. FEMS Microbiol. Lett. 180:21–29 [DOI] [PubMed] [Google Scholar]
- 3. Angel R., Asaf L., Ronen Z., Nejidat A. 2010. Nitrogen transformations and diversity of ammonia-oxidizing bacteria in a desert ephemeral stream receiving untreated wastewater. Microb. Ecol. 59:46–58 [DOI] [PubMed] [Google Scholar]
- 4. Arango C. P., Tank J. L. 2008. Land use influences the spatiotemporal controls on nitrification and denitrification in headwater streams. J. Am. Benthol. Soc. 27:90–107 [Google Scholar]
- 5. Arp D. J., Sayavedra-Soto L. A., Hommes N. G. 2002. Molecular biology and biochemistry of ammonia oxidation by Nitrosomonas europaea. Arch. Microbiol. 178:250–255 [DOI] [PubMed] [Google Scholar]
- 6. Avrahami S., Bohannan B. J. M. 2009. N2O emission rates in a California meadow soil are influenced by fertilizer level, soil moisture and the community structure of ammonia-oxidizing bacteria. Glob. Change Biol. 15:643–655 [Google Scholar]
- 7. Avrahami S., Bohannan B. J. M. 2007. A response of Nitrosospira sp. strain AF-like ammonia-oxidizers to changes in temperature, soil moisture content and fertilizer concentration. Appl. Environ. Microbiol. 73:1166–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Avrahami S., Conrad R. 2005. Cold-temperate climate: a factor for selection of ammonia oxidizers in upland soil? Can. J. Microbiol. 51:709–714 [DOI] [PubMed] [Google Scholar]
- 9. Avrahami S., Conrad R. 2003. Patterns of community change among ammonia oxidizers in meadow soils upon long-term incubation at different temperatures. Appl. Environ. Microbiol. 69:6152–6164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Avrahami S., Conrad R., Braker G. 2002. Effect of soil ammonium concentration on N2O release and on the community structure of ammonia oxidizers and denitrifiers. Appl. Environ. Microbiol. 68:5685–5692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Avrahami S., Liesack W., Conrad R. 2003. Effects of temperature and fertilizer on activity and community structure of soil ammonia oxidizers. Environ. Microbiol. 5:691–705 [DOI] [PubMed] [Google Scholar]
- 12. Beman J. M., Arrigo K. R., Matson P. A. 2005. Agricultural runoff fuels large phytoplankton blooms in vulnerable areas of the ocean. Nature 434:211–214 [DOI] [PubMed] [Google Scholar]
- 13. Brochier-Armanet C., Boussau B., Gribaldo S., Forterre P. 2008. Mesophilic crenarchaeota: proposal for a third archaeal phylum, the Thaumarchaeota. Nat. Rev. Microbiol. 6:245–252 [DOI] [PubMed] [Google Scholar]
- 14. Butturini A., Battin T. J., Sabater F. 2000. Nitrification in stream sediment biofilms: the role of ammonium concentration and DOC quality. Water Res. 34:629–639 [Google Scholar]
- 15. Caffrey J. M., Bano N., Kalanetra K., Hollibaugh J. T. 2007. Ammonia oxidation and ammonia-oxidizing bacteria and archaea from estuaries with differing histories of hypoxia. ISME J. 1:660–662 [DOI] [PubMed] [Google Scholar]
- 16. Cébron A., Berthe T., Garnier J. 2003. Nitrification and nitrifying bacteria in the lower Seine River and estuary (France). Appl. Environ. Microbiol. 69:7091–7100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de la Torre J. R., Walker C. B., Ingalls A. E., Konneke M., Stahl D. A. 2008. Cultivation of a thermophilic ammonia oxidizing archaeon synthesizing crenarchaeol. Environ. Microbiol. 10:810–818 [DOI] [PubMed] [Google Scholar]
- 18. Di H. J., et al. 2010. Ammonia-oxidizing bacteria and archaea grown under contrasting soil nitrogen conditions. FEMS Microbiol. Ecol. 72:386–394 [DOI] [PubMed] [Google Scholar]
- 19. Di H. J., et al. 2009. Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat. Geosci. 2:621–624 [Google Scholar]
- 20. Diebel M. W., Vander Zanden M. J. 2009. Nitrogen stable isotopes in streams: effects of agricultural sources and transformations. Ecol. Appl. 19:1127–1134 [DOI] [PubMed] [Google Scholar]
- 21. Dionisi H. M., et al. 2002. Quantification of Nitrosomonas oligotropha-like ammonia-oxidizing bacteria and Nitrospira spp. from full-scale wastewater treatment plants by competitive PCR. Appl. Environ. Microbiol. 68:245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elser J. J., Marzolf E. R., Goldman C. R. 1990. Phosphorus and nitrogen limitation of phytoplankton growth in the freshwaters of North America: a review and critique of experimental enrichments. Can. J. Fish. Aquat. Sci. 47:1468–1477 [Google Scholar]
- 23. Fierer N., Carney K. M., Horner-Devine M. C., Megonigal J. P. 2009. The biogeography of ammonia-oxidizing bacterial communities in soil. Microb. Ecol. 58:435–445 [DOI] [PubMed] [Google Scholar]
- 24. Francis C. A., Roberts K. J., Beman J. M., Santoro A. E., Oakley B. B. 2005. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. U. S. A. 102:14683–14688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Freitag T. E., Chang L., Prosser J. I. 2006. Changes in the community structure and activity of betaproteobacterial ammonia-oxidizing sediment bacteria along a freshwater-marine gradient. Environ. Microbiol. 8:684–696 [DOI] [PubMed] [Google Scholar]
- 26. Friedrich M. W. 2006. Stable-isotope probing of DNA: insights into the function of uncultivated microorganisms from isotopically labeled metagenomes. Curr. Opin. Biotechnol. 17:59–66 [DOI] [PubMed] [Google Scholar]
- 27. Gomez-Villalba B., Calvo C., Vilchez R., Gonzalez-Lopez J., Rodelas B. 2006. TGGE analysis of the diversity of ammonia-oxidizing and denitrifying bacteria in submerged filter biofilms for the treatment of urban wastewater. Appl. Microbiol. Biotechnol. 72:393–400 [DOI] [PubMed] [Google Scholar]
- 28. Hatzenpichler R., et al. 2008. A moderately thermophilic ammonia-oxidizing crenarchaeote from a hot spring. Proc. Natl. Acad. Sci. U. S. A. 105:2134–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Herrmann M., Saunders A. M., Schramm A. 2009. Effect of lake trophic status and rooted macrophytes on community composition and abundance of ammonia-oxidizing prokaryotes in freshwater sediments. Appl. Environ. Microbiol. 75:3127–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Herrmann M., Scheibe A., Avrahami S., Küsel K. 2011. Ammonium availability affects the ratio of ammonia-oxidizing bacteria to ammonia-oxidizing archaea in simulated creek ecosystems. Appl. Environ. Microbiol. 77:1896–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Horz H. P., Barbrook A., Field C. B., Bohannan B. J. M. 2004. Ammonia-oxidizing bacteria respond to multifactorial global change. Proc. Natl. Acad. Sci. U. S. A. 101:15136–15141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jia Z. J., Conrad R. 2009. Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ. Microbiol. 11:1658–1671 [DOI] [PubMed] [Google Scholar]
- 33. Juretschko S., et al. 1998. Combined molecular and conventional analyses of nitrifying bacterium diversity in activated sludge: Nitrosococcus mobilis and Nitrospira-like bacteria as dominant populations. Appl. Environ. Microbiol. 64:3042–3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Könneke M., et al. 2005. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437:543–546 [DOI] [PubMed] [Google Scholar]
- 35. Koops H. P., Pommerening-Roser A. 2001. Distribution and ecophysiology of the nitrifying bacteria emphasizing cultured species. FEMS Microbiol. Ecol. 37:1–9 [Google Scholar]
- 36. Koops H.-P., Purkhold U., Pommerening-Röser A., Timmermann G., Wagner M. 2003. The lithoautotrophic ammonia-oxidizing bacteria. In Dworkin M., et al. (ed.), The prokaryotes an evolving electronic resource for the microbiological community, 3rd ed., release 3.13. Springer-Verlag, New York, NY [Google Scholar]
- 37. Kowalchuk G. A., Stephen J. R. 2001. Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu. Rev. Microbiol. 55:485–529 [DOI] [PubMed] [Google Scholar]
- 38. Kowalchuk G. A., et al. 1997. Analysis of ammonia-oxidizing bacteria of the beta subdivision of the class Proteobacteria in coastal sand dunes by denaturing gradient gel electrophoresis and sequencing of PCR-amplified 16S ribosomal DNA fragments. Appl. Environ. Microbiol. 63:1489–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kowalchuk G. A., Stienstra A. W., Heilig G. H. J., Stephen J. R., Woldendorp J. W. 2000. Changes in the community structure of ammonia-oxidizing bacteria during secondary succession of calcareous grasslands. Environ. Microbiol. 2:99–110 [DOI] [PubMed] [Google Scholar]
- 40. Kumar S., Nei M., Dudley J., Tamura K. 2008. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinformatics 9:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Küsel K., Drake H. L. 1995. Effects of environmental parameters on the formation and turnover of acetate by forest soils. Appl. Environ. Microbiol. 61:3667–3675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lane D. J. 1991. 16S/23S rRNA sequencing, p. 177–203 In Stackebrandt E., Goodfellow M. (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Chichester, United Kingdom [Google Scholar]
- 43. Leininger S., et al. 2006. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809 [DOI] [PubMed] [Google Scholar]
- 44. Limpiyakorn T., Shinohara Y., Kurisu F., Yagi O. 2005. Communities of ammonia-oxidizing bacteria in activated sludge of various sewage treatment plants in Tokyo. FEMS Microbiol. Ecol. 54:205–217 [DOI] [PubMed] [Google Scholar]
- 45. Ludwig W., et al. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Martens-Habbena W., Berube P. M., Urakawa H., R. de la Torre J., Stahl D. A. 2009. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461:976–979 [DOI] [PubMed] [Google Scholar]
- 47. Mincer T. J., et al. 2007. Quantitative distribution of presumptive archaeal and bacterial nitrifiers in Monterey Bay and the North Pacific Subtropical Gyre. Environ. Microbiol. 9:1162–1175 [DOI] [PubMed] [Google Scholar]
- 48. Minz D., et al. 1999. Diversity of sulfate-reducing bacteria in oxic and anoxic regions of a microbial mat characterized by comparative analysis of dissimilatory sulfite reductase genes. Appl. Environ. Microbiol. 65:4666–4671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mosier A. C., Francis C. A. 2008. Relative abundance and diversity of ammonia-oxidizing archaea and bacteria in the San Francisco Bay estuary. Environ. Microbiol. 10:3002–3016 [DOI] [PubMed] [Google Scholar]
- 50. Neufeld J. D., et al. 2007. DNA stable-isotope probing. Nat. Protoc. 2:860–866 [DOI] [PubMed] [Google Scholar]
- 51. Nicolaisen M. H., Ramsing N. B. 2002. Denaturing gradient gel electrophoresis (DGGE) approaches to study the diversity of ammonia-oxidizing bacteria. J. Microbiol. Methods 50:189–203 [DOI] [PubMed] [Google Scholar]
- 52. Offre P., Prosser J. I., Nicol G. W. 2009. Growth of ammonia-oxidizing archaea in soil microcosms is inhibited by acetylene. FEMS Microbiol. Ecol. 70:99–108 [DOI] [PubMed] [Google Scholar]
- 53. Okano Y., et al. 2004. Application of real-time PCR to study effects of ammonium on population size of ammonia-oxidizing bacteria in soil. Appl. Environ. Microbiol. 70:1008–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Oved T., Shaviv A., Goldrath T., Mandelbaum R. T., Minz D. 2001. Influence of effluent irrigation on community composition and function of ammonia-oxidizing bacteria in soil. Appl. Environ. Microbiol. 67:3426–3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Park H. D., Noguera D. R. 2004. Evaluating the effect of dissolved oxygen on ammonia-oxidizing bacterial communities in activated sludge. Water Res. 38:3275–3286 [DOI] [PubMed] [Google Scholar]
- 56. Pauer J. J., Auer M. T. 2000. Nitrification in the water column and sediment of a hypereutrophic lake and adjoining river system. Water Res. 34:1247–1254 [Google Scholar]
- 57. Peterson B. J., et al. 2001. Control of nitrogen export from watersheds by headwater streams. Science 292:86–90 [DOI] [PubMed] [Google Scholar]
- 58. Prosser J. I., Nicol G. W. 2008. Relative contributions of archaea and bacteria to aerobic ammonia oxidation in the environment. Environ. Microbiol. 10:2931–2941 [DOI] [PubMed] [Google Scholar]
- 59. Purkhold U., Wagner M., Timmermann G., Pommerening-Roser A., Koops H. P. 2003. 16S rRNA and amoA-based phylogeny of 12 novel betaproteobacterial ammonia-oxidizing isolates: extension of the dataset and proposal of a new lineage within the nitrosomonads. Int. J. Syst. Evol. Microbiol. 53:1485–1494 [DOI] [PubMed] [Google Scholar]
- 60. Rotthauwe J. H., Witzel K. P., Liesack W. 1997. The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 63:4704–4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sahan E., Muyzer G. 2008. Diversity and spatio-temporal distribution of ammonia-oxidizing Archaea and Bacteria in sediments of the Westerschelde estuary. FEMS Microbiol. Ecol. 64:175–186 [DOI] [PubMed] [Google Scholar]
- 62. Santoro A. E., Francis C. A., de Sieyes N. R., Boehm A. B. 2008. Shifts in the relative abundance of ammonia-oxidizing bacteria and archaea across physicochemical gradients in a subterranean estuary. Environ. Microbiol. 10:1068–1079 [DOI] [PubMed] [Google Scholar]
- 63. Spang A., et al. 2010. Distinct gene set in two different lineages of ammonia-oxidizing archaea supports the phylum Thaumarchaeota. Trends Microbiol. 18:331–340 [DOI] [PubMed] [Google Scholar]
- 64. Speksnijder A., Kowalchuk G. A., Roest K., Laanbroek H. J. 1998. Recovery of a Nitrosomonas-like 16S rDNA sequence group from freshwater habitats. Syst. Appl. Microbiol. 21:321–330 [DOI] [PubMed] [Google Scholar]
- 65. Stephen J. R., et al. 1999. Effect of toxic metals on indigenous soil beta-subgroup proteobacterium ammonia oxidizer community structure and protection against toxicity by inoculated metal-resistant bacteria. Appl. Environ. Microbiol. 65:95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Szukics U., et al. 2010. Nitrifiers and denitrifiers respond rapidly to changed moisture and increasing temperature in a pristine forest soil. FEMS Microbiol. Ecol. 72:395–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tourna M., Freitag T. E., Nicol G. W., Prosser J. I. 2008. Growth, activity and temperature responses of ammonia-oxidizing archaea and bacteria in soil microcosms. Environ. Microbiol. 10:1357–1364 [DOI] [PubMed] [Google Scholar]
- 68. Tourna M., et al. 2011. Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc. Natl. Acad. Sci. U. S. A. 108:8420–8425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Treusch A. H., et al. 2005. Novel genes for nitrite reductase and Amo-related proteins indicate a role of uncultivated mesophilic crenarchaeota in nitrogen cycling. Environ. Microbiol. 7:1985–1995 [DOI] [PubMed] [Google Scholar]
- 70. Venter J. C., et al. 2004. Environmental genome shotgun sequencing of the Sargasso Sea. Science 304:66–74 [DOI] [PubMed] [Google Scholar]
- 71. Verhamme D. T., Prosser J. I., Nicol G. W. 2011. Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. ISME J. 5:1067–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vitousek P. M., et al. 1997. Human alteration of the global nitrogen cycle: sources and consequences. Ecol. Appl. 7:737–750 [Google Scholar]
- 73. Vitousek P. M., Howarth R. W. 1991. Nitrogen limitation on land and in the sea—how can it occur? Biogeochemistry 13:87–115 [Google Scholar]
- 74. Wagner M., Loy A. 2002. Bacterial community composition and function in sewage treatment systems. Curr. Opin. Biotechnol. 13:218–227 [DOI] [PubMed] [Google Scholar]
- 75. Whitby C. B., et al. 2001. 13C incorporation into DNA as a means of identifying the active components of ammonia-oxidizer populations. Lett. Appl. Microbiol. 32:398–401 [DOI] [PubMed] [Google Scholar]
- 76. Wuchter C., Schouten S., Boschker H. T. S., Sinninghe Damsté J. S. 2003. Bicarbonate uptake by marine Crenarchaeota. FEMS Microbiol. Lett. 219:203–207 [DOI] [PubMed] [Google Scholar]
- 77. Zhang L. M., et al. 2010. Autotrophic ammonia oxidation by soil Thaumarchaea. Proc. Natl. Acad. Sci. U. S. A. 107:17240–17245 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.