Abstract
In Gram-negative methylotrophic bacteria, the first step in methylotrophic growth is the oxidation of methanol to formaldehyde in the periplasm by methanol dehydrogenase. In most organisms studied to date, this enzyme consists of the MxaF and MxaI proteins, which make up the large and small subunits of this heterotetrameric enzyme. The Methylobacterium extorquens AM1 genome contains two homologs of MxaF, XoxF1 and XoxF2, which are ∼50% identical to MxaF and ∼90% identical to each other. It was previously reported that xoxF is not required for methanol growth in M. extorquens AM1, but here we show that when both xoxF homologs are absent, strains are unable to grow in methanol medium and lack methanol dehydrogenase activity. We demonstrate that these defects result from the loss of gene expression from the mxa promoter and suggest that XoxF is part of a complex regulatory cascade involving the 2-component systems MxcQE and MxbDM, which are required for the expression of the methanol dehydrogenase genes.
INTRODUCTION
Methylobacterium extorquens AM1 is a methylotrophic bacterium ubiquitous in the environment and in particular on the undersides of leaves. This organism is able to metabolize both single-carbon compounds like methanol and multicarbon compounds like succinate and pyruvate (1, 11, 19). M. extorquens AM1 is a model organism for understanding the process of methylotrophic growth and has been studied biochemically and genetically for over 50 years (reviewed in reference 7). To utilize methanol as a sole source of carbon and energy, methanol is first oxidized to formaldehyde in the periplasm via methanol dehydrogenase, a soluble quinoprotein (2–4). This enzyme uses pyrroloquinoline quinone (PQQ) to sequentially transfer two electrons to cytochrome cL, which enters the electron transport chain, resulting in ∼1 molecule of ATP per molecule of methanol oxidized (9). Methanol dehydrogenase is a heterotetrameric protein consisting of two 66-kDa large subunits (MxaF) and two small 8.5-kDa subunits (MxaI) (28, 37). The large subunit contains the active-site residues and the PQQ prosthetic group, which is coordinated to a calcium ion in the active site (37). The loss of this enzyme in M. extorquens AM1 eliminates virtually all methanol dehydrogenase activity under any condition tested to date (27).
Previously, it was discovered that the M. extorquens AM1 genome contains a homolog of the large subunit, MxaF, which was named XoxF (6) based on its similarity to the XoxF protein of Paracoccus denitrificans (13). This protein is predicted to be a PQQ-dependent periplasmic alcohol dehydrogenase and is ∼50% identical to MxaF. The region downstream of xoxF contains xoxG, which encodes a putative c-type cytochrome, and xoxJ, which has similarity to mxaJ, a gene of unknown function that was proposed previously to be required for the activation of methanol dehydrogenase and has similarity to periplasmic binding proteins (23, 35). Recently, it was shown in M. extorquens AM1 that mutations in xoxF lead to a 30% decrease in the growth rate in methanol medium and reduced methanol uptake and result in a fitness defect during plant colonization (31). XoxF was purified and shown to bind methanol with a high affinity (11 μM), yet the rate of methanol oxidation was ∼10-fold lower than that with purified methanol dehydrogenase (31). The overexpression of this enzyme did not allow the growth of an mxaF mutant; thus, the in vivo function of XoxF in M. extorquens remained unclear (31).
Some organisms capable of methanol oxidation do not contain the MxaFI-type methanol dehydrogenase but do contain XoxF (10, 15, 18). In Rhodobacter sphaeroides, which does not contain an MxaFI-type methanol dehydrogenase, the loss of xoxF results in the loss of methanol-dependent oxygen uptake and the inability to use methanol as a photosynthetic carbon source, suggesting that XoxF may function as a methanol dehydrogenase in some organisms in vivo (38). In Paracoccus denitrificans, which contains the MxaFI-type methanol dehydrogenase, the loss of xoxF results in a partial growth defect in methanol medium (13). Protein expression studies have shown that in Methylophaga sp., which contains both MxaFI and XoxF, XoxF was highly abundant during growth on dimethylsulfide but not on methanol (30). However, XoxF is also found in nonmethylotrophic organisms, further complicating a predicted role for this enzyme in methylotrophic growth (10). As of yet, in M. extorquens AM1, no in vivo methanol dehydrogenase activity has been directly attributable to XoxF.
Recently, for a variety of other Methylobacterium species, Hibi et al. showed that the rare earth element (REE) La3+ increased methanol dehydrogenase activity up to 5.7-fold in cell extracts (14). Methanol dehydrogenase was purified from cells grown in media containing exogenous La3+ or Ca2+, and upon sequencing, it was determined that the methanol dehydrogenase purified from La3+-grown cells corresponded to XoxF, while the methanol dehydrogenase purified from Ca2+-grown cells corresponded to MxaFI (14). It has not yet been shown whether the effect of La3+ is indirect, possibly by the induction of a stress response system or an alteration of gene expression, or direct, by interacting with XoxF and participating in methanol oxidation.
Recently, the genome sequence for M. extorquens AM1 was reported (36), revealing a second XoxF paralog (XoxF2), which is also ∼50% identical to MxaF and ∼90% identical to XoxF, herein referred to as XoxF1. We show that the loss of both xoxF1 and xoxF2 leads to an inability to grow using methanol as a carbon and energy source and a nearly complete loss of methanol dehydrogenase activity, identical to the phenotype of an mxaF mutant strain. Using promoter fusion constructs, we demonstrate that expression of the genes encoding methanol dehydrogenase is severely repressed in the xoxF1 xoxF2 double mutant strain and that this decrease in expression is likely all or in part due to the decreased expression of the 2-component systems mxbDM and mxcQE, which are required to activate the expression of the methanol dehydrogenase genes (34).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
M. extorquens AM1 strains and plasmids used in this study are described in Table 1. M. extorquens AM1 strains were grown in a minimal salts medium (29) at 28°C to 30°C with methanol (0.5%) and/or succinate (0.4%) as a carbon source. Difco nutrient broth supplemented with Difco BiTek agar (1.5%) was used for conjugation between Escherichia coli helper strain S17-1 (32) and M. extorquens AM1. E. coli strains were grown at 37°C on Luria-Bertani agar. When appropriate, antibiotics were added to the following concentrations: tetracycline (Tc) at 10 μg/ml, kanamycin (Km) at 50 μg/ml, and rifamycin (Rif) at 50 μg/ml.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference(s) |
|---|---|---|
| Strains | ||
| AM1 | Rifr derivative (wild type) | 27 |
| AA31 | mxcE (moxEa); chemically induced mutation | 27 |
| CM194.1 | ΔmxaF | 22 |
| ES890 | xoxF1::Km | This study, 6b |
| ES970 | ΔxoxF2 | This study |
| ES1022 | ΔxoxF2 xoxF1::Km | This study |
| ES1100 | ΔmxaF ΔxoxF2 xoxF1::Km | This study |
| ES1126 | ΔxoxF2 xoxF1::Kmsup; spontaneously isolated suppressor mutant | This study |
| ES1258 | mxbM::Km | This study |
| ES1291 | xoxG::Km | This study |
| ES1296 | ΔxoxF2 xoxG::Km | This study |
| Plasmids | ||
| pAP3 | Expression vector using the META1_3512 promoter (Tcr) | This study |
| pAP5 | Promoterless yfp fusion vector created from pCM62 (Tcr) | This study |
| pALS24 | pHX200 with mxbDM promoter region (Tcr) | 34 |
| pAYC61 | Allelic exchange suicide vector (Tcr Apr) | 5 |
| pCM62 | Expression vector using the lac promoter (Tcr) | 20 |
| pCM80 | Expression vector using the lac and mxa promoters (Tcr) | 20 |
| pCM86 | mxaF in pCM80 (Tcr) | 20 |
| pCM157 | cre expression vector (Tcr) | 21 |
| pCM184 | Allelic exchange suicide vector (Kmr Tcr Apr) | 21 |
| pES134-135 | pCM184 with xoxF2 upstream and downstream flanks (Kmr Tcr Apr) | This study |
| pES222-223 | pCM184 with upstream and downstream xoxG flanks (Kmr Tcr Apr) | This study |
| pES501 | pHX200 with mxcQE promoter region (Tcr) | This study |
| pES500 | xoxF1, xoxG, and xoxJ cloned into pAP3 (Tcr) | This study |
| pES502 | pAP5 with mxa promoter region (Tcr) | This study |
| pES503 | pAP5 with xox1 promoter region (Tcr) | This study |
| pHX200 | Promoterless xylE fusion vector (Tcr) | 39 |
| pLC6168 | pAYC61 with xoxF1upstream and downstream flanks (Kmr Tcr Apr) | 6 |
The mxcE mutant strain was originally reported under the name moxE.
The plasmid used to construct the xoxF1::Km mutant was constructed as described previously (6) and conjugated into the wild-type M. extorquens AM1 strain background used in this study.
Strain construction.
Null mutations were generated in xoxF2 and xoxG using the allelic exchange suicide vector pCM184 as described previously (21). A deletion mutant in xoxF2 was made by the introduction of the cre expression vector pCM157 into the xoxF2::Km mutant strain (21), followed by plasmid curing. Null mutations in xoxF1 and mxbM were made by using pAYC61 as described previously (5), and mutations were confirmed by diagnostic PCR. xoxF1 xoxF2 suppressor mutant strains were spontaneously isolated from all 3 flasks during the growth curve analysis shown in Fig. 1A and described below. After the isolation of the xoxF1 xoxF2 suppressor mutants, the Km insertion in xoxF1 and the absence of xoxF2 were confirmed by diagnostic PCR.
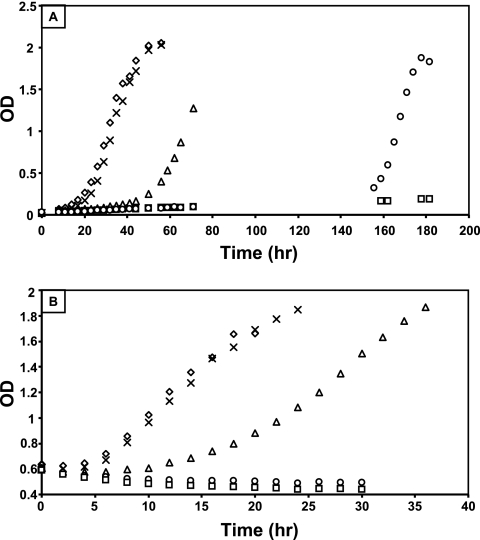
Fig. 1.
Phenotypic growth defects in the xoxF and mxaF mutant strains. (A) Growth of wild-type (diamonds), mxaF (squares), xoxF1 (triangles), xoxF2 (X's), and xoxF1 xoxF2 (circles) strains in minimal medium containing 120 mM methanol as a sole source of carbon. Growth seen past 140 h for the xoxF1 xoxF2 double mutant strain (circles) was due to a second site suppressor mutation. (B) Growth of wild-type (diamonds), mxaF (squares), xoxF1 (triangles), xoxF2 (X's), and xoxF1 xoxF2 (circles) strains after transition of the cells from succinate growth at an OD of 0.6 to growth on methanol. Graphs depict representative data from three biological replicates. The rate of growth varied by ≤8% between replicates.
Phenotypic analysis. (i) Growth.
Growth was assessed for 3 biological replicates both by scoring the colony size on agar plates and by growth curve analysis monitoring the optical density (OD) at 600 nm using a Shimadzu (Kyoto, Japan) UV-2401 PC spectrophotometer. For growth curve analysis, strains were grown at 30°C to late log phase, subcultured (2 ml) into 100 ml of minimal medium in 250-ml flasks containing the appropriate carbon source, and shaken at 200 rpm. For carbon source transition experiments, strains were grown in 100 ml of minimal succinate medium to an OD of ∼0.6 using the conditions described above, pelleted, washed in minimal medium, and resuspended in 100 ml minimal medium containing methanol.
(ii) Complementation studies.
To create a high-level-expression vector, the promoter region of RMQ02531/META1_3512, which was shown in previous microarray experiments to be highly expressed and constitutive in all available array data (29, 33; E. Skovran, unpublished data), was amplified and cloned into the AclI site of pCM62, creating pAP3. The xox1 operon was amplified and inserted into the EcoRI and KpnI sites of pAP3, creating pES500. Complementation was assessed in liquid and on solid medium containing 0.5% methanol.
Enzyme assays.
Cells and cell extracts for enzyme assays were prepared by using a French press as previously described (33). A minimum of 3 biological replicates was used to assay methanol dehydrogenase and catechol dioxygenase (XylE). Assays were performed as previously described (for methanol dehydrogenase [12] and XylE [34]), except that methanol dehydrogenase and XylE were measured in a final volume of 200 μl in a Spectramax 190 plate reader (Molecular Devices, Sunnyvale, CA) at room temperature. Absorbance data were converted to a 1-cm path length by dividing the slope by the conversion factor, 0.541. Protein concentrations were determined by using the Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL). The visualization of methanol dehydrogenase activity was achieved by the isoelectric focusing of cell extracts using a pH range of 3 to 9 and incubation with nitroblue tetrazolium and phenazine methosulfate as described previously (6), except that 20 mM methanol and 15 mM ammonium chloride were used as substrates.
Protein separation.
Cell extracts were prepared as described above and separated (5 to 15 μg) by using standard SDS-polyacrylamide gel electrophoresis on a 12.5% polyacrylamide gel and staining with Coomassie blue R. Precision Plus Protein Kaleidoscope prestained standards (Bio-Rad, Hercules, CA) were used for size estimations.
Promoter fusion assays. (i) Yellow fluorescent protein promoter fusions.
A modified version of yellow fluorescent protein (YFP) that is brighter and more stable in response to pH changes was amplified from the Venus construct (25) by PCR and inserted into pCM62 (20) in the opposite orientation of the lac promoter using the SacI restriction site to generate pAP5. The promoter regions for the mxa operon and the xox1 operon were cloned into the AclI site, creating plasmids pES502 and pES503, respectively. Strains containing the yfp promoter fusion constructs were grown at 29°C using a Spectramax 190 plate reader (Molecular Devices, Sunnyvale, CA) with continuous shaking in a Nunc black optical 96-well microtiter plate with a clear bottom (Nalge Nunc International, Rochester, NY). Cells were grown to the mid-log phase, at which point the relative fluorescence units (RFU) were measured by using an Infinite F500 instrument (Tecan, Männedorf, Switzerland) with excitation and emission spectra of 485 and 535 nm, respectively. The RFU/OD is reported.
(ii) xylE promoter fusions.
The promoter region for the mxcQE genes was cloned into the KpnI and SacI sites in pHX200 (39), creating pES501. Catechol dioxygenase activity was measured as described above.
RESULTS
Loss of both xoxF1 and xoxF2 results in growth phenotypes identical to that of a methanol dehydrogenase null mutant strain (mxaF).
Methanol dehydrogenase is required for the oxidation of methanol, resulting in the inability of an mxaF null mutant strain to grow on methanol medium, but the ability of mxaF null mutant strains to grow using other C1 carbon sources is not altered. To estimate the growth phenotypes of the xox mutant strains, single colonies of the mutant strains were compared for colony size to the wild-type and the mxaF mutant strains on minimal medium containing methanol and other C1 carbon sources (methylamine and formate) (Table 2). The xoxF1 single mutant resulted in a partial growth defect on methanol medium, whereas the xoxF2 single mutant showed no observable growth defect. However, the xoxF1 xoxF2 double mutant strain displayed growth phenotypes identical to that of the mxaF mutant strain: an inability to grow on methanol medium but wild-type growth with the other C1 carbon sources methylamine and formate.
Table 2.
Growth and complementation of the xox and mxaF mutant strainsa
| Strain | Growth with carbon source |
Complementation in methanol medium |
|||||
|---|---|---|---|---|---|---|---|
| MeOH | MA | Formate | pCM80b | pCM86 | pAP3c | pES500 | |
| WT | ++ | ++ | + | ++ | ++ | ++ | ++ |
| mxaF | − | ++ | + | − | + | − | − |
| xoxF1 | + | ++ | + | ND | ND | ND | ND |
| xoxF2 | ++ | ++ | + | ND | ND | ND | ND |
| xoxF1 xoxF2 | − | ++ | + | − | − | − | ++ |
| xoxG | ++ | ++ | + | ND | ND | ND | ND |
| xoxF2 xoxG | ++ | ++ | + | ND | ND | ND | ND |
Abbreviations are as follows: MeOH, methanol; MA, methylamine; WT, wild type; ND, not determined. −, no growth; + or ++, growth (a single plus indicates that colony size is about half that seen for a wild-type strain on methanol medium).
pCM80 is the empty parent vector for pCM86, which harbors mxaF (20).
pAP3 is the empty parent vector for pES500, which harbors the xoxF, xoxG, and xoxJ genes.
The xoxF1 gene is in a putative operon with a cytochrome, xoxG, and a putative periplasmic binding protein, xoxJ. To test if the cytochrome was also required for methanol growth in addition to the XoxF subunit, strains lacking xoxG in both the wild-type and xoxF2 backgrounds were constructed. Interestingly, growth of the xoxG single and xoxF2 xoxG double null mutant strains was similar to that of the wild type, suggesting that only the homolog of the methanol dehydrogenase large subunit is required for methanol growth (Table 2).
Since purified XoxF has methanol dehydrogenase activity in vitro (10-fold decrease in the Vmax compared to that of methanol dehydrogenase [31]), it is possible that XoxF is a methanol dehydrogenase functioning in vivo under unknown conditions. To test whether XoxF and MxaF could substitute for one another, pCM86 containing mxaF and pES500 containing the xox1 cluster (xoxF1, xoxG, and xoxJ) were conjugated into the mxaF and xoxF1 xoxF2 mutant strains and tested for growth on solid methanol medium (Table 2) and liquid methanol medium (data not shown). Under both conditions, pCM86 containing mxaF allowed the partial growth of the mxaF mutant strain, as previously described (20), but did not complement the xoxF1 xoxF2 double mutant strain. pES500 restored wild-type growth to the xoxF1 xoxF2 mutant strain but did not allow the growth of the mxaF mutant strain, confirming that these two similar dehydrogenases cannot substitute for each other under the conditions tested, even when expressed from a complementing plasmid. These results demonstrate that both mxaF and xox have unique and separate roles in methanol metabolism.
For a more detailed analysis of the growth defect in methanol medium, xoxF single and double mutant strains were tested for growth in liquid methanol minimal medium (Fig. 1A) and for their growth responses when the cells were transitioned from succinate to methanol growth during the mid-log phase (Fig. 1B). The xoxF2 mutant strain displayed no phenotypic defect, while the xoxF1 mutant had a slight defect in the growth rate and a reproducibly increased lag (∼50 h) compared to those of the wild-type strain. Like the mxaF mutant, the xoxF1 xoxF2 double mutant strain could not grow in methanol medium. However, suppressor mutations consistently arose ∼150 h postinoculation in the xoxF1 xoxF2 double mutant but not the mxaF mutant strain. Upon retesting, these spontaneous mutants grew like the wild-type strain in methanol medium (data not shown) and are subsequently referred to as xoxF1 xoxF2sup in the text below. Because these suppressor mutants were easily obtained, it seemed likely that the suppression was due to a loss of the function of an unknown gene. Attempts to identify this gene using transposon mutagenesis were unsuccessful. Suppressor mutants bearing a transposon were isolated, but sequencing from the transposons resulted in multiple different sequence identifications and were seemingly random. When M. extorquens AM1 is under stress, many of the ∼200 insertion elements contained in the M. extorquens genome are induced (24), thus making it difficult to use transposon mutagenesis as a method for suppressor identification.
In a previous study, when wild-type chemostat-grown cells were transitioned from succinate growth to methanol growth, the expression levels of both xoxF genes increased, while the expression levels of the mxa genes decreased (33). To determine how the growth of the xoxF mutant strains would respond when the carbon source was switched from succinate to methanol, cells were grown with succinate to an OD of 0.6, washed, and resuspended in methanol medium. The growth of the xoxF1 mutant after the transition showed a larger defect (Fig. 1B) than that in methanol-grown cells alone (Fig. 1A), and the growth of the xoxF1 xoxF2 double mutant strain was identical to that of the mxaF deletion strain, with a continual slight decrease in OD after the transition (Fig. 1B). Taken together, these growth phenotypes are consistent with XoxF acting at the methanol oxidation step.
XoxF is required for methanol dehydrogenase activity in cell extracts.
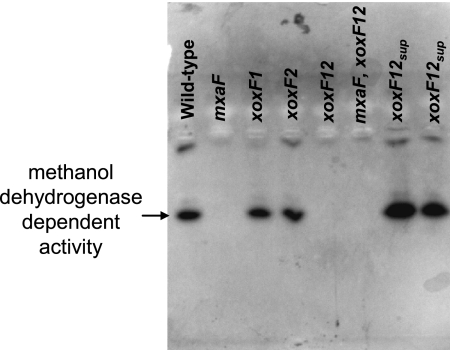
To determine if the cause of the growth defect in the xoxF1 xoxF2 double mutant strain was due to a lack of methanol dehydrogenase activity, cells were grown in medium containing succinate and methanol, and the methanol dehydrogenase activity in cell extracts was visualized by using native isoelectric focusing gel electrophoresis and staining with nitroblue tetrazolium and phenazine methosulfate in the presence of methanol and ammonium chloride (Fig. 2). Methanol dehydrogenase-dependent activity was absent in both the mxaF and xoxF1 xoxF2 double mutant strains but was restored in the xoxF1 xoxF2 suppressor mutant isolates. The absence of a single identical band in both the mxaF and xoxF1 xoxF2 mutant strains is suggestive that both XoxF and MxaF are required for the activity of the same methanol dehydrogenase enzyme.
Fig. 2.
Visualization of methanol dehydrogenase activity in the xoxF and mxaF mutant strains using isoelectric focusing gel electrophoresis. Cells were grown in minimal medium containing both succinate and methanol, and cell extracts from these strains were isoelectrofocused using a pH range of 3 to 9. The presence of methanol dehydrogenase activity was detected by using nitroblue tetrazolium and phenazine methosulfate in the presence of 20 mM methanol and 15 mM ammonium chloride. xoxF12sup refers to two different xoxF1 xoxF2 suppressor mutants that were spontaneously isolated.
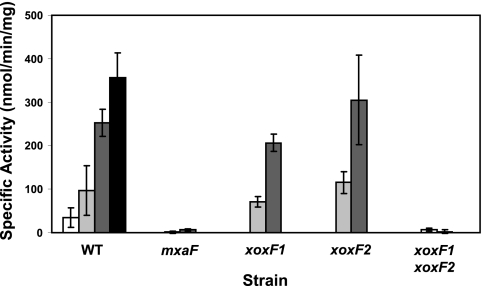
To measure the amount of methanol dehydrogenase activity in the xoxF mutant strains, cells were grown in succinate medium to the mid-log phase (OD of 0.6), washed and resuspended in methanol medium, and harvested at 4 and 10 h posttransition for activity measurements (Fig. 3). Activities in succinate- and methanol-grown cells were also measured for the wild-type strain. The level of methanol dehydrogenase activity was low in wild-type succinate-grown cells and increased over time, similar to the results for chemostat-grown cells transitioned from succinate to methanol growth (33) (Fig. 3A). As was previously reported (27), strains lacking mxaF showed only trace amounts of methanol dehydrogenase activity. Strains lacking both xoxF1 and xoxF2 also had only trace amounts of methanol dehydrogenase activity, suggesting that both mxa and xox are required for the same methanol dehydrogenase step (Fig. 3A).
Fig. 3.
Enzyme activities in the xoxF and mxaF mutant strains. Methanol dehydrogenase activities were measured in cell extracts 4 h (light gray) and 10 h (dark gray) after the transition from succinate to methanol growth. Activities in succinate-grown cells (white bars) and methanol-grown cells (black bars) are shown for the wild-type strain as a reference.
The loss of either xoxF1 or xoxF2 by itself resulted in no major defect in methanol dehydrogenase activity during the transition (Fig. 3A), suggesting that XoxF1 and XoxF2 can substitute for one another, and it is only when both are lacking that this defect is severe enough to be observed during the transition.
Levels of the MxaF protein are decreased in the xoxF1 xoxF2 double mutant strain.
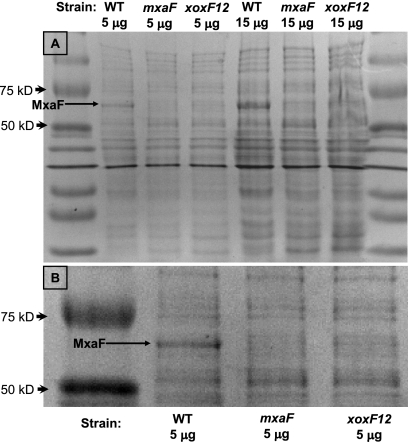
It seemed unlikely that the requirement for Xox was to function directly as a methanol dehydrogenase under the conditions tested, since the loss of the MxaFI methanol dehydrogenase eliminated both growth and activity. This result indicated that XoxF might be required for the activation of methanol dehydrogenase or for the production of the methanol dehydrogenase protein. To determine if methanol dehydrogenase was produced (but not active) in the xoxF1 xoxF2 double mutant strain, proteins in cell extracts were separated by using SDS-polyacrylamide gel electrophoresis. Since MxaF is one of the most abundant proteins in the cell, it is visible after staining with Coomassie blue (Fig. 4). The loss of both xoxF1 and xoxF2 greatly reduced the band corresponding to MxaF, suggesting that XoxF is required for the expression or stability of the methanol dehydrogenase enzyme.
Fig. 4.
Coomassie-stained 12.5% SDS-polyacrylamide gel containing cell extracts from M. extorquens AM1 strains. The size of the MxaF subunit of methanol dehydrogenase is 66 kDa. (A) Entire gel shown containing 5 and 15 μg of cell extract. (B) Enlarged portion of the gel shown in panel A.
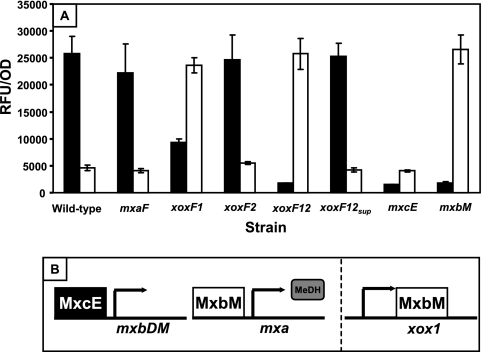
Expression from the mxa and xox1 promoter regions.
To examine the expressions of the mxa and xox operons, transcriptional promoter fusions were created by the insertion of the mxa and xox1 promoter regions upstream of a stable promoterless version of yfp. The RFU/OD was measured for the xoxF and mxaF mutant strains and for strains lacking the response regulators shown to be required for the expression of the mxa operon (34) (Fig. 5A). A model of our current understanding of the regulatory cascade required for mxa expression is depicted in Fig. 5B. In minimal medium containing both succinate and methanol, the wild-type strain exhibited high levels of expression from the mxa promoter (25,800 RFU/OD; average of triplicates) and just above background expression levels from the xox1 promoter region (4,600 RFU/OD; average of triplicates) (Fig. 5A). Background expression was defined as the RFU/OD detected from the promoterless yfp fusion construct pAP5 (3,940 RFU/OD; average of triplicates). A promoter fusion construct was also generated by using a large region upstream and partially within the xoxF2 gene; however, YFP fluorescence measured under multiple growth conditions and strain backgrounds showed only background levels. Thus, it was undetermined if the level of expression of xoxF2 was extremely low in all cases or if the promoter region was located elsewhere. Wild-type levels of expression were observed from both promoters in the mxaF and xoxF2 mutant strains. However, the level of expression from the mxaF promoter was decreased in the xoxF1 mutant strain and reduced to below background levels in the xoxF1 xoxF2 double mutant strain, confirming that XoxF is required for the expression of methanol dehydrogenase. Of interest, the loss of xoxF1 resulted in increased expression from the xox1 promoter to levels observed for the mxa promoter. The xoxF1 xoxF2 suppressor mutants that were spontaneously isolated and described above had wild-type levels of expression from both promoters, explaining their ability to grow in methanol medium.
Fig. 5.
Expression of mxaF-yfp and xox1-yfp promoter fusions in M. extorquens AM1 strains. (A) mxa (black) and xox1 (white) promoter activities were determined by calculating the relative fluorescence units (RFU) per OD from 3 biological replicates grown to the mid-log phase (OD of ∼0.6) in medium containing both succinate and methanol. The background level from a promoterless yfp fusion vector was 3,940 ± 70 RFU/OD. (B) Current model for how MxcE and MxbM regulate expression of the mxa operon. In this model, both the MxcE and MxbM response regulators are required for the expression of the mxa operon, with MxcE having an indirect effect. MeDH represents production of the methanol dehydrogenase gene. MxcE has been shown to be involved in the activation of expression from the mxbDM promoter region, predicting a cascade of regulation, with MxcE activating the expression of mxbM, which, when translated, activates expression from the mxa promoter. The data in panel A added to the model shown passed the dashed line, suggesting that MxbM may act as a repressor of the xox1 operon. The direct binding of both MxcE and MxbM has not yet been demonstrated.
As controls, the activity from these promoters was also measured in the response regulator mutants that were previously shown to be required for the expression of the mxa genes (34) (Fig. 5A). As expected, expression from the mxa promoter was severely repressed, resulting in below-background levels of fluorescence detected in each of the response regulator mutant strains (mxcE and mxbM). Expression from the xox1 promoter was unaffected in the mxcE mutant strain but, interestingly, was derepressed in the mxbM mutant to the high levels seen for the mxa promoter in the wild type and for the xox1 promoter in the xoxF1 mutant strains, suggesting that mxa and xox1 are oppositely regulated by the MxbDM 2-component regulatory system. This opposite expression of the mxa and xox genes was also observed previously during the transition from succinate to methanol growth (33).
Expression of the 2-component regulatory systems required for activation of the mxa genes is repressed in the xoxF1 xoxF2 double mutant strain.
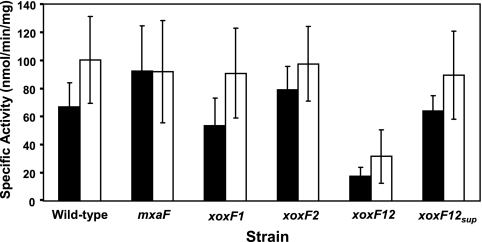
Because xoxF is required for the expression of the mxa genes, it is possible that the XoxF proteins are exerting their effects on regulation through one or more of the 2-component regulatory systems required for the activation of the mxa genes. To test if the expression of the 2-component systems is altered in the xoxF mutant strains, the promoter regions of mxcQE (this work) and mxbDM (34) were cloned upstream of xylE in pHX200 (39) and conjugated into the various mxaF and xoxF strain backgrounds. Only the xoxF1 xoxF2 double mutant strain showed significantly altered expression (Fig. 6). The level of expression from the mxc promoter was decreased to slightly above background levels (17.5 nmol min−1 mg protein−1, versus a background level of 10.9 nmol min−1 mg protein−1, measured using the promoterless xylE construct pHX200), while the level of expression from the mxb promoter was decreased to 31.6 nmol min−1 mg protein−1. Expression levels from both promoters increased to wild-type levels in the xoxF1 xoxF2 suppressor strains.
Fig. 6.
Expression of mxc-xylE and mxb-xylE promoter fusions in M. extorquens AM1 strains. mxc (black) and mxb (white) promoter activities were determined by measuring catechol 2,3-dioxygenase activity (XylE) in crude cell extracts of strains grown in medium containing both succinate and methanol. Cell extracts containing the promoterless xylE construct pHX200 had a background activity of 10.9 ± 2.6 nmol min−1 mg protein−1.
DISCUSSION
The presence of xoxF is widespread in methylotrophs, and it exists even in some nonmethylotrophic bacteria. It is only recently that some understanding of the role of these methanol dehydrogenase-like enzymes has begun to emerge. In organisms like Rhodobacter sphaeroides, in which no MxaFI-type methanol dehydrogenase exists, XoxF was implicated as a methanol dehydrogenase, but methanol dehydrogenase activity in that bacterium was not detected in vivo, thus leaving open the question of whether XoxF was directly or indirectly required for growth with methanol. Schmidt et al. demonstrated that in vitro, XoxF from M. extorquens did indeed have methanol dehydrogenase activity, although the rate of oxidation was low (31). Thus, it was unclear why XoxF was unable to substitute for the methanol dehydrogenase in vivo when expressed from a multicopy plasmid.
In M. extorquens AM1, the existence of xoxF1 has been known for ∼15 years (6), but until the genome was sequenced (36), the presence of the second homolog, xoxF2, was undiscovered. Here we showed that it is only when both xoxF1 and xoxF2 are absent that a strict requirement for growth in methanol medium is uncovered and that this requirement can be explained by a role for XoxF in the expression of the mxa operon encoding methanol dehydrogenase. The presence of the mxaG cytochrome homolog (xoxG) downstream of xoxF1 suggests that XoxF may function as an active dehydrogenase, yet the loss of xoxG had no detectable effect on the ability of M. extorquens AM1 to grow in methanol medium. This suggests either that the dehydrogenase activity is not required for the regulatory role of XoxF or that another cytochrome like MxaG may be able to substitute for XoxG. It is possible that XoxF has a dual function, acting both in a catalytic and in a regulatory role.
One possibility for the role of XoxF is to act as an environmental sensor, possibly by binding to or producing a signal molecule and exerting its effect through a 2-component system. That signal molecule must not be methanol, because a significant expression of the mxa operon occurs in the absence of methanol (33). Free formaldehyde is also unlikely to be the signal produced by XoxF, since the addition of formaldehyde does not allow the growth of the xoxF1 xoxF2 double mutant strain in methanol (data not shown). However, this does not exclude the ability of formaldehyde bound to XoxF to function as the signal that begins the regulatory cascade. However, this is unlikely since there is a high level of expression of methanol dehydrogenase in succinate-grown cells, where formaldehyde should not be present in the periplasm at significant levels (33).
If XoxF does act as an environmental sensor, the binding of a signal molecule may be what begins the regulatory cascade involving the expression of the 2-component systems MxbDM and MxcQE, required for the expression of methanol dehydrogenase. A complex feedback loop appears to be built into this regulatory cascade since (i) Xox is required for normal expression levels of both mxcQE and mxbDM, (ii) MxbDM decreases the expression levels of xox1 (either directly or indirectly), and (iii) MxcQE is required for the expression of mxbDM (34), creating a complex and interwoven regulation scheme. Such a mode of regulation might be very sensitive to changes in the environment, allowing a quick response when needed, or provide a robustness to the system, maintaining homeostasis when reached. The isolation of xoxF1 xoxF2 suppressor mutants suggests that there are other components involved in the regulation of methanol dehydrogenase that are yet unknown. In these suppressor mutants, expression levels of mxa and xox1 as well as expression from the mxbDM and mxcQE promoters were returned to wild-type levels (Fig. 5 and 6), suggesting a loss of a repressor. Further work is needed to determine the specific interactions between the known regulatory components and to identify additional unknown regulatory components involved in the regulation of expression of the methanol dehydrogenase genes.
We demonstrate an absolute requirement for XoxF in the utilization of methanol, specifically at the methanol dehydrogenase step. This requirement is due to a role for XoxF in the expression of the methanol dehydrogenase genes. An examination of expression from the promoters of the known regulators of methanol dehydrogenase demonstrates that XoxF is part of a complex regulatory cascade. This does not exclude XoxF from having a second function, possibly in the oxidation of methanol under unknown conditions or in the oxidation of another unknown substrate.
ACKNOWLEDGMENTS
We thank Mila Chistoserdova and Norma Cecilia Martinez-Gomez for critical reading of the manuscript and Meng Zhang for construction of the mxbM mutant strain.
Funding was provided by the NIH under grant GM058933.
Footnotes
Published ahead of print on 26 August 2011.
REFERENCES
- 1. Anthony C. 1982. The biochemistry of methylotrophs. Academic Press, New York, NY [Google Scholar]
- 2. Anthony C. 1986. The bacterial oxidation of methane and methanol. Adv. Microb. Physiol. 27:113–210 [DOI] [PubMed] [Google Scholar]
- 3. Anthony C. 1992. The c-type cytochromes of methylotrophic bacteria. Biochim. Biophys. Acta 1099:1–15 [PubMed] [Google Scholar]
- 4. Anthony C. 2000. Methanol dehydrogenase, a PQQ-containing quinoprotein dehydrogenase. Subcell. Biochem. 35:73–117 [DOI] [PubMed] [Google Scholar]
- 5. Chistoserdov A. Y., Chistoserdova L. V., McIntire W., Lidstrom M. E. 1994. The genetic organization of the mau gene cluster in Methylobacterium extorquens AM1: complete nucleotide sequence and generation and characteristics of mau mutants. J. Bacteriol. 176:4052–4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chistoserdova L., Lidstrom M. E. 1997. Molecular and mutational analysis of a DNA region separating two methylotrophy gene clusters in Methylobacterium extorquens AM1. Microbiology 143:1729–1736 [DOI] [PubMed] [Google Scholar]
- 7. Chistoserdova L., Chen S. W., Lapidus A., Lidstrom M. E. 2003. Methylotrophy in Methylobacterium extorquens AM1 from a genomic point of view. J. Bacteriol. 185:2980–2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reference deleted.
- 9. Dijkstra M., Frank J., Duine J. A. 1989. Studies on electron transfer from methanol dehydrogenase to cytochrome cL, both purified from Hyphomicrobium X. Biochem. J. 257:87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giovannoni S. J., et al. 2008. The small genome of an abundant coastal ocean methylotroph. Environ. Microbiol. 10:1771–1782 [DOI] [PubMed] [Google Scholar]
- 11. Green P. 1991. The genus Methylobacterium, p. 2342–2349 In Ballows A., Trüper H. G., Dworkin M., Harder W., Schleifer K. H. (ed.), The prokaryotes, 2nd ed Springer, Berlin, Germany [Google Scholar]
- 12. Guo X., Lidstrom M. E. 2006. Physiological analysis of Methylobacterium extorquens AM1 grown in continuous and batch cultures. Arch. Microbiol. 186:139–149 [DOI] [PubMed] [Google Scholar]
- 13. Harms N., et al. 1996. Genetics of C1 metabolism regulation in Paracoccus denitrificans, p. 126–132 In Lidstrom M. E., Tabita F. R. (ed.), Microbial growth on C1 compounds. Kluwer Academic Publishers, Dordrecht, Netherlands [Google Scholar]
- 14. Hibi Y., et al. 2011. Molecular structure of La(3+)-induced methanol dehydrogenase-like protein in Methylobacterium radiotolerans. J. Biosci. Bioeng. 111:547–549 doi:10.1016/j.jbiosc.2010.12.017 [DOI] [PubMed] [Google Scholar]
- 15. Hou S., et al. 2008. Complete genome sequence of the extremely acidophilic methanotroph isolate V4, Methylacidiphilum infernorum, a representative of the bacterial phylum Verrucomicrobia. Biol. Direct 3:26 doi:10.1186/1745-6150-3-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reference deleted.
- 17.Reference deleted.
- 18. Kalyuzhnaya M. G., et al. 2009. Methylophilaceae link methanol oxidation to denitrification in freshwater lake sediment as suggested by stable isotope probing and pure culture analysis. Environ. Microbiol. Rep. 1:385–392 doi:10.1111/j.1758-2229.2009.00046.x [DOI] [PubMed] [Google Scholar]
- 19. Lidstrom M. E. 2006. Aerobic methylotrophic prokaryotes, p. 618–634 In Dworkin M., Falkow S., Rosenberg E., Schleifer K.-H., Stackebrandt E. (ed.), The prokaryotes, vol. 2: ecophysiology and biochemistry Springer-Verlag, New York NY [Google Scholar]
- 20. Marx C. J., Lidstrom M. E. 2001. Development of improved versatile broad-host-range vectors for use in methylotrophs and other Gram-negative bacteria. Microbiology 147:2065–2075 [DOI] [PubMed] [Google Scholar]
- 21. Marx C. J., Lidstrom M. E. 2002. Broad-host-range cre-lox system for antibiotic marker recycling in gram-negative bacteria. Biotechniques 33:1062–1067 [DOI] [PubMed] [Google Scholar]
- 22. Marx C. J., Chistoserdova L., Lidstrom M. E. 2003. Formaldehyde-detoxifying role of the tetrahydromethanopterin-linked pathway in Methylobacterium extorquens AM1. J. Bacteriol. 185:7160–7168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matsushita K., Takahashi K., Adachi O. 1993. A novel quinoprotein methanol dehydrogenase containing an additional 32-kilodalton peptide purified from Acetobacter methanolicus: identification of the peptide as a MoxJ product. Biochemistry 32:5576–5582 [DOI] [PubMed] [Google Scholar]
- 24. Miller J. A. 2009. Formaldehyde stress response in Methylobacterium extorquens AM1. Doctoral thesis. University of Washington, Seattle, WA [Google Scholar]
- 25. Nagai T., et al. 2002. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 20:87–90 [DOI] [PubMed] [Google Scholar]
- 26.Reference deleted.
- 27. Nunn D. N., Lidstrom M. E. 1986. Phenotypic characterization of 10 methanol oxidation mutant classes in Methylobacterium sp. strain AM1. J. Bacteriol. 166:591–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nunn D. N., Day D., Anthony C. 1989. The second subunit of methanol dehydrogenase of Methylobacterium extorquens AM1. Biochem. J. 260:857–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Okubo Y., Skovran E., Guo X., Sivam D., Lidstrom M. E. 2007. Implementation of microarrays for Methylobacterium extorquens AM1. OMICS 11:325–340 [DOI] [PubMed] [Google Scholar]
- 30. Schäfer H. 2007. Isolation of Methylophaga spp. from marine dimethylsulfide-degrading enrichment cultures and identification of polypeptides induced during growth on dimethylsulfide. Appl. Environ. Microbiol. 73:2580–2591 doi:10.1128/AEM.02074-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schmidt S., Christen P., Kiefer P., Vorholt J. A. 2010. Functional investigation of methanol dehydrogenase-like protein XoxF in Methylobacterium extorquens AM1. Microbiology 156:2575–2586 [DOI] [PubMed] [Google Scholar]
- 32. Simon R., Priefer U., Puhler A. 1984. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Biotechnology (NY) 1:784–791 [Google Scholar]
- 33. Skovran E., Crowther G. J., Guo X., Yang S., Lidstrom M. E. 2010. A system biology approach uncovers cellular strategies used by Methylobacterium extorquens AM1 during the switch from multi- to single-carbon growth. PLoS One 5:e14091 doi:10.1371/journal.pone.0014091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Springer A. L., Morris C. J., Lidstrom M. E. 1997. Molecular analysis of MxbD and MxbM, a putative sensor-regulator pair required for oxidation of methanol in Methylobacterium extorquens AM1. Microbiology 143:1737–1744 [DOI] [PubMed] [Google Scholar]
- 35. van Spanning R. J. M., et al. 1991. Isolation and characterization of the moxJ, moxG, moxI, and moxR genes of Paracoccus denitrificans: inactivation of moxJ, moxG, and moxR and the resultant effect on methylotrophic growth. J. Bacteriol. 173:6948–6961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vuilleumier S., et al. 2009. Methylobacterium genome sequences: a reference blueprint to investigate microbial metabolism of C1 compounds from natural and industrial sources. PLoS One 4:e5584 doi:10.1371/journal.pone.0005584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Williams P. A., et al. 2004. The atomic resolution structure of methanol dehydrogenase from Methylobacterium extorquens. J. Mol. Biol. 357:151–162 [DOI] [PubMed] [Google Scholar]
- 38. Wilson S. M., Gleisten M. P., Donohue T. J. 2008. Identification of proteins involved in formaldehyde metabolism by Rhodobacter sphaeroides. Microbiology 154:296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu H. H., Viebahn M., Hanson R. S. 1993. Identification of methanol-regulated promoter sequences from the facultative methylotrophic bacterium Methylobacterium organophilum XX. J. Gen. Microbiol. 139:743–752 [DOI] [PubMed] [Google Scholar]