Abstract
Megasphaera elsdenii is a lactate-fermenting, obligately anaerobic bacterium commonly present in the gastrointestinal tracts of mammals, including humans. Swine M. elsdenii strains were previously shown to have high levels of tetracycline resistance (MIC=64 to >256 μg/ml) and to carry mosaic (recombinant) tetracycline resistance genes. Baby pigs inherit intestinal microbiota from the mother sow. In these investigations we addressed two questions. When do M. elsdenii strains from the sow colonize baby pigs? Can five antibiotic-sensitive M. elsdenii strains administered intragastrically to newborn pigs affect natural colonization of the piglets by antibiotic-resistant (AR) M. elsdenii strains from the mother? M. elsdenii natural colonization of newborn pigs was undetectable (<104 CFU/g [wet weight] of feces) prior to weaning (20 days after birth). After weaning, all pigs became colonized (4 × 105 to 2 × 108 CFU/g feces). In a separate study, 61% (76/125) of M. elsdenii isolates from a gravid sow never exposed to antibiotics were resistant to chlortetracycline, ampicillin, or tylosin. The inoculation of the sow's offspring with mixtures of M. elsdenii antibiotic-sensitive strains prevented colonization of the offspring by maternal AR strains until at least 11 days postweaning. At 25 and 53 days postweaning, however, AR strains predominated. Antibiotic susceptibility phenotypes and single nucleotide polymorphism (SNP)-based identities of M. elsdenii isolated from sow and offspring were unexpectedly diverse. These results suggest that dosing newborn piglets with M. elsdenii antibiotic-sensitive strains delays but does not prevent colonization by maternal resistant strains. M. elsdenii subspecies diversity offers an explanation for the persistence of resistant strains in the absence of antibiotic selection.
INTRODUCTION
Megasphaera elsdenii is a strictly anaerobic, autochthonous member of the gastrointestinal microbiota of ruminants and nonruminants, including humans (2, 16, 28, 39, 40). M. elsdenii has been found at population levels up to 2.4 × 108 CFU/g in fecal samples from humans and pigs (38, 40). Based on its diverse and unique microbiological properties, M. elsdenii has been suggested as a useful archetype commensal, especially for studying factors affecting antibiotic resistance in the intestinal microbiome (38).
Tetracycline-resistant M. elsdenii strains from swine intestinal contents vary both in their tetracycline resistance phenotypes (MIC values ranging from 64 to >256 μg/ml) and in their resistance genotypes (36, 38). Tetracycline-resistant M. elsdenii strains persist at high population levels (107 to 108 CFU/g [wet weight] of feces) in swine in the absence of antibiotic use, that is, in animals raised either organically or traditionally with no known antibiotic exposure (38).
The persistence of antibiotic-resistant bacterial species in intestinal tracts has been noted. Fifteen years after a ban in Sweden on growth-promoting antibiotics, notably the macrolide tylosin, 17% of Enterococcus faecalis isolates were resistant to erythromycin, also a macrolide (1). On 13 Swiss farms and 27 years after tetracyclines were banned in Switzerland as growth promoters, 79% of the enterococci from swine were still tetracycline resistant (3). Withdrawal of all antibiotics from a Kentucky pig herd over a 10-year period led to a decrease in incidence of tetracycline-resistant Escherichia coli from 82 to 42% (25). In recent metagenomic analyses of 44 equivalent bacterial genomes from organic pigs, 10 tetracycline-resistant genes were discovered (21). These and other studies (20, 30, 41) have revealed that, in the absence of antibiotic selection, antibiotic-resistant populations and their resistance genes decrease but persist in mammalian intestinal tracts.
One solution has been proposed for overcoming the persistence of antibiotic resistance in the intestinal tract (45). Namely, exogenously provided, antibiotic-sensitive bacterial strains could be used to displace or replace antibiotic-resistant strains. In these studies, we examined whether or not M. elsdenii antibiotic-sensitive strains would affect sow-to-piglet transmission of antibiotic-resistant strains. Antibiotic-sensitive strains delayed but were unable to prevent colonization of offspring pigs by antibiotic-resistant sow strains. M. elsdenii populations colonizing swine intestines were surprisingly diverse. Subspecies diversity seems a likely basis for M. elsdenii success in the swine intestinal ecosystem and, thus, could contribute to the persistence of antibiotic-resistant M. elsdenii strains in the absence of antibiotic selection.
MATERIALS AND METHODS
M. elsdenii strains and culture conditions.
M. elsdenii strains F101 and F103 were isolated from feral swine on a protected, isolated game preserve in South Carolina. Strains 14-14, 24-50, 26-50, and 33-54 were obtained from domestic swine raised organically (no antibiotic use for the previous 3 to 5 years) on two Iowa farms. M. elsdenii strains were routinely cultured in anaerobic PYG broth or on PYG agar medium (17, 36). PYG is PY medium containing 1% (wt/vol; final concentration) glucose.
M. elsdenii 24-50C is a spontaneous coumermycin-resistant strain and was isolated by plating parent strain 24-50 onto PYG agar medium containing coumermycin A1 (100 μg/ml). Strain 24-50C has a modified nucleotide in gyrB at the equivalent of base position 136 in the E. coli gyrB (5). The base change results in an Arg-to-Leu alteration of the gyrase B subunit and is the likely basis for coumermycin resistance (37; T. B. Stanton, unpublished data). Strains F101, F103, 24-50C, 26-50, and 33-54 are sensitive to chlortetracycline, ampicillin, and tylosin when tested in the patch plate test described below. Strain 14-14 is resistant to all three antibiotics.
For the sake of clarity, the term “strains” will be used in this paper to indicate particular M. elsdenii subspecies previously studied in our laboratory. The term “isolates” will refer to M. elsdenii cells cultured from swine intestinal samples.
Preparation of M. elsdenii cell suspensions for inoculation.
M. elsdenii strains F101, F103, 24-50C, 26-50, and 33-54 were cultured individually in 150 ml of PYG broth to an optical density at 620 nm (OD620) of 1.2 (exponential growth phase; viable cell density of 4 × 107 to 1 × 108 CFU/ml). Cultures were harvested anaerobically by centrifugation (3,000 × g, 5 min) and, inside a flexible film anaerobic chamber (Coy Laboratory Products, Michigan), resuspended in 40 ml of anaerobic PYG plus 10% glycerol as a cryoprotectant. Aliquots (4 ml) of the suspension were injected into sterile 5-ml serum (injection) bottles sealed with thick butyl rubber stoppers. The serum bottles were removed from the chamber and placed in an ethanol bath in a −80°C freezer overnight. Frozen suspensions were stored at −80°C until the day of use. In preliminary studies, the viability of the strictly anaerobic M. elsdenii strains, prepared and stored in this manner, was >75% after 9 weeks.
Swine housing and diet.
Gravid sows with no previous exposure to antibiotics were purchased from a breeder. They arrived at the National Animal Disease Center (NADC) 3 to 4 weeks before the farrowing date. Each sow was kept in an individual room, well isolated from other animals. The rooms were in climate-regulated, access-controlled buildings. Building and room entry by personnel required a clothing change, no prior exposure to swine on that day, and boot dip disinfection. To avoid “nutritional” diarrhea, sows were maintained on their original antibiotic-free diets supplied by the breeder. Farrowing occurred without difficulty. Offspring pigs were each fed commercial antibiotic-free starter diet (TechStart; Kent Feeds). Water for all animals was available ad libitum. All experimental protocols were approved by the NADC Animal Care and Use Committee and followed the guidelines of that committee.
Fecal samples.
From offspring pigs under 20 days old, intestinal contents were sampled by using small or large sterile plastic loops (KV Pet Co.) inserted with a gentle twirling motion into the recta. The loops were weighed before and after sampling to determine wet weight of the intestinal contents (0.3 to 0.5 g/animal). Immediately after weighing and within 2 min of sampling, the loops were placed into 10 ml of anaerobic heart infusion (HI) broth (36) in a tube flushed continuously with an atmosphere of 100% N2 provided by a Hungate-type anaerobic system (18). After 5 to 10 min, the tube was sealed with a rubber stopper, bound with plastic tape, and placed in wet ice.
From sows and offspring pigs over 20 days old, fresh feces (100 to 500 g; within 10 s of being voided) were individually collected, placed in a plastic bag, and immediately put into wet ice. Within 30 min after collection, each specimen in the plastic bag was manually kneaded to create a homogeneous sample and a 5-g aliquot was weighed out and placed into anaerobic HI broth, as described above. Within 1 h after collection, stool samples were transported on ice to the laboratory and transferred into a Coy anaerobic chamber inflated with a mix of N2-H2-CO2 (85:10:5).
M. elsdenii isolation and identification.
Within the Coy anaerobic chamber, intestinal contents taken by sampling loops and within 10-ml tubes were vigorously vortexed three times (0.5 min each) to create a uniform suspension. This concentrated suspension was serially diluted 10-fold in HI broth, and 100 μl of the dilutions was spread-plated onto Me109M agar, a medium developed to selectively isolate M. elsdenii (38).
Within the Coy chamber, stool samples (5 g) in 10 ml of HI broth were diluted into 90 ml of anaerobic HI broth in a sterile Waring blender container (Eberbach; catalog no. EF22337A). The samples were blended three times at high speed in 5-s bursts. The resulting concentrated suspension was diluted and plated as described above.
Inoculated Me109M plates were incubated at 39°C in the anaerobic chamber. After 36 to 48 h, bacterial colonies with the distinct M. elsdenii colony morphology (38) were counted. Individual colonies were subcultured at least twice on PYG agar medium to obtain isolates for antibiotic susceptibility testing and single nucleotide polymorphism (SNP) analysis. Cells in these colonies were examined by microscopy to confirm that they had the distinct M. elsdenii morphology, i.e., very large cocci in pairs or chains (36). Additionally, nearly complete 16S rRNA gene sequences (1,300 to 1,400 bp) were obtained from 12 randomly selected isolates during these investigations and compared with that of LC-1T, type strain of the species M. elsdenii. All isolates had 98.2% or greater 16S rRNA gene sequence identity, consistent with classification as M. elsdenii.
M. elsdenii antibiotic susceptibility tests.
M. elsdenii isolates were examined for susceptibility to chlortetracycline, ampicillin, and tylosin on replicate patch plates as follows. Inside a Coy anaerobic chamber, individual colonies on PYG agar medium were sampled with the flat end of a sterile toothpick and transferred as replicate patches (∼2 mm by 10 mm) onto specific sectors of five agar plates in the sequence PYG plus chlortetracycline (20 μg/ml), PYG plus ampicillin (25 μg/ml), PYG plus tylosin (50 μg/ml), PYG plus coumermycin A1 (25 μg/ml), and PYG (no antibiotics). These antibiotics, except for coumermycin A1, are commonly added to swine diets (7, 15), and antibiotic concentrations in PYG were based on previously determined MIC values for M. elsdenii strains (36) (Stanton, unpublished). M. elsdenii strains 24-50C (Ctcs Amps Tyls Cour) and 14-14 (Ctcr Ampr Tylr Cous) were used as reference strains in the patch plate assays. After 48 h of anaerobic incubation at 39°C, comparisons of M. elsdenii growth on PYG medium without antibiotic with growth on PYG plus antibiotic were used as a basis for judging antibiotic susceptibility. M. elsdenii cells scraped from growth patches on PYG plates were suspended in sterile distilled water (25 μl) and frozen for later use in SNP analyses described below.
Stock solutions of ampicillin (200×), tylosin (200×), and chlortetracycline (100×) were made using water, filter sterilized, and then added to sterile melted PYG agar before pouring the plates. Coumermycin A1 stock solution (200×) was made by adding 10 ml of dimethyl sulfoxide (DMSO) to a vial containing 50 mg of coumermycin A1, and this solution was added directly to the melted agar. All antibiotics were purchased from Sigma (St. Louis, MO).
Experiment A: M. elsdenii natural colonization of offspring pigs.
The time course of sow-to-offspring transmission (colonization of the intestinal tract) of M. elsdenii was monitored. Sow fecal samples were obtained on six separate days before farrowing, and one sample was obtained 5 days after farrowing. Six piglets (group 1) were weaned and separated (same room, different pen) from the sow at 21 days after birth. Fecal samples were taken from these offspring at 11, 18, 25, 32, 46, and 70 days after birth. Another four piglets (group 2) from the same litter were transferred at weaning to a pen in a separate room with different air supply, feed, and water. Fecal samples were taken from group 2 animals at 32 and 70 days after birth.
Experiment B: inoculation of offspring pigs with M. elsdenii antibiotic-sensitive strains.
The ability of antibiotic-sensitive M. elsdenii strains to affect sow-to-piglet transmission of antibiotic-resistant strains was examined. Offspring pigs were inoculated intragastrically with a mixture of five M. elsdenii strains before and after weaning (Fig. 1). On the day of use, frozen suspensions of the five M. elsdenii strains were maintained on dry ice until minutes before piglet inoculation and were then thawed rapidly at 30°C by swirling in a water bath. During inoculation, 0.5-ml samples of each strain were removed and mixed together anaerobically in one sterile injection bottle. The 2.5-ml amount was inoculated by flexible stomach tube into each offspring pig. Pigs in the control group received sterile anaerobic PYG plus 10% glycerol that had been prepared and frozen in parallel with the M. elsdenii inoculum suspensions. Throughout all procedures, M. elsdenii cell suspensions were continually kept anaerobic by flushing the serum bottle contents with oxygen-free nitrogen gas.
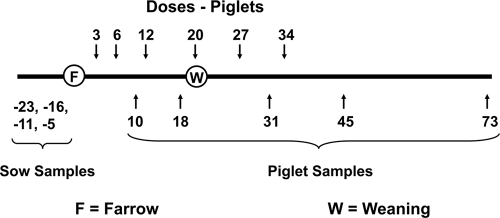
Fig. 1.
Timeline for Megasphaera elsdenii inoculation and fecal sample collection (experiment B). Numbers represent days relative to farrowing (birth) date: above timeline, when offspring pigs were inoculated intragastrically with a mix of five M. elsdenii strains, and below timeline, when fecal samples were taken for enumeration and selective isolation of M. elsdenii. Pigs were weaned at 20 days after farrowing. Three fecal samples were taken from the sow at times indicated and analyzed for M. elsdenii.
Five pigs were inoculated intragastrically with mixtures of M. elsdenii strains and four control pigs received sterile culture medium at 3, 6, 12, 20, 27, and 34 days after birth (Fig. 1). Every experimental pig received approximately 4 × 107 CFU of each M. elsdenii strain at each time point. In order to evaluate M. elsdenii horizontal transmission, inoculated and control pigs were kept in the same pen. Weaning (switch to a solid starter food diet in a pen separate from the sow) occurred at 20 days after birth. Fecal samples were collected at 10, 18, 31, 45, and 73 days after birth and analyzed to determine M. elsdenii total numbers, to evaluate the antibiotic susceptibility of isolates, and to detect the inoculated strains (Fig. 1).
Differentiation of M. elsdenii strains and isolates by SNP analysis of hgd and odt genes.
In preliminary studies, six housekeeping genes of M. elsdenii strain 14-14 were identified during an ongoing genome sequencing project for that strain. The genes were predicted to encode phosphoenolpyruvate synthase, penicillin binding protein, malate/lactate dehydrogenase, DNA gyrase B, a 2-hydroxyglutaryl-coenzyme A (CoA)-dehydratase subunit, and the ATPase subunit for an oligopeptide/dipeptide transporter protein. The sequences of these putative genes were evaluated for differentiating M. elsdenii strains. PCR amplicons of the genes from different swine M. elsdenii strains, including F101, F103, 24-50C, 26-50, and 33-54, were sequenced. SNP differences in the amplicon sequences for the putative 2-hydroxyglutaryl-CoA-dehydratase subunit open reading frame (ORF), designated hgd, and the oligopeptide/dipeptide transporter ORF, designated odt, were chosen for differentiating M. elsdenii strains, as described below.
Samples (10 μl) of frozen M. elsdenii cell suspensions made during the antibiotic patch plate assays were used in PCR amplifications for the hgd and odt genes. Primers for hgd were F-5′ TTCCTACTGCCGCGTCAACA (bp 273 to 292) and R-5′ TTGAAGGTGTGGCCGAGAGC (bp 887 to 868). Primers for odt were F-5′ GACGCCTTGGATCCGCAGAA (bp −38 to −19) and R-5′ AGGGATGGGCCGGACGATAA (bp 751 to 732).
PCR amplification mixtures in 50-μl volumes contained AmpliTaq Gold and GeneAmp reagents (Perkin-Elmer, Applied Biosystems) according to the manufacturer's recommendations, 0.25 μM (each) primer, and M. elsdenii frozen cells or purified DNA (approximately 100 ng of target DNA). The mixtures were heated at 95°C for 10 min to activate the AmpliTaq Gold polymerase. PCR amplification was carried out for 36 cycles each consisting of denaturation (95°C, 1 min), annealing (55°C, 1 min), and extension (72°C, 2 min). An additional extension step (72°C, 8 min) followed the last cycle. The amplicons were sequenced by automated PCR cycle sequencing techniques (12) performed at the Iowa State University DNA Facility (Ames, IA).
The odt amplicon product was 751 bp, and the sequence was subsequently trimmed by removing primer sequences and ambiguous end reads to 627 bp for SNP-based identifications. The hgd amplicon product was 615 bp, and the sequence was trimmed to 476 bp. An 1,108-nucleotide “pseudosequence” was created in a computer file by joining the two sequences with an intervening string of five Gs. This sequence served as a fingerprint for identifying four of the five M. elsdenii dosed strains in fecal samples (Fig. 2). An odt amplicon was not obtained for strain F103 DNA, either because the gene was absent or because, more likely, the PCR primer target sequence(s) was different. Consequently, an M. elsdenii fecal isolate was considered to be F103 both when its odt gene could not be amplified and when its hgd amplicon sequence was identical to that of F103. For computer-based comparisons and SNP analyses of M. elsdenii strains and isolates, programs in Vector NTI v.8 (Invitrogen, Carlsbad, CA) and CLC Genomics Workbench V3.6.5 (CLC Bio, Cambridge, MA) were used.
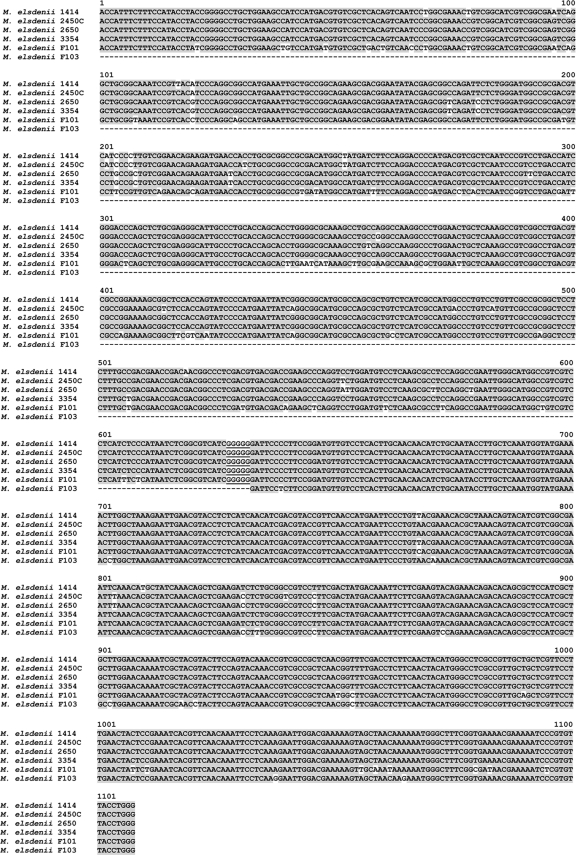
Fig. 2.
SNP differences in hgd-odt assembled sequences of M. elsdenii strain 14-14 and inoculum strains used in these studies. The hgd and odt genes of M. elsdenii strains were amplified and sequenced. Portions of the gene sequences were spliced together at the added GGGGG sequence in the figure. SNPs in the assembled sequences were used to fingerprint M. elsdenii inoculum strains recovered from pigs. Shaded letters represent consensus nucleotides and, when there are equal numbers of nucleotides at a base position, the strain 14-14 nucleotide at that position. An hgd amplicon for strain F103 was not detected. Complete hgd and odt sequences for M. elsdenii strain 14-14 have been deposited in GenBank.
Nucleotide sequence accession numbers.
The two ORF sequences of strain 14-14 have been deposited in GenBank under accession numbers JF297585 and JF297584, respectively.
RESULTS
M. elsdenii natural transmission from sow to offspring.
The natural transmission of M. elsdenii from sow to offspring in the days following birth was examined in experiment A (Table 1). Small numbers of M. elsdenii colonies were obtained when undiluted fecal homogenates from the sow were plated on Me109M selective medium, indicating that the sow was colonized by M. elsdenii. The population levels, however, were beneath the limit of detection (<5 × 103 CFU/g feces).
Table 1.
Experiment A: time course of Megasphaera elsdenii colonization of newborn pigs
| Animal groupa | Day after birth | Day from weaning |
M. elsdenii fecal concnb |
||
|---|---|---|---|---|---|
| Range | Avg (no. of pigs) | SD | |||
| Group 1 | 11 | −10 | <LOD | Und (6) | NA |
| 18 | −3 | <LOD | Und (6) | NA | |
| 25 | +4 | <LOD to 3.5 × 106 | 1.4 × 106 (3) | 1.8 × 106 | |
| 32 | +11 | 1 × 107 to 2.1 × 108 | 1.3 × 107 (6) | 106 | |
| 46 | +25 | 5.2 × 107 to 2 × 108 | 1.4 × 108 (6) | 6 × 107 | |
| 70 | +49 | 4 × 105 to 1 × 108 | 2.4 × 107 (6) | 107 | |
| Group 2 | 32 | +11 | 4.6 × 106 to 1.2 × 107 | 1.2 × 107 (4) | 3.7 × 106 |
| 70 | +49 | 4 × 105 to 1.4 × 106 | 8.5 × 105 (4) | 4.4 × 105 | |
All pigs were littermates weaned at 21 days after birth. At weaning, group 1 pigs (six) were removed to a separate pen in the same room as the mother sow and group 2 pigs (four) were removed to a pen in a separate room.
Concentrations (CFU/g [wet weight] of feces) were determined by plating serial dilutions of fecal suspensions onto Me109M agar medium selective for M. elsdenii growth. SD, sample standard deviation for average fecal counts; Und, no M. elsdenii colonies were obtained; NA, not applicable, insufficient data; <LOD, beneath limit of detection and estimated to be <8,000 CFU/g (wet weight) of feces for loop samples (0.3 g) taken at 11, 18, and 25 days after birth and <5,000 CFU/g (wet weight) of feces for whole fecal (5-g) samples taken at 32, 46, and 70 days after birth.
M. elsdenii colonization of offspring piglets was not detected when the animals were 11 and 18 days old, at 10 and 3 days, respectively, before weaning (group 1; Table 1). A substantial increase in intestinal colonization occurred after weaning. At 4 days after weaning, M. elsdenii cells were cultured from the fecal samples of three of six offspring pigs. At 11, 25, and 49 days after weaning, every pig shed M. elsdenii at detectable levels, averaging 1.3 × 107 to 1.4 × 108 CFU/g feces. M. elsdenii population levels in offspring pigs were several orders of magnitude higher than those detected for the mother sow.
Neither sow nor offspring had ever been exposed to antibiotics. Nevertheless, the antibiotic susceptibility phenotypes of M. elsdenii isolates from both sow and offspring were unexpectedly diverse (Table 2). Of 69 sow isolates, 67 were resistant to one or more of the tested antibiotics. Every resistance combination (single, double, and triple) was represented. In early samples (18, 25, and 32 days after birth) from offspring pigs, M. elsdenii isolates were sensitive to all three antibiotics. At 46 and 70 days after birth, however, M. elsdenii antibiotic-resistant strains with diverse phenotypes were isolated from feces (Table 2).
Table 2.
Experiment A: Megasphaera elsdenii antibiotic resistance phenotypes from mother sow and offspring
| Animala | Day after birth | No. of M. elsdenii isolates testedb | No. of M. elsdenii isolates with antibiotic resistance phenotypec: |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sens | T | A | C | AT | CT | CA | CAT | |||
| Sow | NAd | 69 | 2 | 4 | 15 | 6 | 23 | 1 | 5 | 13 |
| Group 1 offspring (6 pigs) | 11 | Und | ||||||||
| 18 | 2 | 2 | ||||||||
| 25 | 31 | 31 | ||||||||
| 32 | 59 | 59 | ||||||||
| 46 | 60 | 30 | 7 | 13 | 10 | |||||
| 70 | 60 | 19 | 9 | 3 | 2 | 17 | 2 | 8 | ||
| Group 2 offspring (4 pigs) | 70 | 40 | 6 | 6 | 19 | 3 | 6 | |||
At weaning, group 1 pigs were housed in a separate pen in the same room as the mother sow. Group 2 pigs were moved to a separate room.
Ten M. elsdenii colonies (or all colonies, if fewer than 10) were randomly selected for each animal at each sampling time. Sow isolates were obtained from fecal samples taken on six separate days before farrowing and from one sample after farrowing. Und, no M. elsdenii colonies were obtained.
Sens, sensitive to all four antibiotics. T, tylosin resistant; C, chlortetracycline resistant; A, ampicillin resistant. Reference strains 14-14 (Ctcr Ampr Tylr Cous) and 24-50C (Ctcs Amps Tyls Cour) were included as controls in every assay. No coumermycin-resistant isolates were detected.
NA, not applicable.
On the day of weaning, four offspring pigs (group 2) were separated from the litter and moved to a completely different room, well isolated from their group 1 littermates. When tested at 32 and 70 days after birth, group 2 animals had become colonized by M. elsdenii (Table 1). Isolates obtained at 70 days after birth had various antibiotic resistance phenotypes (Table 2).
Given that there was no other environmental source of this obligately anaerobic commensal, the experimental results suggested that M. elsdenii became irreversibly associated with (one or all) offspring pigs before weaning and that attempts to affect sow-to-piglet transmission of M. elsdenii should begin prior to weaning.
M. elsdenii colonization of inoculated pigs.
The ability of five antibiotic-sensitive M. elsdenii strains to colonize and affect sow-to-piglet transmission of antibiotic-resistant strains was examined in experiment B (Tables 3 to 5). Five offspring pigs were inoculated intragastrically with mixtures of five M. elsdenii strains at six time points before, at, and after weaning (Fig. 1). Four control pigs received sterile PYG broth at the same time points. All nine animals were housed in the same pen in order to evaluate horizontal littermate-to-littermate transfer of M. elsdenii. Fecal samples were collected before and after weaning at five time points and screened for M. elsdenii.
Table 3.
Experiment B: Megasphaera elsdenii colonization of inoculated and uninoculated newborn pigsa
| Day after birth | Day from weaning |
M. elsdenii fecal population in pig groupb |
|||||
|---|---|---|---|---|---|---|---|
| Inoculated with M. elsdenii |
Uninoculated (control) |
||||||
| Range (5 pigs) | Avg (total no. of pigs) | SD | Range (4 pigs) | Avg (total no. of pigs) | SD | ||
| 10 | −10 | <LOD to 6.6 × 105 | 3 × 105 (3) | 3.3 × 105 | <LOD to 8.3 × 104 | 8 × 104 (1) | NA |
| 18 | −2 | <LOD to 1.4 × 106 | 7 × 105 (3) | 6.6 × 105 | <LOD | Und (4) | NA |
| 31 | 11 | 1 × 107 to 6 × 107 | 3.3 × 107 (5) | 2.3 × 107 | 8 × 106 to 1.6 × 107 | 107 (4) | 4.6 × 106 |
| 45 | 25 | 6 × 107 to 1.8 × 108 | 2.3 × 108 (5) | 1.6 × 108 | 2.4 × 107 to 1 × 108 | 6.5 × 107 (4) | 4.5 × 107 |
| 73 | 53 | 2.6 × 106 to 2 × 107 | 9.5 × 106 (5) | 7.3 × 106 | 2.6 × 106 to 1 × 108 | 3.1 × 107 (4) | 5.1 × 107 |
Of nine littermate pigs, five were inoculated with M. elsdenii antibiotic-sensitive strains and four control pigs were given sterile medium at various times after birth (Fig. 1). Pigs were weaned at 20 days of age.
CFU/g (wet weight) of feces, as determined by plating dilutions of fecal suspensions onto Me109M agar medium. SD, sample standard deviation for average fecal counts; Und, not detected; <LOD, beneath the limit of detection, defined in Table 1; NA, not applicable, insufficient sample.
Table 5.
Experiment B: SNP-based identification of Megasphaera elsdenii isolates from swine fecal samplesa
| Animal | M. elsdenii treatment | Day after birth | No. of M. elsdenii isolates tested | SNP-based identityb |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inoculated strain |
Sow isolate | Unique isolate | ||||||||
| F101 | F103 | 24-50C | 26-50 | 33-54 | ||||||
| Sow | NAd | NA | 125 | 0 | 0 | 0 | 0 | 0 | 125 | NA |
| Offspring (5 pigs) | Five strains inoculated | 10 | 53 | 0 | 8 | 0 | 45 | 0 | 0 | 0 |
| intragastrically | 18 | 47 | 0 | 0 | 0 | 35 | 12 | 0 | 0 | |
| 31 | 83 | 0 | 0 | 0 | 64 | 19 | 0 | 0 | ||
| 45 | 93 | 0 | 0 | 0 | 28c | 57 | 7 | 1 | ||
| 73 | 92 | 0 | 0 | 0 | 2 | 18 | 52 | 20 | ||
| Offspring (4 pigs) | Sterile medium (control) | 10 | 17 | 0 | 15 | 2 | 0 | 0 | 0 | 0 |
| 18 | 0 | |||||||||
| 31 | 69 | 0 | 0 | 0 | 6 | 0 | 34 | 29 | ||
| 45 | 93 | 0 | 0 | 0 | 0 | 20 | 63 | 10 | ||
| 73 | 89 | 0 | 0 | 0 | 3 | 2 | 83 | 1 | ||
Based on comparisons of hgd-odt sequences (1,108 nucleotides).
A unique isolate did not match any of the five inoculum mixture strains or any of the 125 sow strains.
Three isolates from two pigs had the SNP pattern of M. elsdenii strain 26-50 and were tylosin resistant (Table 4).
NA, not applicable.
M. elsdenii populations in three fecal samples from the mother sow ranged from 4 × 106 to 1.1 × 107 CFU/g (wet weight) of feces. These levels were at least 1,000× higher than the M. elsdenii levels in the sow in experiment A. Of 125 M. elsdenii isolates from the sow, 76 were resistant to one or more antibiotics (Table 4). None of the isolates was resistant to coumermycin A1. None had the SNP identities of the M. elsdenii strains used to inoculate the offspring pigs (Table 5).
Table 4.
Experiment B: Megasphaera elsdenii antibiotic resistance phenotypes from mother sow, inoculated offspring, and control offspringa
| Animal | M. elsdenii treatment | Day after birth | No. of M. elsdenii isolates testedb | No. of M. elsdenii isolates with antibiotic resistance phenotypec |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sens | T | A | C | AT | CT | CA | CAT | Cn | |||||
| Sow | NAd | 125 | 49 | 21 | 7 | 9 | 3 | 17 | 14 | 5 | |||
| Offspring (5 pigs) | Five strains inoculated intragastrically | 10 | 58 | 58 | |||||||||
| 18 | 53 | 53 | |||||||||||
| 31 | 95 | 95 | |||||||||||
| 45 | 93 | 82 | 11 | ||||||||||
| 73 | 93 | 20 | 2 | 1 | 12 | 41 | 1 | 16 | |||||
| Offspring (4 pigs) | Sterile medium (control) | 10 | 19 | 17 | 2 | ||||||||
| 18 | 0 | ||||||||||||
| 31 | 95 | 31 | 5 | 1 | 3 | 4 | 49 | 1 | 1 | ||||
| 45 | 96 | 25 | 17 | 2 | 1 | 8 | 37 | 1 | 5 | ||||
| 73 | 95 | 7 | 69 | 1 | 18 | ||||||||
Sow M. elsdenii isolates were obtained from fecal samples on three separate days, and M. elsdenii isolates were obtained from offspring fecal samples on five separate days (Fig. 1).
Whenever possible, a minimum of 20 isolates were randomly selected from each sample.
Resistance phenotypes are defined in Table 2. All M. elsdenii isolates from fecal samples were coumermycin (Cn) sensitive, except for two isolates from a control pig at day 10. These isolates had the SNP identity of 24-50C (Table 5). Three isolates from two inoculated pigs on day 45 were tylosin resistant (T) and had the SNP pattern of M. elsdenii strain 26-50 (Table 5).
NA, not applicable.
At 10 and 18 days after birth (10 and 2 days before weaning, respectively), M. elsdenii cells were cultured from the fecal samples of three of five inoculated animals (Table 3). The isolates were sensitive to all antibiotics (Table 4), and 100 examined isolates each had the SNP identity of strain F103, 26-50, or 33-54 (Table 5).
One control animal had detectable M. elsdenii fecal levels, 8.3 × 104 CFU/g, at 10 days before weaning (Table 3). Fifteen M. elsdenii isolates from this animal were examined, and all had the SNP identities of strain F103. A second control pig had undetectable numbers of M. elsdenii (<8,000 CFU/g [wet weight] of feces), but two M. elsdenii colonies from this animal were isolated on Me109M medium. The isolates both had the SNP identity of strain 24-50C and were coumermycin resistant (Tables 4 and 5). M. elsdenii was not detected in fecal samples from other control group animals before weaning or from the control animals at 2 days before weaning. These results indicated that four of the five M. elsdenii inoculated strains could be detected and that these survived, at least transiently, in pig intestinal tracts. Additionally, M. elsdenii strains were transmitted between co-penned pigs, most likely via a fecal-oral route. M. elsdenii isolates with the SNP identity of strain F101 were never detected.
M. elsdenii populations were detected in every fecal sample from every pig, inoculated and control, at 31, 45, and 73 days after birth (11, 25, and 53 days after weaning, respectively) (Table 3). M. elsdenii populations ranged from 2.6 × 106 to 1.8 × 108 CFU/g (wet weight), similar to levels seen in postweaning pigs naturally colonized (Table 1).
At 31 days after birth (11 days after weaning), all (95/95) of the tested M. elsdenii isolates from animals dosed with the five antibiotic-sensitive strains of M. elsdenii were antibiotic sensitive (Table 4). Eighty-three tested isolates from the inoculated pigs had the SNP identities of strains 26-50 and 33-54 (Table 5). In contrast, most (64/95) isolates from control, uninoculated animals recovered on the same day were antibiotic resistant (Table 4). Sixty-nine isolates were tested, and in SNP identities, six matched dosed strain 26-50, 34 matched sow strains, and 29 were unique (Table 5).
At 45 days after birth, tylosin-resistant M. elsdenii isolates (11 of 93) appeared in fecal samples from the experimentally inoculated animals (Table 4). Interestingly, three of the tylosin-resistant isolates had the SNP identity of strain 26-50 and came from two different pigs. Seven tylosin-resistant isolates had SNP identities of sow isolates, and one was unique (Table 5). Eighty-two antibiotic-sensitive isolates had the SNP identities of dosed strains 26-50 and 33-54 (Table 5).
M. elsdenii populations in control pigs at 45 days of age had diverse antibiotic resistance phenotypes (Table 4). Twenty of 25 antibiotic-sensitive isolates had the SNP identity of inoculated strain 33-54, indicative again of the easy pig-to-pig transmission of M. elsdenii strains.
By 73 days after birth, M. elsdenii isolates both in the M. elsdenii-inoculated pigs (71/96) and in the control pigs (88/95) were mostly resistant to at least one antibiotic (Table 4). As observed in experiment A, M. elsdenii isolates with diverse antibiotic susceptibility phenotypes were present in fecal samples from both sow and the offspring pigs in the absence of antibiotic selection. Based on these results, inoculating pigs before and after weaning with antibiotic-sensitive M. elsdenii strains at best delayed and did not prevent eventual colonization of the intestinal tract by antibiotic-resistant strains from the mother sow.
DISCUSSION
M. elsdenii, originally known as Peptostreptococcus elsdenii (33), is a commensal (mutualist) species in the gastrointestinal tracts of ruminant and nonruminant mammals, including humans (16, 39, 40). M. elsdenii population densities averaged 6 × 107 CFU/g feces (range, 4 × 105 to 2.1 × 108 CFU/g feces) in 49 samples of postweaning piglets up to 10 weeks old (Tables 1 and 3). These concentrations are comparable to previous estimates of M. elsdenii fecal levels in swine (36, 44) and in humans (40). M. elsdenii population levels are generally low in the bovine rumen but increase dramatically (≥108 CFU/ml) when animals are fed diets containing high levels of starch (44).
M. elsdenii contributes to the overall metabolism that takes place in intestinal microecosystems (2, 28). M. elsdenii metabolizes soluble sugars, amino acids, and lactate (32). In the rumen, for example, M. elsdenii ferments soluble sugars and lactate to acetate, propionate, and butyrate. Propionate is a glucogenic metabolite for the host animal (6, 24), and butyrate is a metabolite promoting healthy mucosal tissues (11, 27). Due to its capacity for lactate metabolism and production of propionate and butyrate, M. elsdenii has been the focus of both prebiotic and probiotic applications for improving animal health (22, 23, 29, 42, 46).
The results of these investigations indicate that the establishment of stable, peak populations of M. elsdenii in young swine intestinal tracts was associated with weaning, both in naturally colonized animals and in animals inoculated with the M. elsdenii strains (Tables 1 and 3). Following the switch from milk to a solid food diet at weaning, the subsequent development of a plant polysaccharide-degrading and lactate-producing microbiota in the pig intestine likely contribute to optimum colonization by M. elsdenii (10, 26, 34).
The offspring of both sows were fed the same grower diet in the two studies, and comparable M. elsdenii populations were detected in the animals (Tables 1 and 3). There were significant differences, however, between M. elsdenii levels in the sows (Tables 1 and 3). During the studies, the sows were maintained on their original diets provided by the breeder. We learned after the experiments that the sow breeder occasionally supplements the standard sow diet with nontraditional feed additives (e.g., granola bars) from a local confectionery factory. The influence of these additives on M. elsdenii populations in the sows is unclear, but their diets were not equivalent. Diet composition is known to alter intestinal microbiota composition (11, 14, 43).
Although sow-to-offspring direct transmission of M. elsdenii prior to weaning is the obvious route for piglet colonization, the recovery of strains 24-50C, F103, 26-50, and 33-54 from control animals copenned with M. elsdenii-inoculated pigs indicates that horizontal transmission of the anaerobe between siblings is also possible. In these experiments with isolated animals, the only environmental source of M. elsdenii for offspring was the sow. Under swine farm management conditions, the opportunity for animal-to-animal and litter-to-litter horizontal transmission of intestinal bacteria would be significantly increased.
Based on SNP identifications, M. elsdenii strains 26-50 and 33-54 were recovered before and after weaning from pigs fed those strains and from control animals after weaning (Table 5). In contrast, strains 24-50C and F103 were detected once and strain F101 was never recovered from fecal samples. An inability to detect these strains in fecal samples indicates that they were present at undetectable levels or were completely eliminated by other M. elsdenii subspecies. Strains F101 and F103 were isolated from feral swine, and strain 24-50C has a modified amino acid in the GyrB subunit of DNA gyrase. Although there were no obvious differences among the five inoculum strains in growth properties or characteristics in culture, the feral strains and 24-50C appear less competitive than 26-50 and 33-54 in the conventional swine intestine. Recovery of the latter two strains decreased over time (Table 5) in the inoculated animals, likely due to competition or dilution by the sow strains.
Three M. elsdenii isolates from two inoculated pigs at 45 days after birth were tylosin resistant and had the SNP identity of strain 26-50. None of 125 tested M. elsdenii isolates from the sow shared SNP identity with strain 26-50 (or any of the other dosed strains). No tylosin-resistant (or any antibiotic-resistant) M. elsdenii isolate with the SNP identity of 26-50 was obtained from control or experimental animals at any other time point. While it cannot completely be ruled out that a sow tylosin-resistant strain with the 26-50 SNP identity escaped detection and colonized the two pigs at high levels at 45 days, a more parsimonious explanation is that the tylosin-resistant isolates resulted from the horizontal transfer of a tylosin resistance determinant to strain 26-50 from a sow M. elsdenii strain (or another intestinal species) in vivo. Although the evidence is circumstantial, these findings, at the very least, emphasize a need for future investigations of antibiotic resistance gene transfer in vitro and in vivo by M. elsdenii.
The diversity of antibiotic susceptibility phenotypes in M. elsdenii is consistent with diverse resistance genotypes detected for this species. M. elsdenii strains have been found to carry tet(O), tet(W), and different recombinant variations of those genes (36, 38). More recently, two ampicillin-resistant M. elsdenii strains were found to have ampicillin resistance genes homologous to a bla resistance determinant from Acidaminococcus fermentans (13). The translated β-lactamase products of the two genes, however, shared 51% amino acid identity, suggesting separate genotype origins (T. B. Stanton et al., unpublished data).
Amplicons of hgd and odt genes were artificially joined in an 1,108-bp sequence in order to differentiate the inoculum strains from each other and to follow their colonization of the swine intestinal tract. One or more of the five dosed strains differed from M. elsdenii strain 14-14 at 104 nucleotide positions (Fig. 2). On this basis, the hgd-odt sequence was selected as a means for detecting the five inoculated strains in swine feces.
An unexpected diversity of hgd-odt genotypes appeared among the M. elsdenii isolates from the sow and offspring. In the course of these studies, 766 M. elsdenii strains and fecal isolates were assigned to 44 SNP genotypes based on the 1,108-bp hgd-odt sequence, as follows: dosed strains (5 genotypes), sow fecal isolates (23 genotypes), and offspring fecal isolates (16 genotypes) (Table 5). The 636 fecal isolates from offspring pigs had SNP identities matching fecal isolates from the sow (239 isolates), dosed strains (336 isolates), or neither (61 isolates). The 61 isolates had 16 unique SNP identities. M. elsdenii isolates with unique SNP identities originated from the sow but were not the predominant subspecies in fecal samples taken before farrowing. It must be emphasized that analysis of the hgd-odt gene sequence was intended as a fingerprint method to identify the inoculated strains and not to evaluate the phylogeny and clade relationships of the isolates.
Dosing offspring swine with antibiotic-sensitive M. elsdenii, at best, delayed but did not prevent colonization of the offspring with antibiotic-resistant strains from the sow. Diversity in both SNP identities and antibiotic susceptibility phenotypes suggests that swine intestinal M. elsdenii populations have subspecies diversity. Such diversity would be consistent with a growing number of reports of subspecies diversity among both pathogenic and nonpathogenic species colonizing the animal and human intestinal tracts (4, 8, 9, 19, 35). Our results indicate that comparable investigations could be insightful for M. elsdenii. Indeed, subspecies diversity, associated with habitat or niche adaptive traits (31), could explain not only the stability of M. elsdenii as an intestinal species but also the persistence of antibiotic-resistant subspecies in the absence of antibiotic selection and in the presence of competing antibiotic-sensitive subspecies.
ACKNOWLEDGMENTS
The authors acknowledge with gratitude the review of the manuscript by Tom Casey and Betsy Bricker.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Footnotes
Published ahead of print on 5 August 2011.
REFERENCES
- 1. Aarestrup F. M., et al. 2002. Antimicrobial resistance among enterococci from pigs in three European countries. Appl. Environ. Microbiol. 68:4127–4129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allison M. J. 1978. Production of branched-chain volatile fatty acids by certain anaerobic bacteria. Appl. Environ. Microbiol. 35:872–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boerlin P., Wissing A., Aarestrup F. M., Frey J., Nicolet J. 2001. Antimicrobial growth promoter ban and resistance to macrolides and vancomycin in enterococci from pigs. J. Clin. Microbiol. 39:4193–4195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Briczinski E. P., et al. 2009. Strain-specific genotyping of Bifidobacterium animalis subsp. lactis by using single-nucleotide polymorphisms, insertions, and deletions. Appl. Environ. Microbiol. 75:7501–7508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Contreras A., Maxwell A. 1992. gyrB mutations which confer coumarin resistance also affect DNA supercoiling and ATP hydrolysis by Escherichia coli DNA gyrase. Mol. Microbiol. 6:1617–1624 [DOI] [PubMed] [Google Scholar]
- 6. Counotte G. H. M., Prins R. A., Janssen R. H. A. M., deBie M. J. A. 1981. Role of Megasphaera elsdenii in the fermentation of DL-[2-13C]lactate in the rumen of dairy cattle. Appl. Environ. Microbiol. 42:649–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dewey C. E., Cox B. D., Straw B. E., Bush E. J., Hurd S. 1999. Use of antimicrobials in swine feeds in the United States. Swine Health Prod. 7:19–25 [Google Scholar]
- 8. Dingle K. E., Colles F. M., Falush D., Maiden M. C. 2005. Sequence typing and comparison of population biology of Campylobacter coli and Campylobacter jejuni. J. Clin. Microbiol. 43:340–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feng P. C., et al. 2007. Genetic diversity among clonal lineages within Escherichia coli O157:H7 stepwise evolutionary model. Emerg. Infect. Dis. 13:1701–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flint H. J., Bayer E. A., Rincon M. T., Lamed R., White B. A. 2008. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat. Rev. Microbiol. 6:121–131 [DOI] [PubMed] [Google Scholar]
- 11. Flint H. J., Petra L., Scott K. P., Duncan S. H. 2007. Commensal bacteria in health and disease, p. 101-115. In Brogden K. A., et al. (ed.), Virulence mechanisms of bacterial pathogens, 4th ed ASM Press, Washington, DC [Google Scholar]
- 12. Frothingham R., Hills H. G., Wilson K. H. 1994. Extensive DNA sequence conservation throughout the Mycobacterium tuberculosis complex. J. Clin. Microbiol. 32:1639–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galan J. C., Reig M., Navas A., Baquero F., Blazquez J. 2000. ACI-1 from Acidaminococcus fermentans: characterization of the first beta-lactamase in anaerobic cocci. Antimicrob. Agents Chemother. 44:3144–3149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herrera P., Kwon Y. M., Ricke S. C. 2009. Ecology and pathogenicity of gastrointestinal Streptococcus bovis. Anaerobe 15:44–54 [DOI] [PubMed] [Google Scholar]
- 15. Herrman T., Sundberg P. 2002. Medicated feed additives for swine. MF-2042, Cooperative Extension Service. Kansas State University, Manhattan, KS [Google Scholar]
- 16. Hespell R. B., Akin D. E., Dehority B. A. 1997. Bacteria, fungi, and protozoa of the rumen, p. 59–141 In Mackie R. I., White B. A., Isaacson R. E. (ed.), Gastrointestinal microbes and host interactions, vol. II Chapman & Hall, New York, NY [Google Scholar]
- 17. Holdeman L. V., Moore W. E. C. 1975. Anaerobe laboratory manual, 3rd ed. Anaerobe Laboratory, Virginia Polytechnic Institute and State University; Blacksburg, VA [Google Scholar]
- 18. Hungate R. E. 1969. A roll tube method for cultivation of strict anaerobes, p. 117–132 In Norris J. R., Ribbons D. W. (ed.), Methods in microbiology, vol. 3B Academic Press Inc., New York, NY [Google Scholar]
- 19. Jost B. H., Trinh H. T., Songer J. G. 2006. Clonal relationships among Clostridium perfringens of porcine origin as determined by multilocus sequence typing. Vet. Microbiol. 116:158–165 [DOI] [PubMed] [Google Scholar]
- 20. Karami N., Nowrouzian F., Adlerberth I., Wold A. E. 2006. Tetracycline resistance in Escherichia coli and persistence in the infantile colonic microbiota. Antimicrob. Agents Chemother. 50:156–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kazimierczak K. A., Scott K. P., Kelly D., Aminov R. I. 2009. Tetracycline resistome of the organic pig gut. Appl. Environ. Microbiol. 75:1717–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klieve A. V., et al. 2003. Establishing populations of Megasphaera elsdenii YE 34 and Butyrivibrio fibrisolvens YE 44 in the rumen of cattle fed high grain diets. J. Appl. Microbiol. 95:621–630 [DOI] [PubMed] [Google Scholar]
- 23. Kung L., Jr., Hession A. O. 1995. Preventing in vitro lactate accumulation in ruminal fermentations by inoculation with Megasphaera elsdenii. J. Anim. Sci. 73:250–256 [DOI] [PubMed] [Google Scholar]
- 24. Ladd J. N., Walker D. J. 1959. The fermentation of lactate and acrylate by the rumen micro-organism LC. Biochem. J. 71:364–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Langlois B. E., Cromwell G. L., Stahly T. S., Dawson K. A., Hays V. W. 1983. Antibiotic resistance of fecal coliforms after long-term withdrawal of therapeutic and subtherapeutic antibiotic use in a swine herd. Appl. Environ. Microbiol. 46:1433–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee A., Gemmell E. 1972. Changes in the mouse intestinal microflora during weaning: role of volatile fatty acids. Infect. Immun. 5:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Louis P., Flint H. J. 2009. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 294:1–8 [DOI] [PubMed] [Google Scholar]
- 28. Miura H., Horiguchi M., Matsumoto T. 1980. Nutritional interdependence among rumen bacteria, Bacteroides amylophilus, Megasphaera elsdenii, and Ruminococcus albus. Appl. Environ. Microbiol. 40:294–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ouwerkerk D., Klieve A. V., Forster R. J. 2002. Enumeration of Megasphaera elsdenii in rumen contents by real-time Taq nuclease assay. J. Appl. Microbiol. 92:753–758 [DOI] [PubMed] [Google Scholar]
- 30. Pallecchi L., et al. 2007. Population structure and resistance genes in antibiotic-resistant bacteria from a remote community with minimal antibiotic exposure. Antimicrob. Agents Chemother. 51:1179–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ponciano J. M., La H. J., Joyce P., Forney L. J. 2009. Evolution of diversity in spatially structured Escherichia coli populations. Appl. Environ. Microbiol. 75:6047–6054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rogosa M. 1984. Genus III. Megasphaera, p. 685 In Krieg N. R., Holt J. G. (ed.), Bergey's manual of systematic bacteriology, vol. 1 Williams & Wilkins, Baltimore, MD [Google Scholar]
- 33. Rogosa M. 1971. Transfer of Peptostreptococcus elsdenii Gutierrez et al. to a new genus, Megasphaera [M. elsdenii (Gutierrez et al.) comb. nov.]. Int. J. Syst. Bacteriol. 21:187–189 [Google Scholar]
- 34. Savage D. C. 1977. Microbial ecology of the gastrointestinal tract. Annu. Rev. Microbiol. 31:107–133 [DOI] [PubMed] [Google Scholar]
- 35. Simpson P. J., Stanton C., Fitzgerald G. F., Ross R. P. 2003. Genomic diversity and relatedness of bifidobacteria isolated from a porcine cecum. J. Bacteriol. 185:2571–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stanton T. B., Humphrey S. B. 2003. Isolation of tetracycline-resistant Megasphaera elsdenii strains with novel mosaic gene combinations of tet(O) and tet(W) from swine. Appl. Environ. Microbiol. 69:3874–3882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stanton T. B., Matson E. G., Humphrey S. B. 2001. Brachyspira (Serpulina) hyodysenteriae gyrB mutants and interstrain transfer of coumermycin A1 resistance. Appl. Environ. Microbiol. 67:2037–2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stanton T. B., McDowall J. S., Rasmussen M. A. 2004. Diverse tetracycline-resistant genotypes of Megasphaera elsdenii strains selectively cultured from swine feces. Appl. Environ. Microbiol. 70:3754–3757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stewart C. S. 1997. Microorganisms in hindgut fermentors, p. 142–186 In Mackie R. I., White B. A., Isaacson R. E. (ed.), Gastrointestinal microbiology II. Gastrointestinal microbes and host interactions, vol. 2 Chapman & Hall, New York, NY [Google Scholar]
- 40. Sugihara P. T., Sutter V. L., Attebery H. R., Bricknell K. S., Finegold S. M. 1974. Isolation of Acidaminococcus fermentans and Megasphaera elsdenii from normal human feces. Appl. Microbiol. 27:274–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thakur S., Tadesse D. A., Morrow M., Gebreyes W. A. 2007. Occurrence of multidrug resistant Salmonella in antimicrobial-free (ABF) swine production systems. Vet. Microbiol. 125:362–367 [DOI] [PubMed] [Google Scholar]
- 42. Tsukahara T., Koyama H., Okada M., Ushida K. 2002. Stimulation of butyrate production by gluconic acid in batch culture of pig cecal digesta and identification of butyrate-producing bacteria. J. Nutr. 132:2229–2234 [DOI] [PubMed] [Google Scholar]
- 43. Turnbaugh P. J., et al. 2009. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 1:6–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vervaeke I. J., Van Assche P. F. 1975. Occurrence of Megasphaera elsdenii in faecal samples of young pigs. Zentralbl. Bakteriol. Hyg. I Abt. Orig. A 231:145–152 [PubMed] [Google Scholar]
- 45. Walker R. D., Buckley M. 2006. Probiotic microbes: the scientific basis. American Academy of Microbiology Colloquium. American Society for Microbiology, Washington, DC: [PubMed] [Google Scholar]
- 46. Yoshida Y., Tsukahara T., Ushida K. 2009. Oral administration of Lactobacillus plantarum Lq80 and Megasphaera elsdenii iNP-001 induces efficient recovery from mucosal atrophy in the small and the large intestines of weaning piglets. Anim. Sci. J. 80:709–715 [DOI] [PubMed] [Google Scholar]