Abstract
Cells of Bacillus subtilis can either be motile or sessile, depending on the expression of mutually exclusive sets of genes that are required for flagellum or biofilm formation, respectively. Both activities are coordinated by the master regulator SinR. We have analyzed the role of the previously uncharacterized ymdB gene for bistable gene expression in B. subtilis. We observed a strong overexpression of the hag gene encoding flagellin and of other genes of the σD-dependent motility regulon in the ymdB mutant, whereas the two major operons for biofilm formation, tapA-sipW-tasA and epsA-O, were not expressed. As a result, the ymdB mutant is unable to form biofilms. An analysis of the individual cells of a population revealed that the ymdB mutant no longer exhibited bistable behavior; instead, all cells are short and motile. The inability of the ymdB mutant to form biofilms is suppressed by the deletion of the sinR gene encoding the master regulator of biofilm formation, indicating that SinR-dependent repression of biofilm genes cannot be relieved in a ymdB mutant. Our studies demonstrate that lack of expression of SlrR, an antagonist of SinR, is responsible for the observed phenotypes. Overexpression of SlrR suppresses the effects of a ymdB mutation.
INTRODUCTION
Bacteria can live their lives in very different ways. In the laboratory, they are usually cultured as uniform populations of individual independent cells. However, in natural habitats the formation of aggregates, so-called biofilms, allows them to get better access to nutrients and to protect themselves against harmful substances such as toxins and antibiotics (1, 47). Moreover, under difficult conditions of extreme nutrient limitation, some bacteria such as the Gram-positive model organism Bacillus subtilis undergo a differentiation program and form dormant spores that can survive for decades.
In the last few years, it became obvious that cultivation of uniform single cells produces laboratory artifacts rather than providing meaningful insights into the real physiology of the bacteria. Instead, the formation of all kinds of cell complexes including biofilms seems to be much more representative for the life of bacteria in their natural environments (30).
For B. subtilis, biofilm formation was discovered only a decade ago (6). Moreover, it turned out that bacteria in liquid culture are not physiologically identical but that individual cells can choose one fate or the other. The latter property is referred to as bistability (19). In liquid cultures of B. subtilis, two types of cells can be observed: short motile cells and long nonmotile cells. The motility correlates with the expression of the major flagellin, which is present only in the motile cells, but the different morphologies of the two cell types suggest that a larger set of genes is subject to bistable expression and that all of these genes are controlled in a coordinate manner. Indeed, both biofilm formation and motility are controlled by a common regulator, the transcription factor SinR (see below).
The formation of flagella, motility, and chemotaxis require a set of genes that is under the control of the alternative RNA polymerase sigma factor, σD (44). The activity of this sigma factor is modulated by a regulatory interaction with its antagonist, FlgM (4). Normally, σD is present in small amounts in the cell. Thus, the availability of σD is a limiting factor for the expression of the motility regulon, and σD is thus a major determinant of bistability. The formation of a biofilm involves the synthesis of an extracellular polysaccharide matrix that is produced by the gene products of the epsA-epsO (epsA-O) operon (28). Moreover, the amyloid-like protein TasA is a major matrix component (41). Both the epsA-O and the tapA-sipW-tasA operons are controlled by the transcription repressor SinR (12). This protein binds its operator sites in the control regions of the biofilm operons in its free form. However, normally SinR is sequestered due to its regulatory interaction with either of its antagonists, SinI or SlrR (3, 8).
Biofilm formation and motility are mutually exclusive lifestyles of B. subtilis: biofilm formation would be impossible if the cells were freely moving. Therefore, there are several checks and balances to control the choice. First, one of the proteins expressed in the eps operon, EpsE, interacts with the flagellar motor switch protein FliG to prevent the rotation of the flagellum (5). In this way, motility is directly inhibited in cells that undergo biofilm formation. Second, SinR not only controls biofilm formation but is also involved in the regulation of motility. In an alternative complex with the transcription factor SlrR, SinR triggers the DNA binding activity of this regulator, resulting in repression of autolysis and motility genes (8). On the other hand, in complex with SlrR, SinR can no longer repress the biofilm operons. Thus, only one of the two sets of the genes can be expressed in a cell at a given time point.
We are interested in RNA degradation in B. subtilis. This process involves the exonucleases RNase J1 and J2 and polynucleotide phosphorylase (15, 17). Recently, the novel essential endoribonuclease RNase Y that is required for the initial steps of RNA degradation was identified (14, 45, 54). The rny gene encoding RNase Y is clustered in all Bacillus species with a previously uncharacterized gene, ymdB. Based on the characterization of a homologous protein from Deinococcus radiodurans, the YmdB protein is a phosphodiesterase of unknown physiological function (46). In the pathogenic firmicute Listeria monocytogenes, ymdB mutants are defective in hemolysis and exhibit intracellular growth defects (56). To gain insight into the role of YmdB in B. subtilis, we analyzed the phenotypes of a ymdB mutant. Our results demonstrate that YmdB is involved in the decision-making for lifestyle selection: the ymdB mutant exhibits a severe overexpression of flagellin and the complete σD regulon; in contrast, the biofilm operons are not expressed in the mutant. Both phenotypes can be traced back to a lack of SlrR expression. In consequence, there is no SlrR-mediated repression of motility genes, and SlrR does not antagonize SinR, which is thus constitutively repressing the biofilm operons.
MATERIALS AND METHODS
B. subtilis strains and growth conditions.
All B. subtilis strains used in this work are derived from the laboratory wild-type strain 168 or the nondomesticated strain NCIB 3610. Mutations were transferred to the NCIB 3610 background using SPP1-mediated generalized transduction (55). All strains are listed in Table 1. B. subtilis was grown in LB medium or in CSE minimal medium containing succinate and glutamate/ammonium as basic sources of carbon and nitrogen, respectively (52). The medium was supplemented with auxotrophic requirements (at 50 mg/liter) and glucose. SP, MSgg, and CSE plates were prepared by the addition of 17 g of Bacto agar/liter (Difco) to SP (8 g of nutrient broth per liter-1 mM MgSO4-13 mM KCl, supplemented after sterilization with 2.5 μM FeSO4, 500 μM CaCl2, and 10 μM MnCl2), MSgg medium (6), or CSE medium, respectively.
Table 1.
B. subtilis strains used in this study
| Strain | Genotype or descriptiona | Reference or sourceb |
|---|---|---|
| 168 | trpC2 | Laboratory collection |
| BP494 | trpC2 bglS::(hag-cfp aphA3) | Paola Bisicchia |
| BP496 | trpC2 amyE::(hag-yfp cat) | Paola Bisicchia |
| DL714 | lacA::p(tapA-yfpermC) in CU1065 (based on 168) | Daniel Lopez |
| DS91 | NCIB 3610 sinI::spc | 28 |
| GP583 | trpC2 ΔymdB::spc | LFH-PCR → 168 |
| GP845 | trpC2bglS::(hag-cfp aphA3) lacA::p(tapA-yfp ermC) | DL714 → BP494 |
| GP847 | trpC2bglS::(hag-cfp aphA3) lacA::p(tapA-yfp ermC) ΔymdB::spc | GP583 → GP845 |
| GP902 | trpC2 Δhag::tet | LFH-PCR → 168 |
| GP904 | trpC2 ΔymdB::spc Δhag::tet | GP902 → GP583 |
| GP906 | trpC2 amyE::(hag1-lacZ aphA3) | pGP1032 → 168 |
| GP907 | trpC2 amyE::(hag2-lacZ aphA3) | pGP1033 → 168 |
| GP908 | trpC2 amyE::(hag3-lacZ aphA3) | pGP1034 → 168 |
| GP909 | trpC2 amyE::(hag4-lacZ cat) | pGP1035 → 168 |
| GP910 | trpC2 amyE::(hag5-lacZ cat) | pGP1036 → 168 |
| GP911 | trpC2 amyE::(hag6-lacZ cat) | pGP1037 → 168 |
| GP912 | trpC2 amyE::(hag7-lacZ cat) | pGP1038 → 168 |
| GP914 | trpC2 amyE::(hag1-lacZ aphA3) ΔymdB::spc | pGP1032 → GP583 |
| GP915 | trpC2 amyE::(hag2-lacZ aphA3) ΔymdB::spc | pGP1033 → GP583 |
| GP916 | trpC2 amyE::(hag3-lacZ aphA3) ΔymdB::spc | pGP1034 → GP583 |
| GP917 | trpC2 amyE::(hag4-lacZ cat) ΔymdB::spc | pGP1035 → GP583 |
| GP918 | trpC2 amyE::(hag5-lacZ cat) ΔymdB::spc | pGP1036 → GP583 |
| GP919 | trpC2 amyE::(hag6-lacZ cat) ΔymdB::spc | pGP1037 → GP583 |
| GP920 | trpC2 amyE::(hag7-lacZ cat) ΔymdB::spc | pGP1038 → GP583 |
| GP921 | ΔymdB::spc | GP583 → NCIB3610 |
| GP922 | trpC2 ΔymdB::cat | LFH-PCR → 168 |
| GP923 | trpC2 ΔymdB::cat ΔsinR::spc | GP922 → TMB079 |
| GP925 | trpC2 ΔymdB::spc amyE::(ppgk-ymdB cat) | pGP1062 → GP583 |
| GP946 | trpC2 sinI-3×FLAG ΔsinR::aphA3 | LFH-PCR → 168 |
| GP947 | trpC2 sinI-3×FLAG ΔsinR::aphA3 ΔymdB::spc | GP946 → GP583 |
| GP948 | trpC2sigD-3×FLAG ermC | pGP1085 → 168 |
| GP950 | trpC2 ΔymdB::spc sigD-3×FLAG ermC | pGP1085 → GP583 |
| GP953 | trpC2 amyE::(hag-yfp cat) ΔymdB::spc | BP496 → GP583 |
| GP959 | trpC2 ΔsinI::spc | DS91 → 168 |
| GP972 | trpC2 ΔymdB::spc lacA::(pxylyfpCaphA3) | GP1173 → GP583 |
| GP973 | trpC2 ΔymdB::spc lacA::(pxylslrR aphA3) | pGP1888 → GP583 |
| GP975 | lacA::(pxylslrR aphA3) ΔymdB::spc | GP973 → GP921 |
| GP976 | trpC2 amyE::(hag-yfp cat) lacA::(pxylslrR aphA3) ΔymdB::spc | pGP1888 → GP953 |
| GP993 | trpC2 amyE::(tapA-lacZ cat) | pGP1926 → 168 |
| GP994 | trpC2 amyE::(tapA-lacZ cat) ΔymdB::spc | pGP1926 → GP583 |
| GP1173 | trpC2 lacA::(pxylyfpCaphA3) | pGP888 → 168 |
| NCIB 3610 | Nondomesticated wild type | Laboratory collection |
| TMB079 | trpC2 ΔsinR::spc | 24 |
yfpC, C-terminal fragment of yfp.
Arrows indicate construction by transformation or transduction. LFH, long-flanking homology.
Assays of complex colony formation.
For colony architecture analysis, bacteria were precultured in LB medium until an optical density at 600 nm (OD600) of 0.6 to 0.8. The culture (1.5 ml) was then pelleted and resuspended in 100 μl of the sterile supernatant. Ten microliters of this cell suspension was then spotted onto minimal MSgg medium (6) containing 1.5% agar and incubated at 22°C for 3 to 5 days.
DNA manipulation and transformation.
Escherichia coli DH5α (43) was used for cloning experiments. Plasmid DNA extraction was performed using standard procedures (43). Restriction enzymes, T4 DNA ligase, and DNA polymerases were used as recommended by the manufacturers. DNA fragments were purified from agarose gels using a QIAquick PCR purification kit (Qiagen, Germany). Phusion DNA polymerase was used for the PCR as recommended by the manufacturer. All primer sequences are provided in Table S1 in the supplemental material. DNA sequences were determined using the dideoxy chain termination method (43). All plasmid inserts derived from PCR products were verified by DNA sequencing. Chromosomal DNA of B. subtilis was isolated as described previously (52).
Transformation and phenotypic analysis.
Standard procedures were used to transform E. coli (43), and transformants were selected on LB plates containing ampicillin (100 μg/ml). B. subtilis was transformed with plasmid or chromosomal DNA according to a two-step protocol described previously (31). Transformants were selected on SP plates containing chloramphenicol (Cm; 5 μg/ml), kanamycin (Km; 10 μg/ml), spectinomycin (Spc; 100 μg/ml), tetracycline (10 μg/ml), or erythromycin (Em) plus lincomycin (Lin) (1 μg/ml and 10 μg/ml, respectively).
In B. subtilis, amylase activity was detected after growth on plates containing nutrient broth (7.5 g/liter), 17 g of Bacto agar/liter (Difco), and 5 g of hydrolyzed starch/liter (Connaught). Starch degradation was detected by sublimating iodine onto the plates.
Quantitative studies of lacZ expression in B. subtilis were performed as follows. Cells were grown in LB medium and harvested throughout growth. β- Galactosidase specific activities were determined with cell extracts obtained by lysozyme treatment as described previously (31). One unit of β-galactosidase is defined as the amount of enzyme which produces 1 nmol of o-nitrophenol per min at 28°C.
Plasmids.
Plasmids pAC6 (48) and pAC7 (53) were used to construct transcriptional and translational fusions, respectively, of the hag or tapA control regions with the lacZ gene. The hag promoter region was amplified using the primer pairs shown in Fig. 5 (see Table S1 in the supplemental material for details), and the PCR products were digested with EcoRI and BamHI and cloned into pAC6 and pAC7 linearized with the same enzymes. The resulting plasmids (Table 1; see also Table S2 in the supplemental material for details) were linearized with PstI and used to transform B. subtilis 168 or GP583. The tapA promoter region was amplified using the primer pair CD226/CD233 (see Table S1 for details), and the PCR product was digested with EcoRI and BamHI and cloned into pAC6 linearized with the same enzymes. The resulting plasmid pGP1926 was linearized with PstI and used to transform B. subtilis 168 or GP583.
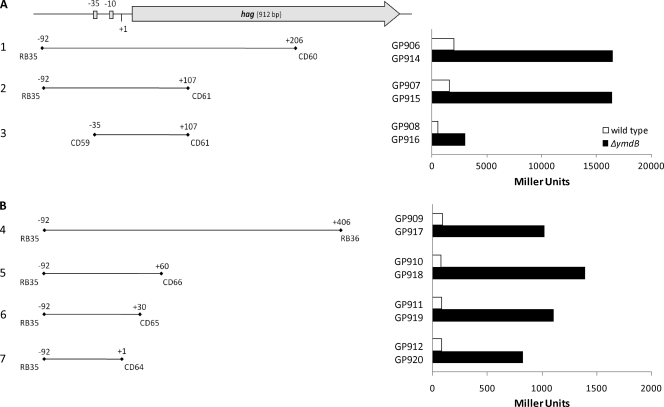
Fig. 5.
YmdB affects hag expression via the core promoter. A deletion analysis of the hag promoter is shown. The region upstream of the hag gene containing the hag promoter region is mapped (top panel). The block arrow represents the hag open reading frame. Bars represent DNA fragments (with their corresponding primer sets) translationally (A) or transcriptionally (B) fused to a lacZ reporter (left panel). For a β-galactosidase assay (right panel) cells containing the hag promoter-lacZ fusions were used, as follows: 1, GP906 and GP914; 2, GP907 and GP915; 3, GP908 and GP916; 4, GP909 and GP917; 5, GP910 and GP918; 6, GP911 and GP919; and 7, GP912 and GP920. The β-galactosidase activities are expressed in Miller Units.
To express a plasmid-borne hag gene in B. subtilis, we constructed plasmid pGP1089. For this purpose the hag gene was amplified with the primers CD172 and CD173 using chromosomal DNA of B. subtilis as a template. The PCR product was digested with BamHI and SalI and cloned into the overexpression vector pBQ200 (36).
Construction of deletion and complementation strains.
Deletion of the ymdB and hag genes was achieved by transformation with PCR products constructed using oligonucleotides (see Table S1 in the supplemental material) to amplify DNA fragments flanking the target genes and intervening antibiotic resistance cassettes (20), as described previously (51).
In order to allow ectopic expression of ymdB, we constructed B. subtilis GP925. In this strain, ymdB is expressed in the amyE locus under the control of the constitutive pgk promoter (35). Strain GP925 was obtained by transformation of B. subtilis GP583 with plasmid pGP1062. This plasmid was obtained as follows. The promoter of the pgk gene and the ymdB gene were amplified using the primer pairs HL51/CD94 and CD95/CD93 and digested with EcoRI/HindIII and HindIII/BamHI, respectively. These fragments were used in a three-arm ligation with pAC6 (48) linearized with EcoRI and BamHI. The resulting plasmid was pGP1062.
Regulated expression of slrR.
To allow the controlled expression of the slrR gene, we placed this gene behind a xylose-regulated promoter and integrated this cassette into the lacA gene in the B. subtilis chromosome. First, the origin of replication and bla resistance gene were amplified from plasmid pUC19 (43) using the primer pair KG50/KG51. This fragment was ligated to the flanking regions of the B. subtilis lacA gene (amplified with KG54/KG55 and KG56/KG57). The resulting plasmid was pGP882. Next, the B. subtilis region containing the xylR gene and the xylA promoter and the aphA3 kanamycin resistance gene from pDG780 (20) were amplified (KG58/KG59 and KG46/KG47, respectively) and cloned between the BamHI and SmaI sites of pGP882. With the oligonucleotide KG59, the translation initiation signals of the strongly expressed gapA gene were attached downstream of the xylA promoter. The resulting plasmid was pGP884. Then, the DNA region corresponding to the C-terminal fragment of the improved yellow fluorescent protein (YFP) from plasmid pIYFP (49) was amplified using the primer pair KG60/KG61. With KG60, a multiple cloning region encompassing XbaI, BamHI, KpnI, and EcoRI sites was added. The fragment was cloned between the BamHI and SalI sites of pGP884 to give pGP888. Finally, the promoterless slrR gene was amplified using the primer pair CD157/ML266 and cloned into pGP888. The resulting plasmid pGP1888 was linearized with ScaI and used to transform B. subtilis GP583.
Analysis of protein expression.
To monitor the expression patterns of σD, this protein was fused to a triple FLAG (3×FLAG) tag at its C terminus. The sigD gene is the penultimate gene of the fla-che operon. To avoid any polar effect on the downstream swrB gene, plasmid pGP1087 was designed, and it allows Campbell-type integration of the plasmid and expression of downstream genes under the control of an isopropyl-ß-d-thiogalactopyranoside (IPTG)-inducible PSPAC promoter. This plasmid was obtained as follows. The triple FLAG tag was amplified from plasmid pGP1331 (32) using the primer pair CD133/CD134, digested with XmaIII, and inserted into XmaIII-linearized pMUTIN-FLAG (25). Next, the 3′ end of the sigD gene was amplified using the oligonucleotides CD129/CD130 and cloned into pGP1087 using the HindIII and ClaI restriction sites. The resulting plasmid was pGP1085 (sigD-3×FLAG).
Real-time qRT-PCR.
For RNA isolation, the cells were grown in CSE minimal medium containing 0.5% (wt/vol) glucose to an OD600 of 0.5 to 0.8 and harvested. Preparation of total RNA was carried out as described previously (35). cDNAs were synthesized using a One-Step reverse transcription-PCR (RT-PCR) kit (Bio-Rad) as described previously (40). Quantitative RT-PCR (qRT-PCR) was carried out on an iCycler instrument (Bio-Rad) following the manufacturer's recommended protocol by using the primers indicated in Table S1 in the supplemental material. The rpsE and rpsJ genes encoding constitutively expressed ribosomal proteins were used as internal controls. Data analysis and the calculation of expression ratios as fold changes were performed as described previously (40). qRT-PCR experiments were performed in duplicate.
Northern blot analysis.
Preparation of total RNA and Northern blot analysis were carried out as described previously (35). Digoxigenin (DIG) RNA probes were obtained by in vitro transcription with T7 RNA polymerase (Roche Diagnostics) using PCR-generated DNA fragments as templates. The primer pairs used to amplify DNA fragments specific for rny, ymdB, hag, and slrR are listed in Table S1 in the supplemental material. The reverse primer contained a T7 RNA polymerase recognition sequence. In vitro RNA labeling, hybridization, and signal detection were carried out according to the instructions of the manufacturer (DIG RNA labeling kit and detection chemicals; Roche Diagnostics). RNA stability was analyzed as described previously (38). Briefly, rifampin was added to logarithmically growing cultures (final concentration, 100 μg/ml), and samples were taken after 0, 5, 15, 30, and 60 min. The quantification was performed using the ImageJ software, version 1.42 (2).
Western blotting.
For Western blot analysis, proteins were separated by 12.5% or 15% SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad) by electroblotting. Rabbit anti-FLAG polyclonal antibodies (1:10,000; Sigma-Aldrich) served as primary antibodies. The antibodies were visualized by using anti-rabbit immunoglobulin alkaline phosphatase secondary antibodies (Promega) and the CDP-Star detection system (Roche Diagnostics), as described previously (13).
In vivo detection of protein-protein interactions.
The isolation of protein complexes from B. subtilis cells was performed by the SPINE technology (21). Briefly, growing cultures of B. subtilis were treated with formaldehyde (0.6% [wt/vol]; 20 min) to facilitate cross-linking of interacting proteins (21). The Strep-tagged proteins and their potential interaction partners were then purified from crude extracts using a Streptactin column (IBA, Göttingen, Germany) and desthiobiotin as the eluent. Interacting proteins were identified by Western blot analysis.
The strain GP946 expressing SinI-FLAG was obtained as follows. First, we constructed plasmid pGP1370 that allows multicopy expression of FLAG-tagged proteins. This plasmid was obtained by amplification of the FLAG tag-encoding region from pGP1331 (32) using the primer pair ML5/ML6 and insertion of the PCR product into the expression vector pBQ200 (36) linearized with HindIII. Next, we amplified sinI and the upstream yqhG gene using the primer pair CD147/CD148. The PCR product was cloned between the BamHI and SalI sites of pGP1370, giving pGP1084. Finally, strain GP946 was constructed by transformation with a PCR product obtained using oligonucleotides (see Table S1 in the supplemental material) to amplify DNA fragments covering the yqhG-sinI-FLAG and tasA regions as well as an intervening kanamycin resistance cassette (20, 51). To allow complementation of sinR that is deleted in GP946, we constructed plasmid pGP1083 that expressed SinR fused to an N-terminal Strep tag. This plasmid was obtained by cloning of the sinR gene (amplified using the oligonucleotides CD137/CD138) between the BamHI and SalI sites of pGP380 (21).
B2H assay.
Primary protein-protein interactions were studied by bacterial two-hybrid (B2H) analysis. The B2H system is based on the interaction-mediated reconstruction of adenylate cyclase (CyaA) activity from Bordetella pertussis in E. coli (26). Briefly, proteins suspected to interact physically were fused with separated domains of the adenylate cyclase as described previously (32). DNA fragments corresponding to the ymdB, sigD, flgM, sinI, and sinR genes were obtained by PCR (for primers, see Table S1 in the supplemental material). The PCR products were digested with KpnI and XbaI and cloned into the vectors of the two-hybrid system that had been linearized with the same enzymes. The resulting plasmids (see Table S2) were used for cotransformations of E. coli BTH101, and the protein-protein interactions were then analyzed by plating the cells on LB plates containing ampicillin (100 μg/ml), kanamycin (50 μg/ml), 5-bromo-4-chloro-3-indolyl-ß-d-galactopyranoside (X-Gal; 40 μg/ml), and IPTG (0.5 mM). The plates were incubated for a maximum of 48 h at 30°C.
Microscopy.
For fluorescence microscopy, cells were grown in LB medium to an OD600 of 0.7 to 1.0, harvested, and resuspended in phosphate-buffered saline (pH 7.5; 50 mM). Fluorescence images were obtained with an Axioskop 40 FL fluorescence microscope, equipped with digital camera AxioCam MRm and AxioVision Rel (version 4.8) software for image processing (Carl Zeiss, Göttingen, Germany) and Neofluar series objective at ×100 primary magnification. The applied filter sets were YFP HC-Filterset (band-pass [BP] 500/24, FT 520, and long-pass [LP] 542/27; AHF Analysentechnik, Tübingen, Germany) for YFP detection and filter set 47 (BP 436/20, FT 455, and LP 480/40; Carl Zeiss) for cyan fluorescent protein (CFP) visualization. All images were taken at the same exposure times. The overlays of fluorescent and phase-contrast images were prepared for presentation with Adobe Photoshop Elements, version 8.0 (Adobe Systems, San Jose, CA).
Time-lapse microscopy of colony formation was performed with Stemi 2000-C instrument equipped with the AxioVision software including the multidimensional acquisition module and the AxioCam ICc1 (Carl Zeiss). Images were taken every 15 min over a time period of 72 h at room temperature. Relative time stamps were added to the images following the format HH:MM:SS:MS (for hour, minute, second, millisecond). The scale bar in the movies corresponds to 0.5 cm.
RESULTS
Overexpression of flagellin in the ymdB mutant.
The B. subtilis ymdB gene is located downstream of the rny gene. While the rny gene product was identified as the novel essential RNase Y, no function has been associated with ymdB (14, 45). A Northern blot analysis revealed that ymdB is the distal gene of the bicistronic rny-ymdB operon (see Fig. S1 in the supplemental material). In a first attempt to identify possible functions of this gene and its product, we made use of the ΔymdB mutant strain GP583. GP583 and the isogenic wild-type B. subtilis 168 were cultivated in LB medium, and the protein patterns of the two strains were compared (Fig. 1). While the general pattern of protein expression was very similar for the two strains, one protein of about 37 kDa was present as an extremely prominent band in the ymdB mutant GP583 but not in the wild-type strain. This band was excised from the gel and identified by mass spectrometry as the flagellin protein Hag. In order to verify that the protein in the prominent band was indeed flagellin, we constructed a hag deletion mutant that was otherwise isogenic to the ymdB mutant GP583. The analysis of the proteome of the hag ymdB double mutant GP904 revealed that the prominent band was absent (Fig. 1). This observation unequivocally confirms the identity of the protein overexpressed in the ymdB mutant as flagellin. To ensure that the overexpression of flagellin was due specifically to the deletion of ymdB and to exclude the possibility of any indirect effect, we performed a complementation analysis by ectopically expressing the ymdB gene under the control of a constitutive promoter. This strain, GP925, is isogenic to the ymdB mutant GP583. As shown in Fig. 1, the intense flagellin band did not appear when expression of ymdB was restored. Thus, a functional ymdB gene is required for proper synthesis of flagellin.
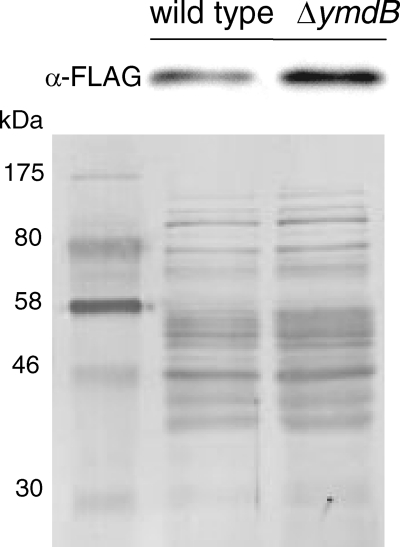
Fig. 1.
Accumulation of the flagellin Hag in the ymdB deletion strain. The proteome of a ymdB mutant strain was compared to the wild-type strain. Crude extracts were isolated from B. subtilis strains grown in complex medium (LB) at 37°C. Lane 1, 168 (wild type); lane 2, GP583 (ΔymdB); lane 3, GP904 (ΔymdB Δhag); lane 4, GP925 (ΔymdB complementation with pGP1062); lane 5, protein weight marker. Fifteen micrograms of crude extract of each culture was loaded on a 12% sodium dodecyl sulfate-polyacrylamide gel. After electrophoresis the gel was stained with Coomassie brilliant blue G-250. The flagellin Hag is indicated with an arrow.
To test whether the accumulation of flagellin in the ymdB mutant is caused at the level of gene expression, we determined the expression of a transcriptional hag-lacZ fusion. For this purpose, the strains GP910 (wild type) and the isogenic ymdB mutant GP918 were grown in CSE minimal medium containing 0.5% glucose, and the β-galactosidase activities driven by the hag promoter were determined. The hag promoter activity resulted in 82 and 1,105 units of β-galactosidase in the wild type and the ymdB mutant strain, respectively. Thus, YmdB is a novel player involved in the control of flagellin gene expression.
YmdB is required for the formation of complex colony architecture.
B. subtilis exhibits “multicellular” behavior by forming complex colonies and biofilms. These properties have been lost during the domestication of strain 168 (37). Therefore, we analyzed the role of YmdB for complex colony formation using the isogenic strain pair derived from NCIB 3610. In good agreement with previous observations (27), the wild-type strain exhibited complex colony architecture. In contrast, smooth nonstructured colonies were detected for the ymdB mutant (Fig. 2; see also movies SM1 and SM2 in the supplemental material). This phenotype might be an indirect result of the overexpression of flagellin in the ymdB mutant. To test this idea, we expressed the hag gene under the control of a strong promoter using plasmid pGP1089 and compared the colony morphology to that of the wild-type strain carrying the empty vector pBQ200. No difference was observed, suggesting that the high expression of flagellin is not the cause for the inability of the ymdB mutant to form complex structures (data not shown). Thus, YmdB is essential for the multicellular lifestyle of B. subtilis.
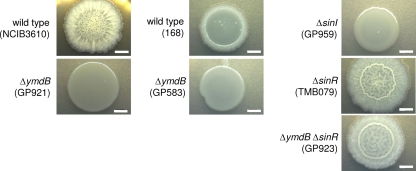
Fig. 2.
Effect of a ymdB deletion on biofilm formation: SinR is epistatic over YmdB. Colony surface architectures of individual colonies grown on MSgg medium are shown. The colonies were filmed (stereomicroscope) after incubation for 3 days at 22°C. The wild-type and mutant strains are as indicated. Scale bar, 250 μm.
Biofilm formation depends on the synthesis of an extracellular polysaccharide and on an amyloid-like fiber protein, TasA. These functions are encoded by genes of the epsA-O and the tapA-sipW-tasA operons (7, 28, 42). To test whether the loss of the complex colony phenotype is accompanied by altered expression of these genes, we studied the activity of a tapA-lacZ fusion in the wild type and the ymdB mutant strain. While about 470 units of β-galactosidase were detected in the wild-type strain GP993, the promoter was inactive (2 units) in the isogenic ymdB mutant GP994. Thus, YmdB is also involved in the control of biofilm gene expression in B. subtilis.
Single-cell expression analysis of motility and biofilm genes.
The experiments described above demonstrate that YmdB directly affects the expression of the hag and the tapA genes. However, the reporter fusion analyses were performed with B. subtilis populations rather than with individual cells. It has been shown previously that hag and tapA have a bistable expression mode; i.e., they are transcribed only in a subset of the cells in a population (27, 34). Thus, YmdB might affect the bistable expression of hag and tapA. To address this issue, we made use of a strain that carries transcriptional fusions of the hag and tapA promoters to the promoterless cfp and yfp genes, respectively. In the strains harboring these fusions, the blue and yellow fluorescence reflects the activities of the hag and tapA promoters, respectively (Fig. 3). In the wild-type strain GP845, we observed two cell types, short and long cells. As reported previously (27), the short cells exhibited a bright blue fluorescence due to the activity of the hag promoter, whereas the long cells had a yellow fluorescence indicative of tapA expression. Notably, the activity of the two promoters was mutually exclusive. The deletion of ymdB in the isogenic strain GP847 completely abolished both the appearance of different cell morphologies and the bistable gene expression: all ymdB mutant cells were short, and all showed a bright blue fluorescence, suggesting that they express the hag gene but not the tapA gene (Fig. 3). Thus, YmdB is required for the differentiation of B. subtilis populations into two distinct cell types.
Fig. 3.
YmdB affects the bistable expression of motility and biofilm genes. Fluorescence microscopy of cells harboring both Phag-cfp and PtapA-yfp fusions in the wild-type strain (GP845) or the isogenic ymdB mutant (GP847). Cells were observed using fluorescence microscopy. Phag-CFP was false-colored blue, and PtapA-YFP is shown in yellow. Cells of GP845 (wild type) and GP847 (ΔymdB) were grown in LB medium and prepared for microscopy in the logarithmic phase of growth. Scale bar, 5 μm.
Implication of YmdB in the expression of the σD regulon.
Recently, it was demonstrated that the bistable expression of hag is due to the limited activity of the sigma factor, σD (16). The lack of bistable hag expression in the ymdB mutant suggests that YmdB might be implicated in the control of σD activity. Therefore, we analyzed the effect of the ymdB mutation on the expression of several σD-dependent genes by real-time quantitative reverse-transcription-PCR (qRT-PCR). This analysis confirmed the high-level expression of the hag gene in the ymdB mutant (Fig. 4). As expected, other σD-dependent genes such as cheV and motA were also more strongly expressed in the ymdB mutant (20- and 25-fold overexpression, respectively) (Fig. 4). Moreover, the expression of the fla-che operon that includes the sigD gene encoding σD was also strongly increased in the ymdB mutant. The expression of the first gene of the operon, flgB, was 7.5-fold elevated in the ymdB mutant, and sigD expression was increased by a factor of 14 (Fig. 4). To exclude any nonspecificity of our assay system, we used the ptsH gene that is expressed under all conditions as a control. Transcription of this gene was not significantly affected by the deletion of ymdB. Thus, the deletion of YmdB results in increased expression of the complete σD regulon.
Fig. 4.
Effect of a ymdB deletion on the expression of genes involved in motility and biofilm formation. Fold changes in the expression of SigD-dependent motility genes (hag, cheV, motA, flgB, and sigD) and SinR-dependent biofilm genes (slrR, tapA, and epsA) in a ΔymdB mutant relative to levels in the wild-type strain (168) are shown. RNA was purified from each strain, and quantitative RT-PCR was performed using primer sets specific for the indicated genes. The gene ptsH was used as a control. Errors bars indicate the standard deviations.
Impact of YmdB on the expression of genes required for biofilm formation.
As shown above, YmdB is not only involved in controlling the expression of the σD regulon but is also required for biofilm formation and for tapA expression (Fig. 2 and 3). To test the role of YmdB in the expression of biofilm genes, we performed qRT-PCR analyses with RNA isolated from the wild-type strain B. subtilis 168 and its isogenic ΔymdB derivative GP583. Again, the constitutively expressed ptsH gene served as a control. As described above, the amount of the ptsH mRNA was not affected by the ymdB allele. The expression of both operons involved in biofilm formation (tapA-sipW-tasA and epsA-O) was strongly affected by the deletion of ymdB. The expression of the promoter-proximal genes of these operons, tapA and epsA, was reduced about 130- and 40-fold, respectively, upon inactivation of ymdB (Fig. 4). Thus, YmdB is required for the expression of the biofilm genes, and the strong reduction of their expression is the likely cause of the inability of the ymdB mutant to form a biofilm.
The hag core promoter is the target for YmdB-dependent regulation.
The results presented above show that YmdB controls the expression of hag and the other genes of the σD regulon. This control could be exerted at the level of transcription initiation or at the level of transcript stability. The localization of the ymdB gene in an operon with the RNase Y-encoding rny gene was suggestive of the latter hypothesis. Therefore, the stability of the hag transcript in the wild-type and ymdB mutant strains was compared. For this purpose, the strains were grown in LB medium, and RNA synthesis was stopped by the addition of rifampin. The amounts of hag-specific mRNA were then probed by Northern blot analysis. As reported previously (22), we observed a single mRNA species that corresponds to a monocistronic hag mRNA. Moreover, this experiment confirmed the strongly increased expression of the hag gene in the ymdB mutant GP583. The analysis of the stability of the hag mRNA revealed a half-life of about 19 min and 32 min in the wild-type strain 168 and the ymdB mutant GP583, respectively (data not shown). These observations suggest that YmdB has a minor effect on the stability of the hag mRNA.
The experiments described above indicate that YmdB may act at the level of both transcription initiation and transcript stability. In order to determine the site of control by YmdB in the hag promoter region more precisely, we constructed a series of hag-lacZ fusions with deletions in the 3′ or 5′ part of the promoter fragment and determined the promoter activities of the remaining fragments by assaying the resulting β-galactosidase activities in isogenic wild-type and ymdB mutant strains. As shown in Fig. 5A, all hag-lacZ translational fusions used in this study responded to the deletion of ymdB by a higher promoter activity. This was the case even if the promoter fragment did not contain any DNA upstream of the core promoter (Fig. 5A, compare GP908 and GP916). Usually, positively acting transcription factors bind their DNA targets upstream of the promoter. Thus, YmdB might control hag expression in a different way. To exclude the implication of the nontranslated mRNA leader in YmdB-dependent expression, we constructed another set of transcriptional hag-lacZ fusions with consecutive deletions of the leader region. Again, all constructs exhibited strongly increased expression in the ymdB mutant background even if the promoter fragment was deleted up to the transcription start point (Fig. 5B, compare GP 912 and GP920). Taken together, these results indicate that YmdB exerts its regulatory effect via the core promoter rather than by binding a target sequence upstream or downstream of the promoter. Thus, it is most likely that σD activity is enhanced in the ymdB mutant.
YmdB controls σD accumulation.
The activity of σD is controlled by a regulatory interaction with the anti-sigma factor FlgM. Increased σD activity may therefore result from high-level expression of σD that titrates FlgM or from an impaired interaction between σD and FlgM that results in higher levels of σD available for transcription initiation. To distinguish between these possibilities, we tested both the cellular amounts of the σD protein and possible protein-protein interactions between σD, FlgM, and YmdB. The amounts of the σD protein in the wild-type strain and in an isogenic ymdB mutant were compared using a sigD allele that fuses a triple FLAG tag to the C terminus of σD. These strains were grown in LB medium, and the presence of SigD-FLAG was tested by Western blot analysis. As shown in Fig. 6, the cellular amount of the σD protein was increased about 2-fold in the ymdB mutant GP950 compared to the level in the isogenic wild-type GP948. Protein-protein interactions were studied using a bacterial two-hybrid system. As reported previously (4), σD and FlgM showed an interaction in this assay. In contrast, YmdB did not interact with SigD or with FlgM (data not shown). These data suggest that the higher expression of σD is the reason for the increased σD activity and the resulting elevated expression of the σD regulon in the ymdB mutant.
Fig. 6.
The cellular amount of SigD is increased in a ymdB mutant. Crude extracts were isolated from the B. subtilis strains GP948 (sigD-FLAG; wild type) and GP950 (sigD-FLAG; ΔymdB strain) grown in complex medium (LB) at 37°C. Five micrograms of crude extract of each culture was loaded on a 12% sodium dodecyl sulfate-polyacrylamide gel. After electrophoresis and blotting onto a polyvinylidene difluoride membrane, the FLAG tag was detected using rabbit polyclonal antibodies. All cultures were harvested in the stationary phase. As a loading control, the membrane was stained with Coomassie brilliant blue G-250. α, anti.
YmdB controls the activity of SinR.
The strongly reduced expression of both biofilm operons suggests that YmdB exerts a general regulatory effect rather than controlling each operon individually. The expression of both operons is repressed by the SinR transcription factor, and induction occurs upon interaction of SinR with its antirepressor, SinI (33). To test whether YmdB interferes with SinI/SinR signaling, we compared the biofilm formation of ymdB, sinI, and sinR mutants. As shown in Fig. 2, the wild-type strain 168 formed colonies with little complexity. The ymdB mutant GP583 formed absolutely smooth colonies. This is in good agreement with the lack of biofilm formation observed in the genetic background of the nondomesticated strain NCIB 3610 (Fig. 2; see also movies SM1 and SM2 in the supplemental material). Similarly, the sinI mutant formed smooth colonies. In contrast and in good agreement with previously published observations (28), the sinR mutant formed complex colonies (see movies SM3 and SM4). To test whether YmdB is involved in this signaling pathway, we constructed the ymdB sinR double mutant GP923 and tested its ability to form structurally complex colonies. As shown in Fig. 2, the phenotype of this double mutant was very similar to that of the sinR mutant TMB079. Thus, the effect of YmdB on biofilm formation is detectable only in the presence of the functional SinR repressor. This suggests that YmdB is involved in the control of SinR activity and, more specifically, that the deletion of ymdB results in permanent transcription repression by SinR.
The results reported above show that YmdB acts like an antagonist of SinR activity, and, indeed, the phenotype of the ymdB mutant with respect to biofilm formation is identical to that of the mutant strain GP959 that lacks the antirepressor SinI (Fig. 2). Thus, YmdB might control the regulatory interaction between SinI and SinR or directly inactivate SinR. To distinguish between these two possibilities, we tested possible interactions of YmdB with SinI and SinR as well as their interaction in a ymdB mutant. The possible interactions between SinI, SinR, and YmdB were studied using the bacterial two-hybrid system. In good agreement with previous reports (33), we observed an interaction between SinI and SinR, whereas YmdB did not interact with either of the two proteins (data not shown). Additionally, we tested the in vivo interaction between SinI and SinR in the wild-type strain and a ymdB mutant by copurification of Strep-tagged SinR with interaction partners from strains that express FLAG-tagged SinI. The two proteins were copurified irrespective of the status of the ymdB gene (data not shown). These results exclude the possibility that YmdB interferes with the regulatory protein-protein interaction between SinI and SinR and suggest that the altered activity of SinR in the ymdB mutant is brought about by a different mechanism.
Reduced SlrR expression is responsible for the phenotypes of the ymdB mutant.
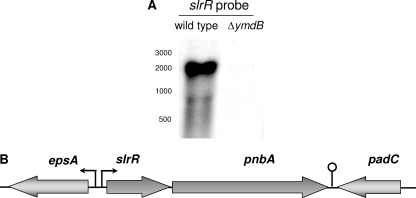
Both biofilm operons are under the control of SinR, and the evidence presented above demonstrates that YmdB is involved in the control of SinR activity in an SinI-independent manner. Therefore, we considered the possibility that additional factors related to SinR might be affected by the ymdB mutation. Indeed, the expression of the slrR gene is repressed by SinR, and the SlrR protein in turn controls the activity of SinR and acts as a direct regulator of motility genes (9, 10, 29). Thus, we compared the expression of the slrR gene in the wild-type strain 168 and its isogenic ymdB mutant GP583 by qRT-PCR analysis. As shown in Fig. 4, the deletion of ymdB resulted in a strong (about 65-fold) reduction of slrR expression. To gain insight into the expression of slrR, we performed a Northern blot analysis using a probe specific for slrR. We detected a signal at a size of 2,000 nucleotides that corresponds to an slrR-pnbA operon (Fig. 7). The pnbA gene encodes a para-nitrobenzyl esterase of unknown physiological function (39, 57). The slrR-pnbA transcript was not detectable in the ymdB mutant, thus confirming the qRT-PCR analysis. This result is in excellent agreement with the idea that the complete SinR regulon is constitutively repressed in the ymdB mutant.
Fig. 7.
YmdB affects the expression of the slrR mRNA. (A) Northern blot analyses to test the effect of YmdB on the expression of the slrR mRNA. The strains B. subtilis 168 (wild type) and GP583 (ymdB mutant) were grown in CSE minimal medium containing 0.5% glucose. The cells were harvested in the exponential phase of growth. The transcripts were detected with riboprobes specific for slrR. (B) Schematic illustration of the slrR-pnbA operon.
However, the experiments described above did not allow us to conclude whether the reduced slrR expression is just another result of the deletion of the ymdB gene or whether there is a causal relation between slrR expression in the mutant and the other phenotypes observed. To address this problem, we constructed a strain that allows expression of the slrR gene under the control of the xylose operon promoter. In this strain, GP975, the slrR gene is expressed if xylose is added to the medium. First, we determined the colony architecture of the ymdB mutant in the absence or presence of xylose, i.e., with or without an expressed slrR gene. As shown in Fig. 8, the wild-type strain NCIB 3610 showed a complex colony architecture that was completely lost when ymdB was deleted (GP921). The same result was obtained with GP975 in the absence of xylose, i.e., when slrR was not expressed. In contrast, induction of slrR expression in GP975 suppressed the phenotype of the ymdB mutation, suggesting that, indeed, the weak expression of slrR was responsible for the lack of complex colony architecture in the ymdB mutant.
Fig. 8.
The defective expression of motility and biofilm genes in the ymdB mutant can be restored by slrR overexpression. (A) Fold changes in expression of hag, sigD, tapA, and epsA were investigated in the ΔymdB mutant (GP973) with and without induction of slrR. In the strain GP973, the slrR gene was placed under the control of a xylose-inducible promoter. The strains were grown in CSE minimal medium containing 0.5% glucose in the presence or absence of xylose. RNA was purified from each strain, and quantitative RT-PCR was performed using primer sets specific to the indicated genes. ptsH was used as a control. Errors bars represent the standard deviations of two replicates. (B) Colony surface architectures of individual colonies grown on MSgg medium with and without xylose are shown. The colonies were filmed (stereomicroscope) after incubation for 4 days at 22°C. The wild-type and mutant strains are as indicated. Scale bar, 250 μm. (C) Fluorescence microscopy of cells (GP976) harboring a Phag-yfp fusion and an inducible slrR expression cassette. Cells of GP976 (ΔymdB) were grown in LB medium with and without xylose and prepared for microscopy in the logarithmic phase of growth. Cells were observed using fluorescence microscopy. Phag-YFP was false-colored yellow. Live cells are shown on the right. Scale bar, 5 μm.
If the weak expression of slrR is responsible for all effects of the ymdB mutation, one would expect that the expression levels of the affected motility and biofilm genes in the ymdB mutant would be reversed upon expression of slrR. This hypothesis was addressed by comparing the expression levels of the corresponding genes in the isogenic strains GP972 and GP973. In both strains the ymdB gene was deleted, and in addition the slrR gene was expressed from a xylose-controlled promoter in GP973. As in the previous experiments, we used the ptsH gene as the control, and expression of this gene was not affected by slrR expression (Fig. 8). As shown in Fig. 8, the expression of the hag gene was essentially abolished upon overexpression of SlrR. This is in perfect agreement with the established role of SlrR as a transcription repressor of hag and other motility genes (9). Similarly, expression of the sigD gene was also reduced when slrR was expressed. The effect was different when the biofilm operons were analyzed. While the ymdB mutation resulted in loss of expression of the tapA-sipW-tasA and epsA-O operons (Fig. 4), the expression of slrR in the ymdB mutant restored the expression of both operons (Fig. 8). This observation is in good agreement with the suppression of the ymdB mutant phenotype with respect to colony architecture upon slrR overexpression.
The reversal of the effects of the ymdB mutation upon slrR overexpression was also tested for the hag-yfp fusion. As shown in Fig. 8C, overexpression of slrR resulted in loss of fluorescence, indicating that the hag promoter was not active when SlrR was overexpressed. This result fits very well with the data from the qRT-PCR analysis of hag expression.
In conclusion, the defective expression of slrR in the ymdB mutant is responsible for the changes in gene expression of motility and biofilm genes when the ymdB gene is deleted.
DISCUSSION
During logarithmic growth, the cells of a B. subtilis population choose one of two alternative ways of life: one portion of a culture forms small motile cells, whereas the other cells are long and may consequently form multicellular structures such as biofilms and pellicles on solid surfaces and in liquid medium, respectively. These different cellular fates result from the expression of distinct sets of genes: the short motile cells express flagellin and the other proteins of the large motility regulon controlled by σD, whereas the longer cells seem to express genes involved in biofilm formation (34). The work presented here demonstrates that the YmdB protein is implicated in this choice: cells that are devoid of an active YmdB protein have no choice but expressing the σD regulon. In contrast, biofilm formation is defective in such cells due to the lack of expression of the epsA-O and tapA-sipW-tasA operons. Thus, YmdB is a novel component of the regulatory machinery that determines bistable gene expression and the ultimate fate of the individual cells.
The SinR transcription factor is the master regulator for bistable gene expression in B. subtilis. On one hand, SinR represses the expression of the genes required for the synthesis of the biofilm matrix. On the other hand, the gene for an additional transcription factor, SlrR, is repressed by SinR. SlrR, in turn, is a repressor of σD-dependent genes for autolysis and motility, including the hag gene (10). The activity of SinR is controlled by regulatory interactions with its cognate antirepressor, SinI, and with SlrR (10, 33). Moreover, expression of the sinIR operon encoding SinR and its antagonist is activated by the master regulator of sporulation initiation, Spo0A, in its phosphorylated form. Spo0A itself is phosphorylated in only a fraction of the population (11, 18). Thus, bistability conferred by Spo0A may influence motility and biofilm formation via the expression and activity of SinR.
Bistable gene expression may be affected by any mutation that interferes with the expression, accumulation, and/or activity of the regulators that control the transcription of the bistable target genes. Recently, it has been shown that increased expression of the sigD gene results in the accumulation of active σD. This may result from the inability of the anti-sigma factor FlgM to inactivate the increased amounts of the sigma factor due to the shift in the stoichiometry between the two proteins. As a consequence, expression of the σD regulon including the hag gene encoding flagellin no longer exhibits bistability but occurs in all cells of the population (16, 50). The larger amount of σD in the ymdB mutant may also explain the increased expression of the σD regulon and the expression of flagellin in all cells of the population (Fig. 3, 4, and 6).
The data presented in this study demonstrate that YmdB affects the expression of both the σD and SinR regulons by controlling the expression of SlrR, a bifunctional transcription repressor of motility genes and an antagonistic interaction partner of SinR. As a member of the SinR regulon, the slrR gene is poorly expressed in the ymdB mutant, and establishing a causal relation is a problem similar to that of the hen and the egg. However, the overexpression of SlrR in the ymdB mutant strain restored the expression of biofilm genes and resulted in decreased expression of the members of the σD regulon. Moreover, overexpression of SlrR allowed the ymdB mutant to form complex colonies. Thus, overexpression of slrR suppresses all known phenotypes of the ymdB mutant strain. This suggests that YmdB and SlrR are part of one signaling chain that ultimately controls the expression of motility and biofilm genes in an antagonistic manner. Based on our results, YmdB might sense some signal(s) and transduce this information to the expression and/or activity of SlrR, which in turn might repress the expression of motility genes and release active SinR to repress biofilm formation. The antagonistic interactions of SlrR with SlrA and SinR as well as of SinR with SinI and SlrR have been intensively studied (8, 29, 33); however, the nature of input signals that control these interactions is largely unknown. This work establishes YmdB as the most upstream factor in the signaling pathway for motility and biofilm formation.
The key question that remains open is related to the molecular mechanism that mediates this control. Based on its similarity to a phosphodiesterase from D. radiodurans (46), YmdB may have the same biochemical activity. It is well established that phosphodiesterases play a crucial role in many signaling processes. In E. coli, the cyclic AMP (cAMP) phosphodiesterase CpdA degrades cAMP and is thus a player in carbon catabolite repression (23). On the other hand, all RNases are also phosphodiesterases that participate in gene regulation. The elucidation of the activity of YmdB at the molecular level and the link of this activity to the control of slrR expression will be the subject of further work.
Supplementary Material
ACKNOWLEDGMENTS
Dörte Becher (University of Greifswald) is acknowledged for the identification of the Hag band by mass spectrometry. Ricarda Banse, Stefan Wicht, and Boris Görke are acknowledged for help with some experiments. We are grateful to Paola Bisicchia, Sina Jordan, Oscar Kuipers, Fabian Commichau, Daniel López, and Thorsten Mascher for the gift of strains and plasmids.
This work was supported by grants of the Deutsche Forschungsgemeinschaft (SFB860) and the Fonds der Chemischen Industrie to J.S.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 19 August 2011.
REFERENCES
- 1. Abee T., Kovács A. T., Kuipers O. P., van der Veen S. 2011. Biofilm formation and dispersal in Gram-positive bacteria. Curr. Opin. Biotechnol. 22:172–179 [DOI] [PubMed] [Google Scholar]
- 2. Abramoff M. D., Magelhaes P. J., Ram S. J. 2004. Image processing with ImageJ. Biophotonics Int. 11:36–42 [Google Scholar]
- 3. Bai U., Mandic-Mulec I., Smith I. 1993. SinI modulates the activity of SinR, a developmental switch protein of Bacillus subtilis, by protein-protein interaction. Genes Dev. 7:139–148 [DOI] [PubMed] [Google Scholar]
- 4. Bertero M. G., Gonzales B., Tarricone C., Ceciliani F., Galizzi A. 1999. Overproduction and characterization of the Bacillus subtilis anti-sigma factor FlgM. J. Biol. Chem. 274:12103–12107 [DOI] [PubMed] [Google Scholar]
- 5. Blair K. M., Turner L., Winkelmann J. T., Berg H. C., Kearns D. B. 2008. A molecular clutch disables flagella in the Bacillus subtilis biofilm. Science 320:1636–1638 [DOI] [PubMed] [Google Scholar]
- 6. Branda S. S., González-Pastor J. E., Ben-Yehuda S., Losick R., Kolter R. 2001. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 98:11621–11626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Branda S. S., Chu F., Kearns D. B., Losick R., Kolter R. 2006. A major protein component of the Bacillus subtilis biofilm matrix. Mol. Microbiol. 59:1229–1238 [DOI] [PubMed] [Google Scholar]
- 8. Chai Y., Kolter R., Losick R. 2009. Paralogous antirepressors acting on the master regulator for biofilm formation in Bacillus subtilis. Mol. Microbiol. 74:876–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chai Y., Losick R., Kolter R. 2010. Reversal of an epigenetic switch governing cell chaining in Bacillus subtilis by protein stability. Mol. Microbiol. 78:218–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chai Y., Norman T., Losick R., Kolter R. 2010. An epigenetic switch governing daughter cell separation in Bacillus subtilis. Genes Dev. 24:754–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chastanet A., et al. 2010. Broadly heterogenous activation of the master regulator for sporulation in Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 107:8486–8491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chu F., Kearns D. B., Branda S. S. S. S., Kolter R., Losick R. 2006. Targets of the master regulator of biofilm formation in Bacillus subtilis. Mol. Microbiol. 59:1216–1228 [DOI] [PubMed] [Google Scholar]
- 13. Commichau F. M., Herzberg C., Tripal P., Valerius O., Stülke J. 2007. A regulatory protein-protein interaction governs glutamate biosynthesis in Bacillus subtilis: the glutamate dehydrogenase RocG moonlights in controlling the transcription factor GltC. Mol. Microbiol. 65:642–654 [DOI] [PubMed] [Google Scholar]
- 14. Commichau F. M., et al. 2009. Novel activities of glycolytic enzymes in Bacillus subtilis: interactions with essential proteins involved in mRNA processing. Mol. Cell Proteomics 8:1350–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Condon C. 2010. What is the role of RNase J in RNA turnover? RNA Biol. 7:316–321 [DOI] [PubMed] [Google Scholar]
- 16. Cozy L. M., Kearns D. B. 2010. Gene position in a long operon governs motility development in Bacillus subtilis. Mol. Microbiol. 76:273–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deikus G., Bechhofer D. H. 2009. Bacillus subtilis trp leader RNA: RNase J1 endonuclease cleavage specificity and PNPase processing. J. Biol. Chem. 284:26394–26401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Jong I. G., Veening J. W., Kuipers O. P. 2010. Heterochronic phosphorelay gene expression as a source of heterogeneity in Bacillus subtilis spore formation. J. Bacteriol. 192:2053–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dubnau D., Losick R. 2006. Bistability in bacteria. Mol. Microbiol. 61:564–572 [DOI] [PubMed] [Google Scholar]
- 20. Guérout-Fleury A. M., Shazand K., Frandsen N., Stragier P. 1995. Antibiotic resistance cassettes for Bacillus subtilis. Gene 167:335–336 [DOI] [PubMed] [Google Scholar]
- 21. Herzberg C., et al. 2007. SPINE: a method for the rapid detection and analysis of protein-protein interactions in vivo. Proteomics 7:4032–4035 [DOI] [PubMed] [Google Scholar]
- 22. Horsburgh M. J., Thackray P. D., Moir A. 2001. Transcriptional responses during outgrowth of Bacillus subtilis endospores. Microbiology 147:2933–2941 [DOI] [PubMed] [Google Scholar]
- 23. Inamura R., et al. 1996. Identification of the cpdA gene encoding cyclic 3′,5′adenosine monophosphate phosphodiesterase in Escherichia coli. J. Biol. Chem. 271:25423–25429 [DOI] [PubMed] [Google Scholar]
- 24. Jordan S., et al. 2007. LiaRS-dependent gene expression is embedded in transition state regulation in Bacillus subtilis. Microbiology 153:2530–2540 [DOI] [PubMed] [Google Scholar]
- 25. Kaltwasser M., Wiegert T., Schumann W. 2002. Construction and application of epitope- and green fluorescent protein-tagging integration vectors for Bacillus subtilis. Appl. Env. Microbiol. 68:2624–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karimova G., Pidoux J., Ullmann A., Ladant D. 1998. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. U. S. A. 95:5752–5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kearns D. B., Losick R. 2005. Cell population heterogeneity during growth of Bacillus subtilis. Genes Dev. 19:3083–3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kearns D. B., Chu F., Branda S. S., Kolter R., Losick R. 2005. A master regulator for biofilm formation by Bacillus subtilis. Mol. Microbiol. 55:739–749 [DOI] [PubMed] [Google Scholar]
- 29. Kobayashi K. 2008. SlrR/SlrA controls the initiation of biofilm formation in Bacillus subtilis. Mol. Microbiol. 69:1399–1410 [DOI] [PubMed] [Google Scholar]
- 30. Kolter R. 2010. Biofilms in lab and nature: a molecular geneticist's voyage to microbial ecology. Int. Microbiol. 13:1–7 [DOI] [PubMed] [Google Scholar]
- 31. Kunst F., Rapoport G. 1995. Salt stress is an environmental signal affecting degradative enzyme synthesis in Bacillus subtilis. J. Bacteriol. 177:2403–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lehnik-Habrink M., et al. 2010. The RNA degradosome in Bacillus subtilis: identification of CshA as the major RNA helicase in the multi-protein complex. Mol. Microbiol. 77:958–971 [DOI] [PubMed] [Google Scholar]
- 33. Lewis R. J., Brannigan J. A., Offen W. A., Smith I., Wilkinson A. J. 1998. An evolutionary link between sporulation and prophage induction in the structure of a repressor:anti-repressor complex. J. Mol. Biol. 283:907–912 [DOI] [PubMed] [Google Scholar]
- 34. López D., Vlamakis H., Kolter R. 2010. Biofilms. Cold Spring Harb. Perspect. Biol. 2:a000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ludwig H., et al. 2001. Transcription of glycolytic genes and operons in Bacillus subtilis: evidence for the presence of multiple levels of control of the gapA operon. Mol. Microbiol. 41:409–422 [DOI] [PubMed] [Google Scholar]
- 36. Martin-Verstraete I., Débarbouillé M., Klier A., Rapoport G. 1994. Interaction of wild-type truncated LevR of Bacillus subtilis with the upstream activating sequence of the levanase operon. J. Mol. Biol. 241:178–192 [DOI] [PubMed] [Google Scholar]
- 37. McLoon A. L., Guttenplan S. B., Kearns D. B., Kolter R., Losick R. 2011. Tracing the domestication of a biofilm-forming bacterium. J. Bacteriol. 193:2027–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meinken C., Blencke H. M., Ludwig H., Stülke J. 2003. Expression of the glycolytic gapA operon in Bacillus subtilis: differential syntheses of proteins encoded by the operon. Microbiology 149:751–761 [DOI] [PubMed] [Google Scholar]
- 39. Ribitsch D., et al. 2011. Hydrolysis of polyethyleneterephthalate by p-nitrobenzylesterase from Bacillus subtilis. Biotechnol. Prog. [Epub ahead of print.] doi:10.1002/btpr.610 [DOI] [PubMed] [Google Scholar]
- 40. Rietkötter E., Hoyer D., Mascher T. 2008. Bacitracin sensing in Bacillus subtilis. Mol. Microbiol. 68:768–785 [DOI] [PubMed] [Google Scholar]
- 41. Romero D., Aguilar C., Losick R., Kolter R. 2010. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc. Natl. Acad. Sci. U. S. A. 107:2230–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Romero D., Vlamakis H., Losick R., Kolter R. 2011. An accessory protein required for anchoring and assembly of amyloid fibres in B. subtilis biofilms. Mol. Microbiol. [Epub ahead of the print.] doi:10.1111/j.1365-2958.2011.07653.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 44. Serizawa M., et al. 2004. Systematic analysis of SigD-regulated genes in Bacillus subtilis by DNA microarray and Northern blotting analyses. Gene 329:125–136 [DOI] [PubMed] [Google Scholar]
- 45. Shahbabian K., Jamalli A., Zig L., Putzer H. 2009. RNase Y, a novel endoribonuclease, initiates riboswitch turnover in Bacillus subtilis. EMBO J. 28:3523–3533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shin D. H., et al. 2008. Structural and enzymatic characterization of DR1281: a chalcineurin-like phosphoesterase from Deinococcus radiodurans. Proteins 70:1000–1009 [DOI] [PubMed] [Google Scholar]
- 47. Stoodley P., Sauer K., Davies D. G., Costerton J. W. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187–209 [DOI] [PubMed] [Google Scholar]
- 48. Stülke J., et al. 1997. Induction of the Bacillus subtilis ptsGHI operon by glucose is controlled by a novel antiterminator, GlcT. Mol. Microbiol. 25:65–78 [DOI] [PubMed] [Google Scholar]
- 49. Veening J. W., Smits W. K., Hamoen L. W., Jongbloed J. D., Kuipers O. P. 2004. Visualization of differential gene expression by improved cyan fluorescent protein and yellow fluorescent protein production in Bacillus subtilis. Appl. Environ. Microbiol. 70:6809–6815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Veening J. W., Kuipers O. P. 2010. Gene position within a long transcript as a determinant for stochastic switching in bacteria. Mol. Microbiol. 76:269–272 [DOI] [PubMed] [Google Scholar]
- 51. Wach A. 1996. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast 12:259–265 [DOI] [PubMed] [Google Scholar]
- 52. Wacker I., et al. 2003. The regulatory link between carbon and nitrogen metabolism in Bacillus subtilis: regulation of the gltAB operon by the catabolite control protein CcpA. Microbiology 149:3001–3009 [DOI] [PubMed] [Google Scholar]
- 53. Weinrauch Y., Msadek T., Kunst F., Dubnau D. 1991. Sequence and properties of comQ, a new competence regulatory gene of Bacillus subtilis. J. Bacteriol. 173:5685–5693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yao S., Bechhofer D. H. 2010. Initiation of decay of Bacillus subtilis rpsO mRNA by endoribonuclease RNase Y. J. Bacteriol. 192:3279–3286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yasbin R. E., Young F. E. 1974. Transduction in Bacillus subtilis by bacteriophage SPP1. J. Virol. 14:1343–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zemansky J., et al. 2009. Development of a mariner-based transposon and identification of Listeria monocytogenes determinants, including the peptidyl-prolyl isomerase PrsA2, that contribute to its hemolytic phenotype. J. Bacteriol. 191:3950–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zock J., et al. 1994. The Bacillus subtilis pnbA gene encoding p-nitrobenzyl esterase: cloning, sequence and high-level expression in Escherichia coli. Gene 151:37–43 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.