Abstract
Burkholderia pseudomallei is a saprophytic bacterium which is the causative agent of melioidosis, a common cause of fatal bacterial pneumonia and sepsis in the tropics. The incidence of melioidosis is clustered spatially and temporally and is heavily linked to rainfall and extreme weather events. Clinical case clustering has recently been reported in Townsville, Australia, and has implicated Castle Hill, a granite monolith in the city center, as a potential reservoir of infection. Topsoil and water from seasonal groundwater seeps were collected around the base of Castle Hill and analyzed by quantitative real-time PCR targeting the type III secretion system genes for the presence of B. pseudomallei. The organism was identified in 65% (95% confidence interval [CI], 49.5 to 80.4) of soil samples (n = 40) and 92.5% (95% CI, 83.9 to 100) of seasonal groundwater samples (n = 40). Further sampling of water collected from roads and gutters in nearby residential areas after an intense rainfall event found that 88.2% (95% CI, 72.9 to 100) of samples (n = 16) contained viable B. pseudomallei at concentrations up to 113 CFU/ml. Comparison of isolates using multilocus sequence typing demonstrated clinical matches and close associations between environmental isolates and isolates derived from clinical samples from patients in Townsville. This study demonstrated that waterborne B. pseudomallei from groundwater seeps around Castle Hill may facilitate exposure to B. pseudomallei and contribute to the clinical clustering at this site. Access to this type of information will advise the development and implementation of public health measures to reduce the incidence of melioidosis.
INTRODUCTION
The Gram-negative bacillus Burkholderia pseudomallei is the etiological agent of melioidosis, a clinically diverse and often fatal cause of community-acquired pneumonia in Southeast Asia and northern Australia (4). The disease epidemiology is poorly understood; however, infection is believed to follow traumatic inoculation, inhalation, or ingestion of the organism, which is often isolated from the environment in regions where the disease is endemic. The incidence of disease increases following extreme rainfall events (6). Disease incidence correlates with a high prevalence of B. pseudomallei in the environment, in which it appears to be unevenly distributed over large scales, resulting in spatial clustering of clinical incidence (21, 22). As no vaccine is available, the management of melioidosis is focused on exposure reduction through awareness programs (10, 12). Only through a better understanding of the ecology and epidemiology of melioidosis in regions of endemicity can these programs be informed.
Townsville, Australia, is one of two important foci of endemic melioidosis in Queensland (14). While early studies in the region described the isolation of B. pseudomallei from the environment (23), epidemiological studies attempting to link environmental isolates to melioidosis patients have been unsuccessful (15). Recently, Corkeron et al. (3) reported the spatial distribution of clinical disease in Townsville and implicated Castle Hill, an isolated granite monolith in the city, as a potential reservoir for melioidosis. The aims of this study were to provide epidemiological evidence to support this hypothesis and to study environmental factors that may enhance the transmission of melioidosis in this urban environment.
MATERIALS AND METHODS
Environmental samples from Castle Hill.
Environmental samples were collected from the piedmont slopes of Castle Hill (19°15′S, 146°47′E) in early March 2010, near the end of the wet season in northern Queensland. Water samples (n = 40) were obtained from individual groundwater seeps found in the vicinity of a previously confirmed region where B. pseudomallei is endemic, on the southwestern slopes of Castle Hill (unpublished data). Briefly, sterile, disposable 3-ml pipettes (Sarstedt, Nümbrecht, Germany) were used to fill sterile 50-ml Falcon tubes (Sarstedt) with fresh groundwater at the immediate point of seepage from the ground. Samples were stored at ambient temperature and further processed the same day. Top soil (n = 40) was collected from a 300-mm depth along four 100-m transects at 10-m intervals. For sites at which boring was not possible due to large granite outcrops, boring was done at the nearest location where soil was present. Four aliquots of soil were collected at each sampling point in 40-ml specimen containers using an auger that was washed and then sanitized with 70% ethanol between bores. GPS (global positioning system) coordinates were recorded for each sample, and the location was marked for future reference. Resampling of the soil (n = 40) was performed in the dry season in August 2010 by collecting soil from additional bores drilled approximately 300 mm from the original bores. No groundwater seeps were available for sampling during the dry season. Soil water content analysis was performed using standard laboratory methods. Briefly, soil samples were weighed and then oven dried at 105°C for 24 h prior to reweighing and calculation of gravimetric soil water content (25). Statistical comparison of gravimetric soil water content was performed by OpenEpi software using an independent t test (8).
Environmental samples from residential areas.
After environmental sampling on Castle Hill, groundwater seeps were followed into tributaries and into residential areas. Additional water samples from these large tributaries (n = 16) were collected from roads and gutters adjacent to residential properties surrounding Castle Hill during late March 2010, after a 24-h period of intense rainfall. Water was collected into sterile 400-ml screw-top containers (Sarstedt) which were submersed in the flow, capped with zero headspace, disinfected with 70% ethanol prior to labeling, transported to the laboratory, and refrigerated at 4°C to limit replication of B. pseudomallei (24) and potentially antagonistic organisms (17, 18) which might hinder recovery of the organism. The following day, 100 μl of this water was plated in triplicate and incubated at 37°C for 7 days on modified Ashdown's agar (1) containing 15 g/liter technical agar no. 3 (Oxoid, Australia), 15 g/liter tryptone (Oxoid), 40 ml/liter glycerol (Ajax Finechem, Australia), 5 mg/liter crystal violet, 50 mg/liter neutral red, and 50 mg/liter colistin (Sigma, Australia). Plates were observed at 24-h intervals over 7 days for the presence of B. pseudomallei colonies. Suspected colonies were enumerated and subcultured for confirmatory molecular identification.
Extraction of DNA and PCR.
Groundwater and road water samples (50 ml) were transferred aseptically to an equal volume of double-strength Ashdown's broth (2) containing 15 g/liter tryptone (Oxoid), 5 mg/liter crystal violet, and 50 mg/liter colistin (Sigma) in 500-ml conical Pyrex culture flasks which were sealed and then incubated at 37°C with agitation at 100 rpm for 24 h. Soil samples (50 g) were also cultivated in sealed 500-ml conical Pyrex culture flasks containing 100 ml of single-strength Ashdown's isolation broth under the same conditions as the water samples. After broth enrichment of soil and water samples, a single-use 10-μl inoculation loop (Sarstedt) of culture broth was removed and subcultivated on Ashdown's agar in a streak-plate fashion. Incubation at 37°C for 24 h on the agar was performed to further enrich B. pseudomallei, lowering the concentration of PCR-inhibiting compounds relative to the original soil enrichment broth (unpublished data). A large loop of the primary inoculum was scraped from the agar and suspended in 50 μl of Prepman Ultra sample preparation reagent (Applied Biosystems) in 1.5-ml O-ring screw-top microcentrifuge tubes (Sarstedt), vortexed vigorously, and then incubated in a block heater at 100°C for 10 min. Samples were centrifuged at 16,000 × g for 2 min, and the supernatant was removed to new 1.5-ml O-ring screw-top microcentrifuge tubes. No-template controls were performed in triplicate utilizing Prepman Ultra sample preparation reagent without the addition of bacterial inoculum and were processed as the other samples.
Burkholderia pseudomallei DNA was detected using quantitative real-time PCR (qPCR) targeting a 115-bp region within orf2 of the sequence encoding the type III secretion system as described by Novak et al. (20) on a Rotor-Gene 6000 series thermocycler (Corbett Life Science, Australia). The assay was previously determined to be significantly more sensitive than cultivation-based techniques, with no evidence of false-positive results (16). Briefly, 20-μl reaction mixtures consisted of 1× GoTaq colorless master mix (Promega, Australia), 256 nM FAM (6-carboxyfluorescein)-Black Hole Quencher (BHQ)-labeled probe (BpTT4208P [5′-FAM-CCGGAATCTGGATCACCACCACTTTCC-BHQ-3′]), two primers at 400 nM each (BpTT4176F [5′-CGTCTCTATACTGTCGAGCAATCG-3′] and BpTT4290R [5′-CGTGCACACCGGTCAGTATC-3′]), and molecular biology-grade H2O (Sigma) to 20 μl. The template was 1 μl of Prepman Ultra sample preparation reagent as previously prepared (including DNA extraction controls) or molecular biology-grade H2O (Sigma) for no-template qPCR controls. Cycling comprised an initial denaturation period of 3 min at 95°C, followed by 45 cycles of 95°C for 15 s and 59°C for 15 s. All DNA preparation and qPCRs were performed in duplicate on all samples. Statistical comparison of B. pseudomallei prevalences was performed by OpenEpi software using Fisher's exact test (8).
Molecular assay sensitivity.
Control soil for assay sensitivity testing was obtained from an area inside Townsville where 267 soil samples had previously tested negative for the presence of B. pseudomallei using cultivation and qPCR but from which numerous isolates of antagonistic Burkholderia ubonensis-like organisms were recovered (unpublished data). To determine the lowest limit of detection, soils were inoculated with serial dilutions of environmental B. pseudomallei strain K43 from 0 to 60 CFU/g soil as confirmed by standard plate counts, which were performed in duplicate. Each inoculation was performed in quadruplicate and qPCR was performed in triplicate on each sample.
Molecular epidemiology.
BOX-PCR was used to screen subcultured B. pseudomallei isolates for clonality (5). High-purity DNA was extracted using a Promega Wizard SV genomic DNA purification system (Promega, Australia) as per the manufacturer's directions and was quantified and qualified with a NanoPhotometer (Implen, Germany). PCRs using 30-μl reaction mixtures were carried out in 200-μl thin-walled PCR tubes (Sarstedt) and consisted of 1× GoTaq colorless master mix (Promega, Australia), 0.4 μM BOX-A1R primer (5′-CTACGGCAAGGCGACGCTGACG-3′) (5), 2 ng of DNA template, and molecular biology-grade H2O (Sigma) to 30 μl. Thermal cycling was performed by Mastercycler (Eppendorf, Germany) and comprised an initial denaturation period of 3 min at 95°C followed by 40 cycles of 95°C for 30 s, 52°C for 30 s, and 72°C for 180 s and a final elongation of 72°C for 10 min. BOX-PCR products were analyzed by electrophoresis using a 1.5% agarose gel. Based on this analysis, 20 B. pseudomallei isolates with different BOX-PCR profiles were selected for multilocus sequence typing (MLST). PCRs for MLST contained 50 ng template DNA, 1× GoTaq colorless master mix (Promega, Australia), 0.8 μM mixed primers, and molecular biology-grade H2O (Sigma) to 30 μl. Primers for MLST were as described previously (13) with the recommended amendments listed on the B. pseudomallei MLST website (http://bpseudomallei.mlst.net). Cycling conditions consisted of an initial denaturation period of 3 min at 95°C followed by 40 cycles of 95°C for 30 s, 62°C for 30 s, and 72°C for 30 s and a final elongation of 72°C for 10 min. Sequencing products were analyzed by electrophoresis using a 1.5% agarose gel to ascertain correct fragment size, concentration, and purity against a 100-bp DNA marker (Real Biotech Corporation, Taiwan). Reaction products were purified and sequenced by Macrogen (Seoul, South Korea) using ABI PRISM3700 automated sequencing instrumentation (Applied Biosystems). New alleles and sequence types (STs) were submitted to the B. pseudomallei MLST database curator. Analysis of MLST data was performed with eBURST software (11) on the entire MLST data set.
RESULTS
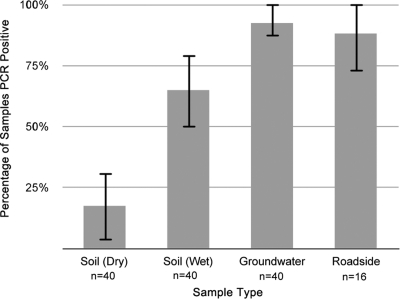
The qPCR assay sensitivity testing revealed agreement across quadruplicate replicates that were seeded with 5 CFU of B. pseudomallei per g of soil. Reproducibility of the qPCR assay analyzing field samples was 100%, with complete correlation between duplicate assays. Burkholderia pseudomallei DNA was detected by qPCR in 7 of 40 (17.5% [95% confidence interval {CI}, 5.2 to 29.8]) of the soil samples collected during the dry season, 26 of 40 (65% [95% CI, 49.5 to 80.4]) of the soil samples collected during the wet season, and 37 of 40 (92.5% [95% CI, 83.9 to 100]) of the water samples from seasonal groundwater seeps at the base of Castle Hill (Fig. 1). Analysis with Fisher's exact test calculated a significant difference between all three proportions (P = 0.005), while the independent t test determined that mean soil water content between seasons was significantly different (P < 0.001), with 13.7% (standard deviation, 4.8) gravimetric water content in the wet season and 3.4% (standard deviation, 2.2) in the dry season.
Fig. 1.
Percentage of environmental samples testing qPCR positive for B. pseudomallei. Differences between values for soil collected during the wet season and soil collected during the dry season are statistically significant (P = 0.005). The highest prevalence was in water from seasonal groundwater seeps around Castle Hill (P = 0.005). Error bars represent 95% confidence intervals.
Burkholderia pseudomallei DNA was detected in 14 of 16 (88.2% [95% CI, 72.9 to 100]) of the roadside water samples collected from Castle Hill (Fig. 1). Triplicate 100-μl spread plates yielded B. pseudomallei colonies from 12 of the 14 qPCR-positive roadside water samples. The mean level of B. pseudomallei from qPCR-positive roadside waters was 14 CFU/ml, with a median of 5 CFU/ml. The highest recovery of the organism was from the southwestern side of Castle Hill, where 113 CFU/ml were recovered using the direct plating method.
Genotyping with MLST resolved 10 sequence types. Eight isolates consisted of three STs which directly matched clinical isolates previously recovered from patients in the Townsville hospital. The remaining seven STs were previously undescribed, of which four were single-locus variants (SLVs) of Townsville clinical isolates (Fig. 2).
Fig. 2.
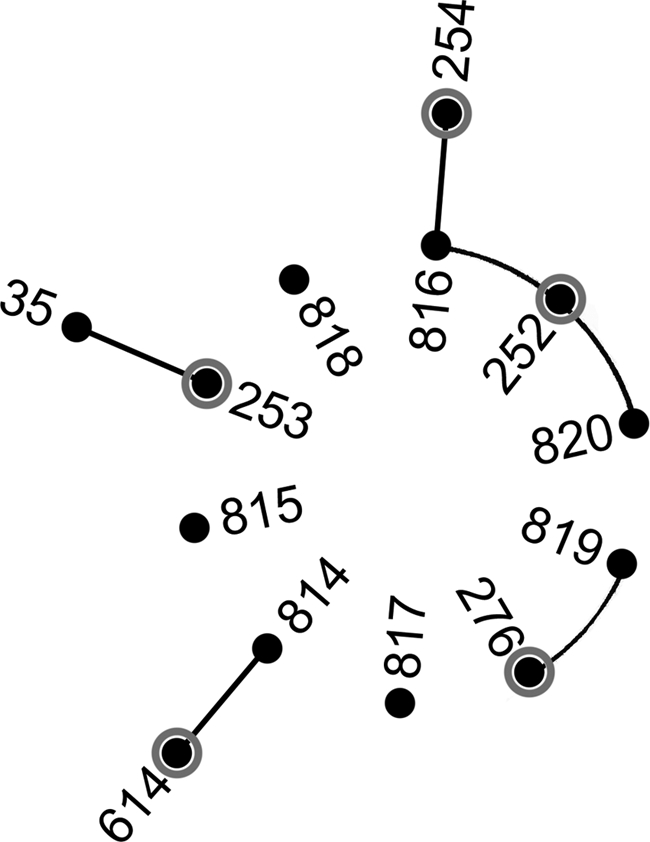
Modified eBURST diagram using MLST data for Townsville groundwater B. pseudomallei isolates and all SLVs identified from the global MLST database. The isolates in the central ring are those isolated from groundwater runoff adjacent to residential properties; isolates with gray circles are sequence types previously recovered from patients in the Townsville hospital (26). Connecting lines represent SLVs. Of the 20 isolates recovered, five belonged to ST276, three to ST814 and ST816, and two to ST252 and ST817. The remaining STs were represented by a single isolate.
DISCUSSION
We have demonstrated that viable B. pseudomallei is discharged from naturally occurring seasonal groundwater seeps in an urban environment overrepresented by clinical melioidosis. Moreover, molecular comparison of these environmental isolates to clinical isolates obtained from patients in the Townsville hospital suggests that seasonal groundwater seeps may be a contributor to melioidosis case clustering around Castle Hill.
Groundwater from Castle Hill can be observed trickling through residential properties and over the surrounding suburban roads for several weeks following heavy rainfall, especially during the wet season. Despite the small quantity of water flowing from individual seeps, collective flow into common tributaries can result in large volumes of groundwater carrying high numbers of viable B. pseudomallei organisms. While prior research has confirmed that man-made water bores represent a reservoir for B. pseudomallei (7, 9, 15), this study has demonstrated B. pseudomallei in natural groundwater seeps.
Molecular typing of these waterborne isolates has identified multiple matches to clinical melioidosis isolates from patients in the area. The molecular matches and high prevalence of B. pseudomallei in water samples from the area constitute compelling evidence that exposure to seasonal groundwater may pose a significant risk factor for acquiring melioidosis in this region. Furthermore, it is likely that the seasonal groundwater seeps are influential in the temporal and spatial clustering of clinical incidence in the area. The findings of this study will help to raise awareness of the dangers associated with seasonal groundwater and associated runoff in regions where melioidosis is endemic.
Questions remain regarding the factors responsible for persistence of B. pseudomallei around Castle Hill. The comparatively low water content of soil on Castle Hill recorded during the dry season of this study is not conducive to long-term survival of the organism (24), and this may be reflected in the significant seasonal prevalences observed. Recent studies in Northern Territory have determined that the organism is more frequently isolated from bores with a low pH, low salinity, and high iron content (9). The piedmont slopes of Castle Hill were previously determined to match these conditions of slight acidity (pH. 5.9 to 6.2) and low total soluble salt content (0.007%) below 300 mm, while the dark red to yellowish red soil color is indicative of high iron oxide content (19). Furthermore, the well-drained granite and sandy loam structure of Castle Hill lends itself to the formation of seasonal groundwater seeps. Although the prevalence of B. pseudomallei in soil increases in the wet season, further studies are required to determine if this phenomenon is due to reseeding of waterlogged areas with the organism that has persisted and multiplied in favorable below-ground conditions. Previous studies indicated that B. pseudomallei may undergo vertical migration in conjunction with the water table after intense rainfall and proliferation in the soil during warmer months (23), which supports this hypothesis. Certainly, Castle Hill represents a landform in which a temporary localized water table is located above the surrounding areas and can be observed draining into the lower-lying urban areas surrounding it.
In conclusion, this study has demonstrated that transport of B. pseudomallei from a primary reservoir source can be facilitated by groundwater seeps and, by extension, that the hydrology of the surrounding areas may be an important determinant of clinical melioidosis. Raising public awareness and implementing appropriate urban drainage management strategies are practical steps that can be taken to reduce the incidence of melioidosis in regions of endemicity where seasonal groundwater seeps are common.
ACKNOWLEDGMENTS
We thank Joseph Kemei at CSIRO Land and Water for performing the gravimetric soil water content analysis, Robert Norton and David Porter at the Townsville Hospital and Maree Corkeron at the Queensland University of Technology for their assistance in providing epidemiological data of melioidosis in Townsville, and Mark Mayo at the Menzies School in Darwin for providing MLST data pertaining to Townsville clinical isolates.
Footnotes
Published ahead of print on 26 August 2011.
REFERENCES
- 1. Ashdown L. R. 1979. An improved screening technique for isolation of Pseudomonas pseudomallei from clinical specimens. Pathology 11:293–297 [DOI] [PubMed] [Google Scholar]
- 2. Ashdown L. R., Clarke S. G. 1992. Evaluation of culture techniques for isolation of Pseudomonas pseudomallei from soil. Appl. Environ. Microbiol. 58:4011–4015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Corkeron M., Norton R., Nelson P. N. 2010. Spatial analysis of melioidosis distribution in the Townsville region of North Queensland. Epidemiol. Infect. 138:1345–1352 [DOI] [PubMed] [Google Scholar]
- 4. Currie B. J., et al. 2000. Endemic melioidosis in tropical northern Australia: a 10-year prospective study and review of the literature. Clin. Infect. Dis. 31:981–986 [DOI] [PubMed] [Google Scholar]
- 5. Currie B. J., et al. 2007. Using BOX-PCR to exclude a clonal outbreak of melioidosis. BMC Infect. Dis. 7:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Currie B. J., Jacups S. P. 2003. Intensity of rainfall and severity of melioidosis, Australia. Emerg. Infect. Dis. 9:1538–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Currie B. J., et al. 2001. A cluster of melioidosis cases from an endemic region is clonal and is linked to the water supply using molecular typing of Burkholderia pseudomallei isolates. Am. J. Trop. Med. Hyg. 65:177–179 [DOI] [PubMed] [Google Scholar]
- 8. Dean A. G., Sullivan K. M., Soe M. M. 23 June 2011, posting date. OpenEpi: open source epidemiologic statistics for public health, version 2.3.1. www.OpenEpi.com
- 9. Draper A. D., et al. 2010. Association of the melioidosis agent Burkholderia pseudomallei with water parameters in rural water supplies in northern Australia. Appl. Environ. Microbiol. 76:5305–5307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faa A. G., Holt P. J. 2002. Melioidosis in the Torres Strait islands of far North Queensland. Commun. Dis. Intell. 26:279–283 [PubMed] [Google Scholar]
- 11. Feil E. J., Li B. C., Aanensen D. M., Hanage W. P., Spratt B. G. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 186:1518–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gal D., et al. 2004. Contamination of hand wash detergent linked to occupationally acquired melioidosis. Am. J. Trop. Med. Hyg. 71:360–362 [PubMed] [Google Scholar]
- 13. Godoy D., et al. 2003. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. J. Clin. Microbiol. 41:2068–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanna J. N., Humphreys J. L., Brookes D. L., Messina T., Raulli A. 2010. Melioidosis in north Queensland, 2000–2009. Commun. Dis. Intell. 34:444–447 [PubMed] [Google Scholar]
- 15. Inglis T. J., et al. 2004. Preliminary report on the northern Australian melioidosis environmental surveillance project. Epidemiol. Infect. 132:813–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaestli M., et al. 2007. Sensitive and specific molecular detection of Burkholderia pseudomallei, the causative agent of melioidosis, in the soil of tropical northern Australia. Appl. Environ. Microbiol. 73:6891–6897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin H. H., et al. 2011. Burkholderia multivorans acts as an antagonist against the growth of Burkholderia pseudomallei in soil. Microbiol. Immunol. doi:10.1111/j.1348-0421.2011.00354.x. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 18. Marshall K., et al. 2010. Antibiosis of Burkholderia ubonensis against Burkholderia pseudomallei, the causative agent for melioidosis. Southeast Asian J. Trop. Med. Public Health 41:904–912 [PubMed] [Google Scholar]
- 19. Murtha G. G. 1975. Soils and land use on the northern section of the Townsville coastal plain, north Queensland. Soils and Land Use Series, no. 55. CSIRO Australia, Division of Soils.
- 20. Novak R. T., et al. 2006. Development and evaluation of a real-time PCR assay targeting the type III secretion system of Burkholderia pseudomallei. J. Clin. Microbiol. 44:85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palasatien S., Lertsirivorakul R., Royros P., Wongratanacheewin S., Sermswan R. W. 2008. Soil physicochemical properties related to the presence of Burkholderia pseudomallei. Trans. R. Soc. Trop. Med. Hyg. 102(Suppl. 1):S5–S9 [DOI] [PubMed] [Google Scholar]
- 22. Suputtamongkol Y., et al. 1994. The epidemiology of melioidosis in Ubon Ratchatani, northeast Thailand. Int. J. Epidemiol. 23:1082–1090 [DOI] [PubMed] [Google Scholar]
- 23. Thomas A. D., Forbes-Faulkner J., Parker M. 1979. Isolation of Pseudomonas pseudomallei from clay layers at defined depths. Am. J. Epidemiol. 110:515–521 [DOI] [PubMed] [Google Scholar]
- 24. Tong S., Yang S., Lu Z., He W. 1996. Laboratory investigation of ecological factors influencing the environmental presence of Burkholderia pseudomallei. Microbiol. Immunol. 40:451–453 [DOI] [PubMed] [Google Scholar]
- 25. Topp G. C., Ferre P. A. 2002. Methods for measurement of soil water content: thermogravimetric using convective oven-drying, p. 422–424 In Jane D. H., Topp G. C.(ed.), Methods of soil analysis, part 4: physical methods. Soil Science Society of America, Madison, WI [Google Scholar]
- 26. Ulett G. C., et al. 2001. Burkholderia pseudomallei virulence: definition, stability and association with clonality. Microbes Infect. 3:621–631 [DOI] [PubMed] [Google Scholar]