Abstract
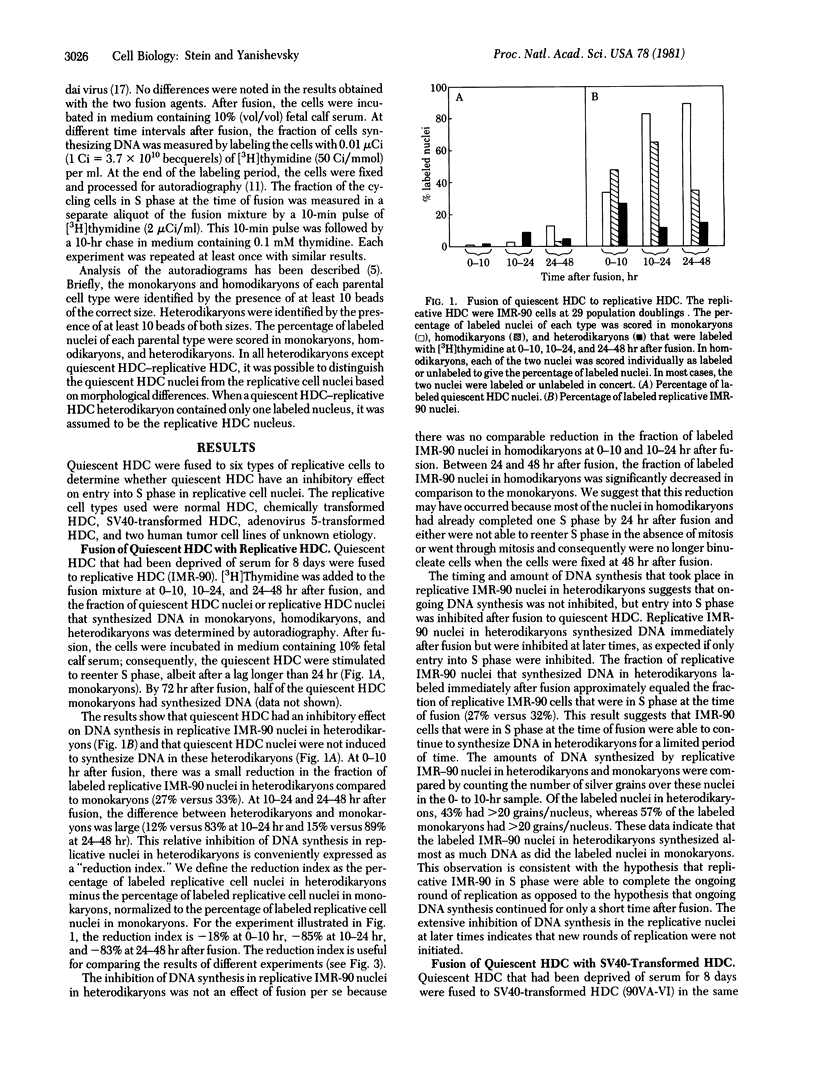
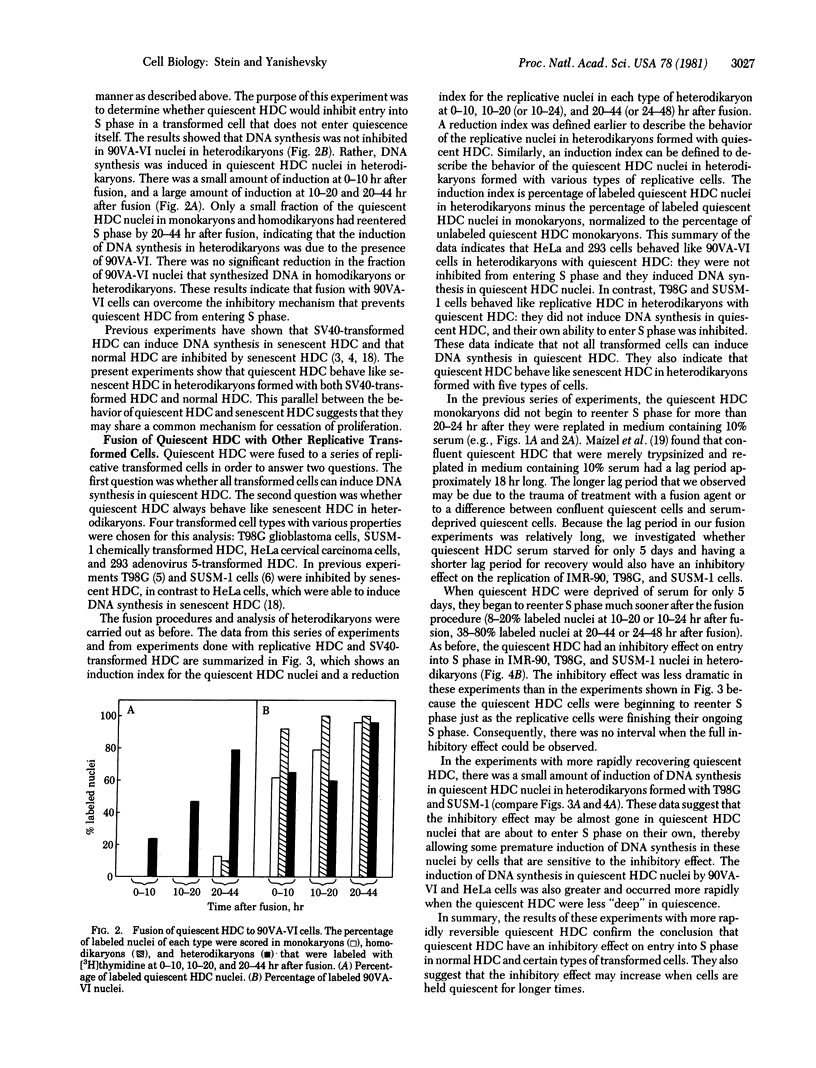
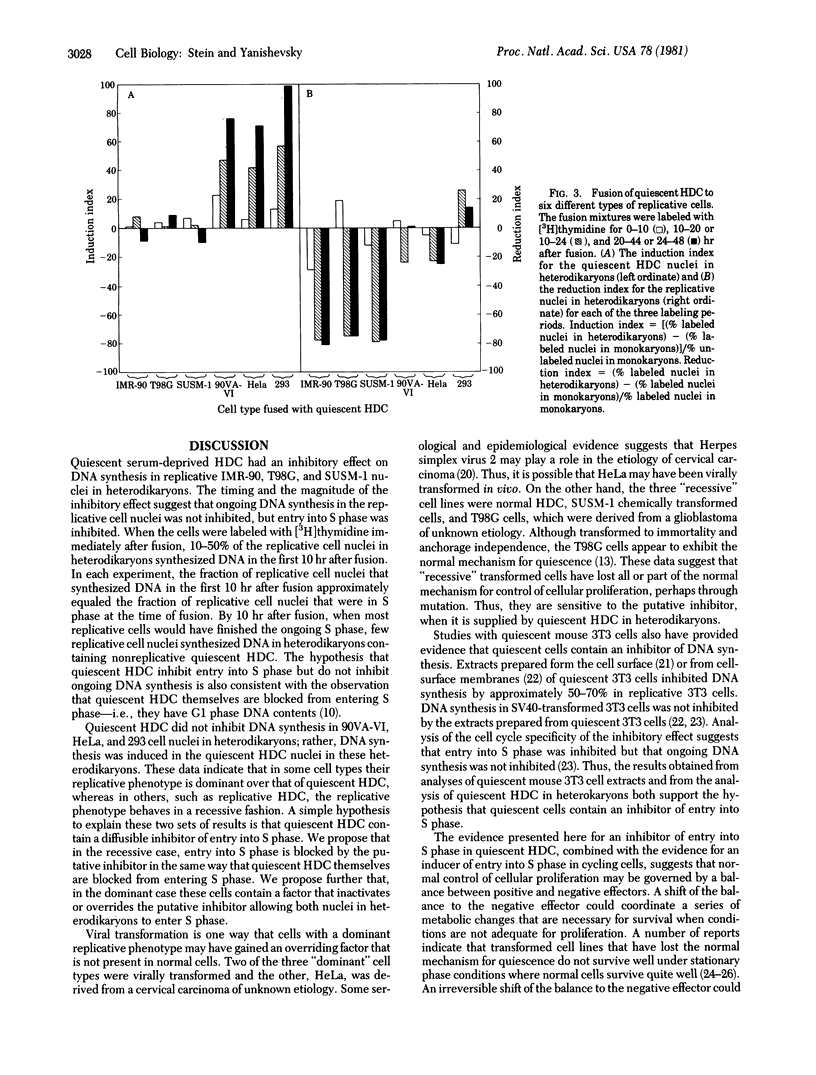
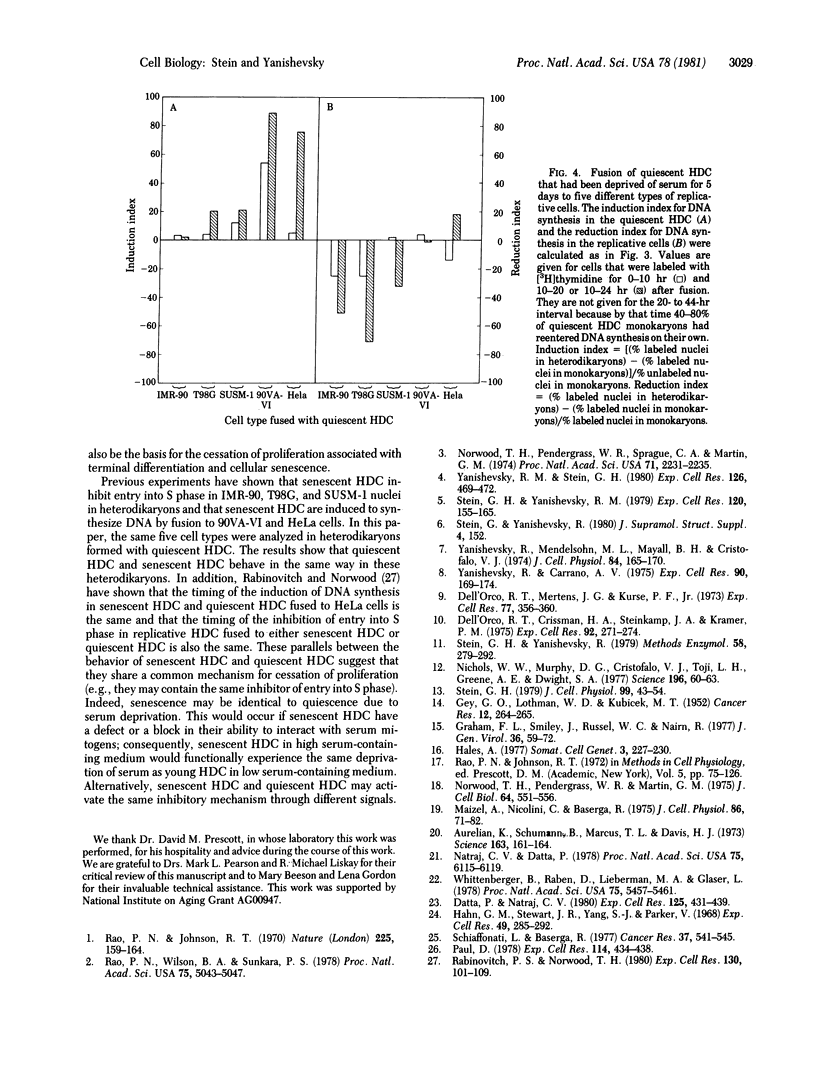
Serum-deprived quiescent human diploid cells (HDC) were fused to replicative HDC, and DNA synthesis was monitored in the resulting heterodikaryons. Quiescent HDC had an inhibitory effect on DNA synthesis in replicative HDC nuclei in heterodikaryons. The timing of the inhibitory effect suggests that entry into S phase was inhibited but ongoing DNA synthesis was not inhibited in the replicative HDC nuclei. When quiescent HDC were fused to T98G human glioblastoma cells or SUSM-1 chemically transformed human cells, entry into S phase was similarly inhibited. However, when quiescent HDC were fused to simian virus 40-transformed human cells, adenovirus 5-transformed human cells, or HeLa cells, DNA synthesis was induced in the quiescent HDC nuclei. A simple hypothesis to explain these results is that quiescent HDC contain an inhibitor of entry into S phase. Transformed cells with a dominant replicative phenotype may have gained a factor that overrides the putative inhibitor, perhaps through viral transformation, whereas recessive transformed cells may ahve lost the normal inhibitory mechanism, perhaps through mutation. Senescent HDC behave like quiescent HDC in heterodikaryons formed with the same types of replicative cells, which suggest that senescent HDC and quiescent HDC share elements of a common mechanism for cessation of proliferation.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aurelian L., Schumann B., Marcus R. L., Davis H. J. Antibody to HSV-2 induced tumor specific antigens in serums from patients with cervical carcinoma. Science. 1973 Jul 13;181(4095):161–164. doi: 10.1126/science.181.4095.161. [DOI] [PubMed] [Google Scholar]
- Datta P., Natraj C. V. Fibroblast growth regulatory factor inhibits DNA synthesis in BALB/c 3T3 cells by arresting in G1. Exp Cell Res. 1980 Feb;125(2):431–439. doi: 10.1016/0014-4827(80)90137-8. [DOI] [PubMed] [Google Scholar]
- Dell'Orco R. T., Mertens J. G., Kruse P. F., Jr Doubling potential, calendar time, and senescence of human diploid cells in culture. Exp Cell Res. 1973 Mar 15;77(1):356–360. doi: 10.1016/0014-4827(73)90588-0. [DOI] [PubMed] [Google Scholar]
- Dell'orco R. T., Crissman H. A., Steinkamp J. A., Kraemer P. M. Population analysis of arrested human diploid fibroblasts by flow microfluorometry. Exp Cell Res. 1975 May;92(2):271–274. doi: 10.1016/0014-4827(75)90380-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Hahn G. M., Stewart J. R., Yang S. J., Parker V. Chinese hamster cell monolayer cultures. I. Changes in cell dynamics and modifications of the cell cycle with the period of growth. Exp Cell Res. 1968 Feb;49(2):285–292. doi: 10.1016/0014-4827(68)90179-1. [DOI] [PubMed] [Google Scholar]
- Hales A. A procedure for the fusion of cells in suspension by means of polyethylene glycol. Somatic Cell Genet. 1977 Mar;3(2):227–230. doi: 10.1007/BF01551817. [DOI] [PubMed] [Google Scholar]
- Maizel A., Nicolini C., Baserga R. Effect of cell trypsinization on nuclear proteins of WI-38 fibroblasts in culture. J Cell Physiol. 1975 Aug;86(1):71–82. doi: 10.1002/jcp.1040860109. [DOI] [PubMed] [Google Scholar]
- Natraj C. V., Datta P. Control of DNA synthesis in growing BALB/c 3T3 mouse cells by a fibroblast growth regulatory factor. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6115–6119. doi: 10.1073/pnas.75.12.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols W. W., Murphy D. G., Cristofalo V. J., Toji L. H., Greene A. E., Dwight S. A. Characterization of a new human diploid cell strain, IMR-90. Science. 1977 Apr 1;196(4285):60–63. doi: 10.1126/science.841339. [DOI] [PubMed] [Google Scholar]
- Norwood T. H., Pendergrass W. R., Martin G. M. Reinitiation of DNA synthesis in senescent human fibroblasts upon fusion with cells of unlimited growth potential. J Cell Biol. 1975 Mar;64(3):551–556. doi: 10.1083/jcb.64.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwood T. H., Pendergrass W. R., Sprague C. A., Martin G. M. Dominance of the senescent phenotype in heterokaryons between replicative and post-replicative human fibroblast-like cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2231–2235. doi: 10.1073/pnas.71.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch P. S., Norwood T. H. Comparative heterokaryon study of cellular senescence and the serum-deprived state. Exp Cell Res. 1980 Nov;130(1):101–109. doi: 10.1016/0014-4827(80)90046-4. [DOI] [PubMed] [Google Scholar]
- Rao P. N., Johnson R. T. Mammalian cell fusion: studies on the regulation of DNA synthesis and mitosis. Nature. 1970 Jan 10;225(5228):159–164. doi: 10.1038/225159a0. [DOI] [PubMed] [Google Scholar]
- Rao P. N., Wilson B. A., Sunkara P. S. Inducers of DNA synthesis present during mitosis of mammalian cells lacking G1 and G2 phases. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5043–5047. doi: 10.1073/pnas.75.10.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffonati L., Baserga R. Different survival of normal and transformed cells exposed to nutritional conditions nonpermissive for growth. Cancer Res. 1977 Feb;37(2):541–545. [PubMed] [Google Scholar]
- Stein G. H. T98G: an anchorage-independent human tumor cell line that exhibits stationary phase G1 arrest in vitro. J Cell Physiol. 1979 Apr;99(1):43–54. doi: 10.1002/jcp.1040990107. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Yanishevsky R. M. Entry into S phase is inhibited in two immortal cell lines fused to senescent human diploid cells. Exp Cell Res. 1979 Apr;120(1):155–165. doi: 10.1016/0014-4827(79)90546-9. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Yanishevsky R. Autoradiography. Methods Enzymol. 1979;58:279–292. doi: 10.1016/s0076-6879(79)58143-9. [DOI] [PubMed] [Google Scholar]
- Whittenberger B., Raben D., Lieberman M. A., Glaser L. Inhibition of growth of 3T3 cells by extract of surface membranes. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5457–5461. doi: 10.1073/pnas.75.11.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanishevsky R. M., Stein G. H. Ongoing DNA synthesis continues in young human diploid cells (HDC) fused to senescent HDC, but entry into S phase is inhibited. Exp Cell Res. 1980 Apr;126(2):469–472. doi: 10.1016/0014-4827(80)90290-6. [DOI] [PubMed] [Google Scholar]
- Yanishevsky R., Carrano A. V. Prematurely condensed chromosomes of dividing and non-dividing cells in aging human cell cultures. Exp Cell Res. 1975 Jan;90(1):169–174. doi: 10.1016/0014-4827(75)90370-5. [DOI] [PubMed] [Google Scholar]
- Yanishevsky R., Mendelsohn M. L., Mayall B. H., Cristofalo V. J. Proliferative capacity and DNA content of aging human diploid cells in culture: a cytophotometric and autoradiographic analysis. J Cell Physiol. 1974 Oct;84(2):165–170. doi: 10.1002/jcp.1040840202. [DOI] [PubMed] [Google Scholar]


