Abstract
PrsA2 is a conserved posttranslocation chaperone and a peptidyl prolyl cis-trans isomerase (PPIase) that contributes to the virulence of the Gram-positive intracellular pathogen Listeria monocytogenes. One of the phenotypes associated with a prsA2 mutant is decreased activity of the broad-range phospholipase C (PC-PLC). PC-PLC is made as a proenzyme whose maturation is mediated by a metalloprotease (Mpl). The proforms of PC-PLC and Mpl accumulate at the membrane-cell wall interface until a decrease in pH triggers their maturation and rapid secretion into the host cell. In this study, we examined the mechanism by which PrsA2 regulates the activity of PC-PLC. We observed that in the absence of PrsA2, the proenzymes are secreted at physiological pH and do not mature upon a decrease in pH. The sensitivity of the prsA2 mutant to cell wall hydrolases was modified. However, no apparent changes in cell wall porosity were detected. Interestingly, synthesis of PC-PLC in the absence of its propeptide lead to the secretion of a fully active enzyme in the cytosol of host cells independent of PrsA2, indicating that neither the propeptide of PC-PLC nor PrsA2 is required for native folding of the catalytic domain, although both influence secretion of the enzyme. Taken together, these results suggest that PrsA2 regulates compartmentalization of Mpl and PC-PLC, possibly by influencing cell wall properties and interacting with the PC-PLC propeptide. Moreover, the ability of these proproteins to respond to a decrease in pH during intracellular growth depends on their localization at the membrane-cell wall interface.
INTRODUCTION
Protein secretion in Gram-positive bacteria is comprised of two steps. Proteins are first translocated across the bacterial cytoplasmic membrane to the membrane-cell wall interface. These proteins are then secreted across the bacterial cell wall. Following translocation, proteins rely on various folding factors to reach their native state. These folding factors, located on the trans side of the cell membrane, include disulfide bond oxidoreductases, peptidyl prolyl cis-trans isomerases (PPIases), and chaperones (10, 34). In general, chaperones assist proteins folding into their native conformations by inhibiting improper protein-protein interactions and altering the activation energy necessary for proper folding (11). PPIases serve as chaperones in addition to their isomerase activity and contribute to cell envelope integrity (10). PrsA, a parvulin-type PPIase of the Gram-positive bacterium Bacillus subtilis, contributes to the folding of penicillin binding proteins (PBPs) (18). PBPs catalyze the cross-linking of glycan chains with short peptides forming peptidoglycan and transglycosylase reactions during cell wall biosynthesis (15). Peptidoglycan of the B. subtilis prsA mutant shows a cross-linking defect, causing changes in cell morphology and a decrease in viability (44). PrsA2, a PrsA ortholog from the Gram-positive pathogen Listeria monocytogenes, is not essential for viability during extracellular growth, but the mutant leaks cytoplasmic proteins, suggesting that it is important for cell envelope homeostasis (1, 3, 47).
Listeria monocytogenes is a facultative intracellular pathogen and the causative agent of the food-borne disease listeriosis (43). Following entry into a host cell, membrane-bound bacteria rapidly escape vacuoles to access the host cytosol where they multiply (42). L. monocytogenes uses an actin-based mechanism of motility to spread from cell to cell without exiting to the extracellular milieu. During cell-to-cell spread, the bacteria become entrapped in double-membrane vacuoles from which they must escape to perpetuate their intracellular growth cycle. Escape from vacuoles is mediated by a pore-forming hemolysin called listeriolysin O (LLO) (14) and two phospholipases of type C (9, 39). PrsA2 contributes to LLO folding and pore-forming ability and to the activity of the broad-range phospholipase C (PC-PLC) (2, 47). Accordingly, the intracellular growth and cell-to-cell spread of L. monocytogenes are affected in the absence of PrsA2. In addition, L. monocytogenes is highly attenuated in vivo in the absence of PrsA2. Interestingly, the PPIase activity of PrsA2 is not required for PC-PLC activity, but it is needed for optimal LLO pore-forming ability and L. monocytogenes virulence (3).
PC-PLC and the metalloprotease of L. monocytogenes (Mpl) are synthesized as inactive proenzymes containing N-terminal propeptides. During intracytosolic growth, these proteins translocate across the bacterial cytoplasmic membrane and accumulate at the membrane-cell wall interface (28, 31, 40). Upon cell-to-cell spread, bacteria in vacuoles experience a decrease in pH, which initiates Mpl autocatalysis, leading to the proteolytic maturation of PC-PLC by mature Mpl (6, 12, 27). Concomitant to maturation, both proteins are rapidly secreted across the bacterial cell wall. The secretion of PC-PLC in response to pH is dependent on Mpl, but the secretion of Mpl is not dependent on PC-PLC (12, 46).
In this study, we investigated the mechanism by which PrsA2 affects the activity of PC-PLC. Our results indicated that PrsA2 is required to maintain the Mpl zymogen and the proform of PC-PLC associated with bacteria during infection, possibly as a result of its influence on cell wall composition. Interestingly, the secreted proenzymes did not undergo maturation upon a decrease in intracellular pH, indicating that Mpl autocatalysis is spatially controlled in addition to being controlled by pH. Also, phospholipase activity was independent of PrsA2 when PC-PLC was produced in the absence of its propeptide. Therefore, it appears that the PC-PLC maturation defect observed in the absence of PrsA2 results from premature secretion of the Mpl zymogen, possibly caused by changes in the biochemical properties of the cell wall, and from the inability of the zymogen to undergo autocatalysis postsecretion.
MATERIALS AND METHODS
Bacterial strains.
All Listeria monocytogenes strains used in this study are listed in Table 1. L. monocytogenes strains were grown in brain heart infusion (BHI) medium or Luria-Bertani (LB) broth supplemented with 50 mM morpholinepropanesulfonic acid (MOPS) adjusted to pH 7.3, 0.2% activated charcoal, and 25 mM glucose-1-phosphate (LB-MOPS-G1P). Escherichia coli bacteria with pKSV7-derived plasmids were cultured in LB broth supplemented with ampicillin (100 μg/ml). E. coli bacteria containing pPL2-derived plasmids were cultured in LB broth supplemented with chloramphenicol (25 μg/ml). L. monocytogenes strains harboring pPL2-derived or pKSV7-derived plasmids were cultured in BHI medium supplemented with chloramphenicol (10 μg/ml).
Table 1.
L. monocytogenes strains and plasmids used in this study
| Strain or plasmid | Genotype and/or relevant feature(s) | Reference |
|---|---|---|
| Strains | ||
| 10403S | Wild-type (serotype 1/2a) | 5 |
| NF-L943 | prfA with G155S mutation in 10403S background (overexpresses PrfA-dependent genes) | 37 |
| DP-L1075 | 10403S prfA::Tn917-LTV3 | 13 |
| DP-L1935 | Internal in-frame deletion of plcB in 10403S background | 39 |
| DP-L2296 | Internal in-frame deletion of mpl in 10403S background | 26 |
| DP-L2343 | Deletion of mpl structural gene in 10403S background | 36 |
| DP-L5596 | 10403S prsA2::Himar1 | 47 |
| DP-L5601 | Internal in-frame deletion of prsA2 from positions 29 to 291 in 10403S background (ΔprsA2) | 47 |
| DP-L5603 | DP-L5601 carrying pJZ065 integrated at the tRNAArg site | 47 |
| DP-L5749 | 10403S carrying pBHE573 | 35 |
| DP-L5750 | 10403S expressing PSA bacteriophage holin/lysin and carrying pBHE573 | 35 |
| DP-L5751 | DP-L5601 carrying pBHE573 | This study |
| DP-L5755 | 10403S carrying pJZ095 integrated at the tRNAArg site | This study |
| EJ-L12 | 10403S ΔinlA | 4 |
| HEL-335 | 10403S plcBΔpro | 46 |
| HEL-981 | NF-L943 Mpl-FlagN-cata | 12 |
| HEL-1216 | DP-L2296 prsA2::Himar1 | This study |
| HEL-1230 | NF-L943 Mpl-FlagN-catprsA2::Himar1 | This study |
| HEL-1232 | NF-L943 Mpl-FlagN-catprsA2::Himar1 carrying pJZ065 integrated at the tRNAArg site | This study |
| HEL-1405 | HEL-335 prsA2::Himar1 | This study |
| NF-L1166 | 10403S with actA-gus-neo-plcB and prfA with the L140F mutation | 30 |
| Plasmids | ||
| pBHE573 | Firefly luciferase with the cytomegalovirus promoter | 35 |
| pJZ065 | prsA2 promoter, without prfA box, and open reading frame cloned into pPL2 | 47 |
| pJZ095 | prsA2 promoter-gus cloned into pPL2 (PPrsA2-gus) | This study |
| pPL2 | Site-specific shuttle integration vector | 22 |
Mpl-FlagN-cat is an Mpl construct that contains a Flag tag at the N terminus of its catalytic domain.
Transduction of prsA2::Himar1.
Transductions were performed as described previously (17, 47). Briefly, U153 phage grown on donor strain L. monocytogenes 10403S prsA2::Himar1 (DP-L5596) was used to infect recipient Listeria strains (Table 1). Transductants were selected at 37°C on semisolid medium supplemented with lincomycin (25 μg/ml) and erythromycin (2 μg/ml).
Construction of prsA2 promoter-gus fusion plasmid.
Splicing by overlap extension (SOEing) PCR was used to fuse the promoter of prsA2 to gus (gene encoding β-glucuronidase [Gus]). The promoter of prsA2 and the gus structural gene were amplified by PCR using primer pair JZ-241 (47) and BK-011 (5′-GTTTCTACAGGACGGACCATTAAATAAAACACACTCCTTAGTTGTTGGAA-3′) and primer pair BK-012 (5′-TTCCAACAACTAAGGAGTGTGTTTTATTTAATGGTCCGTCCTGTAGAAAC-3′) and BK-013 (5′-TTTGTCGACTCATTGTTTGCCTCCCTGCTG-3′), respectively, and L. monocytogenes NF-L1166 (kindly provided by Nancy Freitag) genomic DNA as the template (Table 1). The respective 307-bp and 1,851-bp PCR products were used in a SOEing PCR with primers JZ-241 and BK-013. The 2,108-bp product was digested with EagI and SalI and ligated into the shuttle integration vector pPL2, thus generating pJZ095 (Table 1).
Conjugation experiments.
All pPL2-derived plasmids (Table 1) were introduced into Listeria by conjugation as described previously (32). Chloramphenicol-resistant conjugants were screened for plasmid integration at the attB site of the tRNAArg gene by PCR using primers NC16 and PL95 (22).
Metabolic labeling and immunoprecipitation assays.
The specific detection of metabolically labeled broad-range phospholipase C (PC-PLC) or metalloprotease (Mpl) synthesized by intracellular bacteria was performed as described previously (31, 38, 40). Secreted PC-PLC and Mpl were immunoprecipitated from cleared host cell lysates, whereas bacterium-associated PC-PLC was immunoprecipitated from the lysate of intracellular bacteria. For pulse-chase experiments, radiolabeled infected cells were chased in tissue culture medium containing unlabeled methionine (5 mM), cysteine (1 mM), and chloramphenicol (20 μg/ml) for the times indicated below in Results and the figure legends. To detect PC-PLC or Mpl as a function of pH, radiolabeled infected cells were chased in a potassium-based buffer adjusted to either pH 7.3 or pH 6.5 and supplemented with nigericin (10 μM). Immunoprecipitates were resolved by SDS-PAGE and detected by autoradiography or phosphorimaging.
To determine the half-life of PC-PLC, the intensities of protein bands detected by phosphorimaging from pulse-chase experiments were quantified using ImageJ (rsbweb.nih.gov/ij/). A regression curve was generated and used to calculate the half-life of PC-PLC.
Immunofluorescence staining of Mpl.
Bacterium-associated Mpl-FlagN-cat (an Mpl construct that contains a Flag tag at the N terminus of its catalytic domain) was detected by immunofluorescence as described previously (12). Briefly, HeLa cells infected with L. monocytogenes were fixed and then treated with purified L. monocytogenes-specific phage endolysin Ply118. To detect bacterium-associated Flag-tagged Mpl (Mpl-Flag), mouse monoclonal anti-Flag M2 (Sigma) was used followed by a donkey anti-mouse antibody conjugated to fluorescein isothiocyanate (FITC). Bis-benzimide (Hoechst 33258) was used to detect Listeria.
Expression of prsA2.
Levels of prsA2 expression were measured using a quantitative Gus assay as described previously (37). L. monocytogenes strains containing pJZ095 were grown in BHI medium for 8 h. At each hour, optical density measurements (optical density at 600 nm [OD600]) were taken, and bacterial pellets were resuspended in 100 mM potassium phosphate (pH 7.0), 100 mM NaCl, and 0.1% Triton X-100. Gus substrate (4-methylumbelliferyl-β-d-glucuronide [4-MUGlcU]) was added to lysates at a concentration of 0.4 mg/ml for 1 h at room temperature. Fluorescence measurements were taken after adding 200 mM sodium carbonate to each sample. Fluorescence measurements (λexcitation = 360 nm; λemission = 460 nm) were made using a Horiba-FluoroMax-3 fluorometer and normalized to OD600 readings. Normalized fluorescence measurements were converted to concentrations of Gus using a standard curve of fluorescence intensity to concentrations of 4-methylumbelliferone.
Preparation of cell wall fractions.
Bacterial cell wall fractions were isolated as described previously (40). Briefly, bacteria were grown in LB-MOPS-G1P to an OD600 of ∼1.2. Bacterial cells were suspended in protoplast buffer supplemented with phage endolysin Ply118 to a calculated OD600 of 12. Protoplast formation was monitored by microscopy. The protoplasts were pelleted by centrifugation at 15,000 × g for 5 min at 4°C, and the cell wall proteins were recovered in the supernatant. The proteins were precipitated using 5% trichloroacetic acid, resolved by SDS-PAGE, and detected with Coomassie brilliant blue R-250. The purity of the cell wall fraction was verified by performing Western immunoblotting for detection of InlA, a cell wall protein, and PrfA, a cytoplasmic protein as described previously (40).
Cell wall hydrolysis assays.
Bacteria were grown in BHI medium at 37°C and 200 rpm to an OD600 of ∼0.5, pelleted, and suspended in 20 mM Tris-HCl (pH 8.0) to a calculated OD600 of 1.0. Chicken egg white lysozyme (Sigma) or phage endolysin Ply118 was added to a final concentration of 50 μg/ml. OD600 measurements were taken every minute for 30 min at 37°C using a BioTek PowerWave XS microplate spectrophotometer. Three experimental replicate assays were performed.
Bacteriolysis luciferase assay.
The luciferase assay to test for bacterial lysis in infected cells was performed as described previously (35). Briefly, bone marrow-derived macrophages from IFNAR−/− mice (IFNAR stands for IFN-α/β receptor) were infected with L. monocytogenes strains expressing firefly luciferase under the control of a cytomegalovirus promoter (pBHE573; Table 1). Six hours postinfection, luciferase reagent (Sigma) was added to infected host cell lysates, and luciferase activity was measured.
Detection of phospholipase activity.
PC-PLC activity was detected on LB egg yolk agar plates as described previously (46). Bacteria were spot inoculated onto plates and incubated for 48 h at 37°C.
Plaque assay.
The efficiency of the cell-to-cell spread of intracellular bacteria was measured using the plaque assay as described previously (41). The infected monolayers of mouse L2 fibroblast cells were stained with neutral red, and plaque areas were calculated. Plaque area is reported as a percentage of the wild-type plaque size. Three independent experiments were performed.
Statistical analysis.
Cell wall hydrolysis assay results were analyzed using a two-tailed paired t test to determine whether the prsA2 mutant was more sensitive to cell wall hydrolases than the wild-type strain. Plaque assay results were analyzed using a one-way analysis of variance (ANOVA) with a Tukey-Kramer multiple-comparison test to determine whether the plaque size of each of the strains tested was significantly different. A significance level (α) of 0.05 was set for both tests.
RESULTS
PC-PLC and Mpl are no longer found associated with bacteria in the absence of PrsA2.
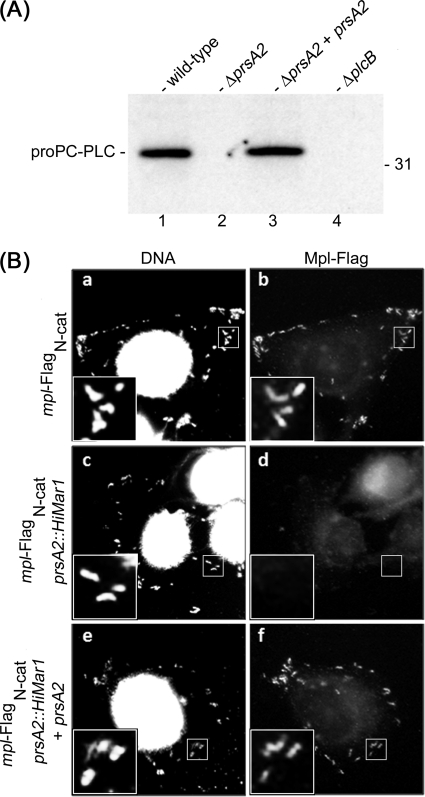
Previous studies have reported that broad-range phospholipase C (PC-PLC) secretion and activity are reduced in the absence of PrsA2 (2, 47). PC-PLC and metalloprotease of L. monocytogenes (Mpl) are translocated across the bacterial membrane as inactive proenzymes and accumulate at the membrane-cell wall interface (40). Upon a decrease in pH, which is normally experienced when bacteria are in vacuoles, Mpl undergoes autocatalysis and mediates the maturation of PC-PLC (6, 12). This maturation process is followed by the rapid secretion of these two enzymes across the bacterial cell wall (12, 28, 40). We questioned the role of PrsA2 in regulating the compartmentalization of PC-PLC and Mpl. Infected cells were pulse-labeled, and bacterium-associated PC-PLC was immunoprecipitated from lysates of intracellular bacteria. The proform of PC-PLC (proPC-PLC) was detected from the wild-type but not from the prsA2 bacterium lysate (Fig. 1A). We were unsuccessful at detecting Mpl from immunoprecipitates using either anti-Flag or anti-Mpl antibodies, possibly as a result of nonspecific interactions between Mpl and other proteins from the bacterial lysates or a result of proteolytic degradation. Therefore, we used an immunofluorescence assay to detect bacterium-associated Mpl. We observed Mpl associated with bacteria in cells infected with wild-type bacteria, but not in cells infected with the prsA2 mutant (Fig. 1B). Complementation of the mutant restored the wild-type phenotype for PC-PLC and Mpl. This result indicated that PrsA2 is necessary for the accumulation of PC-PLC and Mpl within bacteria, suggesting that these proteins are degraded rapidly posttranslocation or secreted prematurely in the absence of PrsA2.
Fig. 1.
Broad-range phospholipase C (PC-PLC) and metalloprotease of L. monocytogenes (Mpl) are not associated with bacteria in the absence of PrsA2. (A) Bacterium-associated PC-PLC was immunoprecipitated from intracellular bacterial cell lysates. L. monocytogenes strains 10403S (wild type), DP-L5601 (ΔprsA2 mutant) (lane 2), DP-L5603 (ΔprsA2 mutant carrying pJZ065, which contains the prsA2 gene), and DP-L1935 (ΔplcB mutant) were tested. The position of the 31-kDa protein standard is indicated to the right of the gel, and the position of the proform of PC-PLC (proPC-PLC) is indicated to the left of the gel. (B) Infected HeLa cells were fixed and stained for fluorescence microscopy. Bis-benzamide was used to detect host cell nuclei and bacterial chromosome, and anti-Flag M2 antibody was used to detect Flag-tagged Mpl (Mpl-Flag). L. monocytogenes strains HEL-981 (a and b), HEL-1230 (c and d), and HEL-1232 (e and f) were studied. Mpl-FlagN-cat is an Mpl construct that contains a Flag tag at the N terminus of its catalytic domain.
PC-PLC and Mpl are prematurely secreted from bacteria in the absence of PrsA2.
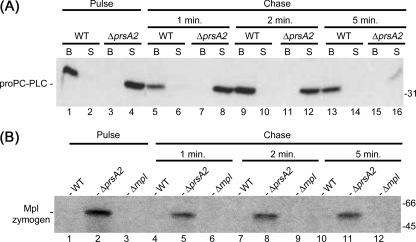
The rate of protein secretion across the bacterial cell wall could be influenced by the biophysical and biochemical properties of the cell wall, protein structure, protein-protein interactions, and ionic interactions. Considering that PrsA2 is likely to act as a chaperone and therefore assist in the folding of proteins posttranslocation, we hypothesized that the absence of PrsA2 could influence the rate of PC-PLC and Mpl secretion. Therefore, we assessed whether PC-PLC and Mpl were secreted prematurely in the absence of PrsA2. Infected cells were pulse-labeled and then chased for 1, 2, or 5 min postinfection. PC-PLC was immunoprecipitated from the secreted bacterial fraction in lysates of infected cells and from lysates of intracellular bacteria. The results showed that PC-PLC was found exclusively in the secreted fraction of the prsA2 mutant immediately after the pulse but remained associated with bacteria for the entire time of the chase with the wild-type strain (Fig. 2A). Mpl was also immunoprecipitated from the secreted bacterial fraction in lysates of infected cells. Similar to PC-PLC, Mpl was secreted rapidly from the prsA2 mutant, but not from wild-type bacteria (Fig. 2B). Although we were unable to isolate Mpl from intracellular bacterial lysates, these results indicated that wild-type bacteria maintain a pool of Mpl, whereas the prsA2 mutant does not (Fig. 1B).
Fig. 2.
PC-PLC and Mpl are prematurely secreted into the host cytosol in the absence of PrsA2. Infected mouse J774 macrophage-like cells were pulse-labeled and then chased with cold Met/Cys and chloramphenicol for either 1, 2, or 5 min as indicated in the figure. (A) Bacterium-associated (B) and secreted (S) PC-PLC were immunoprecipitated from bacterial and host cell lysates, respectively. L. monocytogenes strains 10403S (wild type [WT]) and DP-L5601 (ΔprsA2 mutant) were tested. (B) Secreted Mpl was immunoprecipitated from the host cell lysates. L. monocytogenes strains 10403S (WT), DP-L5601 (ΔprsA2 mutant), and DP-L2343 (Δmpl mutant) were studied. The positions of molecular mass markers (in kilodaltons) are indicated to the right of the gel.
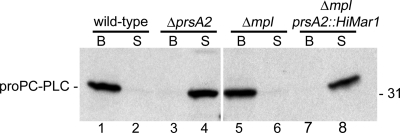
Mpl controls the secretion of PC-PLC across the cell wall. In the absence of Mpl, PC-PLC remains associated with bacteria upon a decrease in pH. The ability of Mpl to facilitate the secretion of PC-PLC is independent of its role in mediating the maturation of PC-PLC, suggesting that perhaps Mpl serves to transport PC-PLC across the cell wall (46). Therefore, we asked whether the secretion of PC-PLC from the prsA2 mutant was dependent on Mpl. Infected cells were pulse-labeled, and PC-PLC was immunoprecipitated from bacteria that lacked either Mpl and/or PrsA2. As previously reported, PC-PLC remained associated with bacteria at physiological pH in the presence or absence of Mpl (Fig. 3, lanes 1 and 5). However, in the absence of PrsA2, PC-PLC was secreted independently of Mpl (Fig. 3, lanes 4 and 8). Together, these results indicated that, at physiological pH, PrsA2 is required (i) to prevent the premature secretion of PC-PLC and Mpl across the bacterial cell wall and (ii) to enable Mpl to control PC-PLC secretion.
Fig. 3.
PC-PLC is secreted into the host cytosol independent of Mpl in the absence of PrsA2. Infected mouse J774 macrophage-like cells were pulse-labeled. Bacterium-associated (B) and secreted (S) PC-PLC were immunoprecipitated from bacterial and host cell lysates, respectively. L. monocytogenes strains 10403S (wild type), DP-L5601 (ΔprsA2 mutant), DP-L2343 (Δmpl mutant), and HEL-1216 (Δmpl prsA2::Himar1 mutant) were tested. The position of the 31-kDa protein standard is indicated to the right of the gel.
Maturation of PC-PLC and Mpl does not occur in the absence of PrsA2 during intracellular infection.
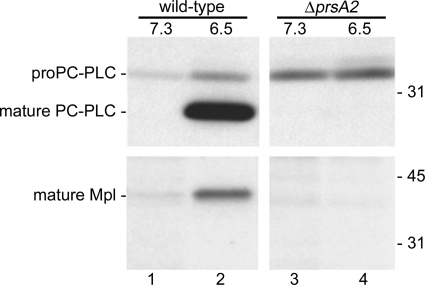
We previously hypothesized that the maturation of PC-PLC by Mpl in response to a decrease in pH occurs prior to secretion because secretion of PC-PLC synthesized in the absence of its propeptide is very rapid as opposed to secretion of the proform (46). To test this hypothesis, we assessed whether a decrease in pH would lead to the maturation of PC-PLC in the cytosol of infected cells, as the prsA2 mutant does not retain the proform of PC-PLC and Mpl associated with bacteria. Infected cells were pulse-labeled with [35S]methionine/cysteine and chased under conditions that maintain the host cell cytosol at physiological pH or mimic the acidified vacuolar pH to induce PC-PLC maturation. Secreted PC-PLC was immunoprecipitated from host cell lysates and detected by autoradiography. As previously reported, mature PC-PLC was detected in the host cell lysate following a decrease in intracellular pH in cells infected with wild-type bacteria (Fig. 4). However, pH-mediated maturation of PC-PLC did not occur in cells infected with the prsA2 mutant.
Fig. 4.
pH-dependent PC-PLC maturation and Mpl autocatalysis do not occur in the absence of PrsA2. Infected mouse J774 macrophage-like cells were pulse-labeled and then chased in a nigericin-containing potassium buffer equilibrated to pH 7.3 or pH 6.5. Secreted PC-PLC and Mpl were immunoprecipitated from host cell lysates. L. monocytogenes strains 10403S (wild type) and DP-L5601 (ΔprsA2 mutant) were tested. The positions of molecular mass markers (in kilodaltons) are indicated to the right of the gel.
The absence of PC-PLC maturation in response to a decrease in pH could be due to a failure by Mpl either to undergo autocatalysis or to process PC-PLC postsecretion. To answer this question, we performed the same experiment as described above, except that secreted Mpl, not PC-PLC, was immunoprecipitated. Mature Mpl was detected in the host cell lysate following a decrease in intracellular pH in cells infected with wild-type bacteria (Fig. 4). However, Mpl maturation did not occur in response to a decrease in pH in cells infected with the prsA2 mutant. Together, these results suggested that the effect of pH on Mpl autocatalysis and Mpl-mediated maturation of PC-PLC is spatially controlled and that PrsA2 is required to maintain the proproteins at the membrane-cell wall interface where maturation occurs.
Cell wall permeability does not appear to be increased in the absence of PrsA2.
Since the B. subtilis PrsA is important for peptidoglycan cross-linking (18) and the L. monocytogenes PrsA2 is associated with cell wall integrity (3), we suspected that perhaps the cell wall of the prsA2 mutant was more porous than the cell wall of wild-type bacteria. To assess differences in cell wall porosity, we opted to examine the profile of proteins that are present within the cell wall or are retained at the membrane-cell wall interface of L. monocytogenes. Before performing this experiment, we determined the temporal regulation of prsA2 expression in broth culture to assess when a defect in prsA2 expression was most likely to affect cell wall integrity. A prsA2 promoter and gus structural gene transcriptional fusion was constructed, and levels of β-glucuronidase (Gus) were measured (Fig. 5). Gus was detected early during exponential growth phase, peaked in late log phase, and dropped during stationary phase, suggesting that PrsA2 was most important during the exponential phase of bacterial growth.
Fig. 5.
prsA2 is expressed during exponential growth in broth culture. L. monocytogenes strain 10403S carrying pJZ095 (strain DP-L5755) was grown for 8 h in BHI medium. Each hour, an OD600 measurement was taken, and the β-glucuronidase (Gus) level from 1 ml of culture was measured. The levels of Gus were normalized to the OD600 measurements of the culture at each time point. The error bars represent the standard errors of the means. Data were collected from duplicate samples from three independent experiments.
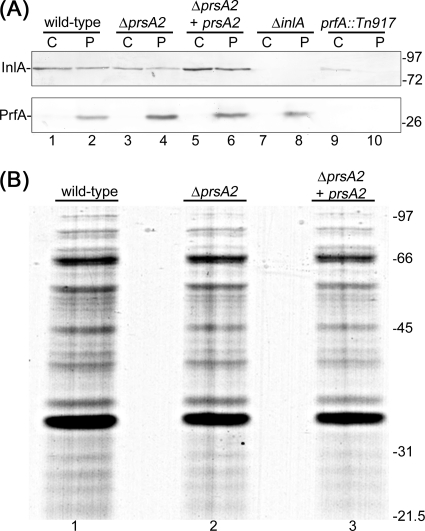
Cell wall porosity was assessed using bacteria in the exponential phase of growth. Protoplasts were generated using a purified cell wall hydrolase. To verify the purity of the cell wall fraction, we assessed the presence of a cell wall protein, InlA, and of a cytoplasmic protein, PrfA, by Western immunoblotting (40). The results confirmed the presence of InlA and the absence of PrfA in the cell wall fraction of each sample, indicating that it was devoid of cytoplasmic proteins (Fig. 6A).
Fig. 6.
(A) Detection of InlA and PrfA from cell wall (C) and protoplast (P) fractions by Western immunoblotting. L. monocytogenes strains 10403S (wild type), DP-L5601 (ΔprsA2 mutant), DP-L5603 (ΔprsA2 mutant carrying pJZ065, which contains the prsA2 gene), EJL-12 (ΔinlA mutant), and DP-L1075 (prfA::Tn917) were tested. The positions of molecular mass markers (in kilodaltons) are indicated to the right of the gel. (B) Protein profiles of L. monocytogenes wild-type and prsA2 mutant cell wall fractions. Proteins that localize in the cell wall and at the membrane-cell wall interface were isolated as described in Materials and Methods. Equivalent amounts of culture OD units were loaded per lane. Proteins were resolved by SDS-PAGE and detected by Coomassie brilliant blue staining. The heavy band above the 31-kDa marker corresponds to phage endolysin Ply118. L. monocytogenes strains 10403S (wild type), DP-L5601 (ΔprsA2 mutant), and DP-L5603 (ΔprsA2 mutant carrying pJZ065, which contains the prsA2 gene) were tested. The positions of molecular mass markers (in kilodaltons) are indicated to the right of the gel.
This cell wall fraction is expected to contain cell wall-anchored proteins as well as translocated proteins that have not yet been secreted across the cell wall. If the prsA2 mutant cell wall were more porous, we would expect to detect either fewer or fainter protein bands in this fraction. Although we observed minor differences, overall the protein patterns for the wild-type strain, the prsA2 mutant, and the complemented mutant strain were very similar (Fig. 6B). These results suggested that there is no gross difference in the cell wall porosity of the wild-type L. monocytogenes and the prsA2 mutant.
Susceptibility of L. monocytogenes to cell wall hydrolases is modified in the absence of PrsA2.
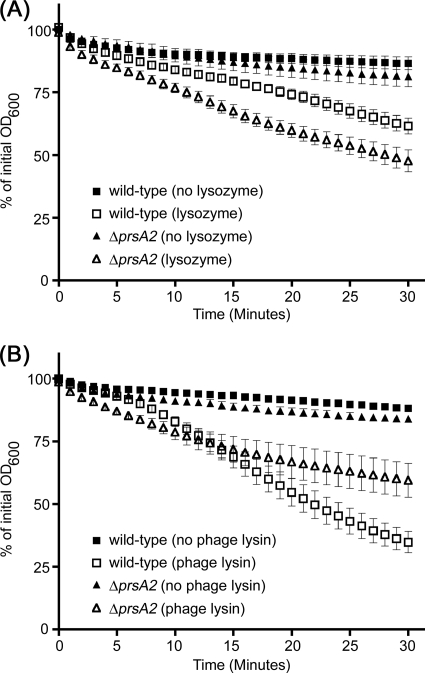
To further investigate potential differences in the biochemical properties of the cell wall from wild-type and prsA2 mutant bacteria, we assessed the susceptibility of the cell wall to lysozyme and to an L. monocytogenes-specific cell wall hydrolase. Lysozyme cleaves the β(1,4) linkage between the N-acetylglucosamine and N-acetylmuramic acid residues of peptidoglycan. Independent of its enzymatic activity, lysozyme has also been shown to induce cell lysis through the activation of autolysins (21). To determine whether the prsA2 mutant was more susceptible to lysozyme-mediated lysis, the optical density of exponential-phase wild-type and prsA2 bacteria incubated with or without lysozyme was measured as a function of time (Fig. 7A). In the presence of lysozyme, the prsA2 mutant exhibited a slight but significant increase in the rate of lysis compared to wild-type bacteria, beginning at 10 min (P < 0.05). The same assay was performed with purified Ply118, an L. monocytogenes-specific phage endolysin that cleaves the peptidoglycan peptide backbone between the l-alanine and d-glutamate residues (24). The prsA2 mutant was slightly more sensitive to Ply118 during the first 10 min of incubation (Fig. 7B). However, as incubation progressed, the mutant showed more resistance to Ply118 than wild-type bacteria. This difference was significant beginning at 25 min (P < 0.05) (Fig. 7B). Taken together, these results indicated that there are biochemical differences, albeit minor, in the peptidoglycan cell wall between the wild type and the prsA2 mutant.
Fig. 7.
Deletion of prsA2 affects the cell wall of L. monocytogenes. The sensitivity of mid-log phase bacteria to lysis in the presence (open symbols) or absence (filled symbols) of lysozyme (A) or phage endolysin Ply118 (B) was monitored by spectrometry. OD600 measurements were normalized to the initial optical density prior to incubation. The error bars represent the standard errors of the means from three independent experiments. The L. monocytogenes strains tested include 10403S (wild type) (squares) and DP-L5601 (ΔprsA2 mutant) (triangles).
The absence of PrsA2 does not increase the susceptibility of L. monocytogenes to intracellular lysis.
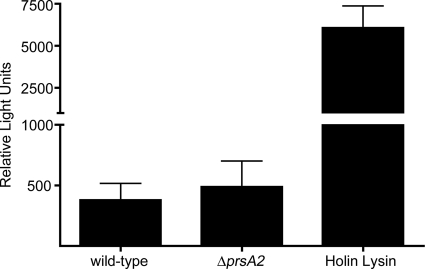
Next, we investigated whether differential susceptibility of wild-type and prsA2 mutant bacteria to cell wall hydrolases would predispose the prsA2 mutant to lysis during intracellular growth. Host cells were infected with bacteria carrying the gene coding for luciferase on a plasmid (35). The luciferase-coding gene is under the control of a cytomegalovirus promoter whose expression is dependent on host cell transcription factors, thus on bacterial lysis. No significant differences were observed between host cells infected with the wild-type and prsA2 mutant bacteria (Fig. 8). For a positive control for this assay, we infected cells with bacteria expressing PSA bacteriophage holin and lysin. This bacterial strain underwent autolysis, and the infected host cells generated high levels of luciferase activity. This result indicated that intracellular bacteria lacking PrsA2 are not predisposed to intracellular lysis to a larger extent than wild-type bacteria.
Fig. 8.
The prsA2 mutant does not show an increased sensitivity to lysis during intracellular growth. Intracellular bacterial lysis was measured using the luciferase reporter plasmid pBHE573 in infected macrophage cells. The error bars represent the standard deviations from three independent experiments. The L. monocytogenes strains tested include the wild type (strain DP-L5749), ΔprsA2 mutant (DP-L5751), and a 10403S strain expressing holin/lysin (DP-L5750).
PC-PLC is less stable when produced in the absence of PrsA2.
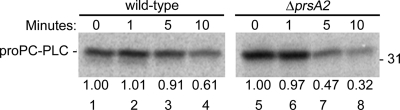
The rapid secretion of PC-PLC and Mpl across the bacterial cell wall in the absence of PrsA2 could result from modifications in protein structure. To assess whether PrsA2 assists in the folding of PC-PLC and Mpl, the stability of the proteins secreted in the cytosol of infected cells was assessed. Pulse-labeled bacterial proteins from infected cells were chased for incremental periods of time. Secreted PC-PLC and Mpl were immunoprecipitated from the host cell lysate, fractionated by SDS-PAGE, and detected by phosphorimaging. Because wild-type bacteria secrete smaller amounts of proproteins than the prsA2 mutant, the wild-type sample gels were exposed two to three times longer than the prsA2 mutant sample gels were. The intensities of protein bands were quantified using ImageJ (Fig. 9), and protein half-lives were calculated. The half-lives of PC-PLC made by the prsA2 mutant and wild-type strain were 5 min and greater than 10 min, respectively (Fig. 9 and 2A, respectively). In contrast, Mpl was very stable in the absence or presence of PrsA2 (Fig. 2B and data not shown). These results suggested that the secreted proform of PC-PLC, but not the Mpl zymogen, is folded differently in the absence of PrsA2, and thus more sensible to proteolysis.
Fig. 9.
PC-PLC is less stable when produced in the absence of PrsA2. Infected mouse J774 macrophage-like cells were pulse-labeled for 5 min and chased for 0, 1, 5, and 10 minutes. Secreted PC-PLC was then immunoprecipitated as described in Materials and Methods and detected by phosphorimaging. The relative amount of protein detected at each time point in comparison to the 0-min lanes was measured using ImageJ. L. monocytogenes strains 10403S (wild type) and DP-L5601 (ΔprsA2 mutant) were tested. The position of the 31-kDa protein standard is indicated to the right of the gel.
Deletion of the propeptide rescues the PC-PLC activity defect in the absence of PrsA2.
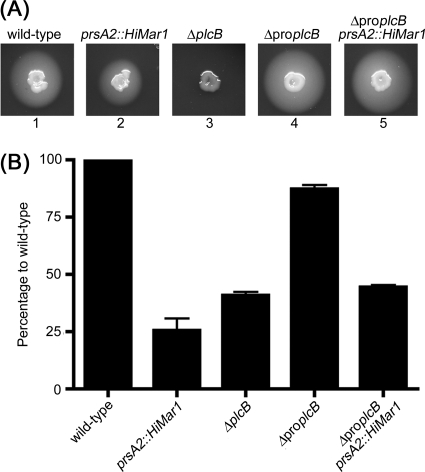
PC-PLC synthesized in the absence of its propeptide is rapidly secreted across the bacterial cell wall as an active enzyme, and this phenomenon is independent of pH and of Mpl (46). We questioned whether PrsA2 contributes to the folding of the catalytic domain of PC-PLC by examining phospholipase activity generated by a propeptide deletion mutant (ΔproplcB). Wild-type and mutant bacteria were spotted on egg yolk agar, where a zone of opacity around the colony is indicative of PC-PLC activity. As previously reported, the prsA2 mutant generated less activity than the wild-type strain, and the propeptide deletion mutant generated a higher level of phospholipase activity than the wild-type strain (46, 47) (Fig. 10A, panels 1, 2, and 4). Interestingly, the level of phospholipase activity generated by the propeptide deletion mutant was independent of PrsA2 (Fig. 10A, panel 5).
Fig. 10.
Deletion of the PC-PLC propeptide restores phospholipase activity in the prsA2 mutant. (A) Detection of PC-PLC activity on LB egg yolk agar plates. A zone of opacity indicates PC-PLC activity. L. monocytogenes strains 10403S (wild type) (panel 1), DP-L5596 (prsA2::Himar1) (panel 2), DP-L1935 (ΔplcB mutant) (panel 3), HEL-335 (propeptide deletion mutant [ΔproplcB]) (panel 4), and HEL-1405 (HEL-335 prsA2::Himar1) (panel 5) were tested. (B) Plaque assay to measure L. monocytogenes cell-to-cell spread in mouse L2 fibroblast cells. Plaque area is reported as a percentage of the wild-type plaque area. The error bars represent the standard deviations from three independent experiments. The strains tested were the same as those tested in panel A. Statistical significance was tested using ANOVA with Tukey-Kramer multiple-comparison test. All comparisons were significantly different (P ≤ 0.05) except between strains DP-L1935 and HEL-1405.
We next wished to determine whether deleting the propeptide of PC-PLC would partially restore the cell-to-cell spread defect of the prsA2 mutant in infected cells. To address this question, we used a plaque assay in which the size of the plaques is proportional to the efficiency of cell-to-cell spread of the bacteria (Fig. 10B). As previously reported, the prsA2 mutant makes plaques that are 25.5% the size of wild-type plaques (2, 47), whereas the plcB mutant generates plaques that are 40.8% the size of wild-type plaques, indicating that the plaque defect observed in the prsA2 mutant is not due to its effect on PC-PLC alone. The ΔproplcB mutant makes plaques that are close in size to wild-type plaques (87.3% of wild-type) (45). The prsA2 ΔproplcB double mutant makes plaques that are 44.5% the size of wild-type plaques, which is an intermediate size between the prsA2 and ΔproplcB single mutants. Together, these results indicated that deleting the propeptide of PC-PLC rescues the PC-PLC activity defect associated with the absence of PrsA2 but only partially restores the cell-to-cell spread defect. This absence of full complementation can possibly be attributed to a decrease in the ability of listeriolysin O (LLO) to form pores in the absence of PrsA2 (2, 47).
DISCUSSION
L. monocytogenes relies on numerous virulence factors to facilitate its intracellular lifestyle. Two of these factors, broad-range phospholipase C (PC-PLC) and metalloprotease of L. monocytogenes (Mpl), contribute to vacuolar lysis. Both proteins are produced as proenzymes that are translocated across the bacterial membrane and retained at the membrane-cell wall interface until a decrease in vacuolar pH triggers Mpl autocatalysis followed by Mpl-mediated maturation of PC-PLC (28, 31, 40). This maturation process correlates with the detection of Mpl and PC-PLC in the host cell. PrsA2, a peptidyl prolyl cis-trans isomerase (PPIase) and posttranslocation chaperone, was identified as contributing to the activity of PC-PLC (2, 3, 47). In this study, we assessed the mechanism by which PrsA2 controls PC-PLC activity. Our data showed that, in the absence of PrsA2, the proforms of PC-PLC and Mpl are prematurely secreted across the cell wall and that a decrease in intracellular pH did not lead to maturation of either protein. This phenomenon does not appear to result from a change in cell wall porosity, although there is biochemical evidence that the cell wall of the prsA2 mutant is slightly modified compared to the cell wall of the wild type. The stability of the secreted proform of PC-PLC was compromised in the absence of PrsA2, whereas the secreted Mpl zymogen was very stable. However, the PC-PLC activity defect observed in the absence of PrsA2 was rescued by deleting the propeptide of PC-PLC. We conclude that the PC-PLC activity defect observed in the absence of PrsA2 results from the premature secretion of the Mpl zymogen, possibly caused by changes in the biochemical properties of the cell wall, and from the inability of the zymogen to undergo autocatalysis postsecretion.
The ability of Mpl to localize at the membrane-cell wall interface during intracellular growth is imperative to its function (31). This fact was emphasized in this study by the observation that, in the absence of PrsA2, Mpl was prematurely secreted and did not undergo maturation upon a decrease in pH. This phenomenon is probably not due to a change in Mpl conformation, as the prematurely secreted enzyme was very stable. This was not surprising, as Mpl folding is unlikely to be assisted by PrsA2 considering that the individual propeptides of metalloproteases have been shown to act as folding chaperones (8, 25). If Mpl autocatalysis occurs when the protein localizes at the membrane-cell wall interface, then Mpl-mediated maturation of PC-PLC is likely to occur before secretion of these two proteins across the cell wall, as the confined area within the cell envelope would favor an encounter between the proform of PC-PLC and mature Mpl. Control of Mpl-mediated activation of PC-PLC is less stringent when the proteins are secreted by bacteria grown on complex medium, as some PC-PLC activity was detected around the colonies formed by the prsA2 mutant. However, it is difficult to compare extracellular and intracellular conditions. The pH stability experienced by bacteria multiplying in the cytosol of host cells cannot be reproduced when L. monocytogenes is grown in complex medium. Consequently, the Mpl zymogen may be exposed to acidic conditions during translocation across the bacterial cell wall, enabling immediate autocatalysis. However, the fact that some level of PC-PLC was detected on complex medium in the absence of PrsA2 argues that PrsA2 is not required for Mpl folding. We conclude that Mpl autocatalysis is controlled by factors and/or conditions that prevail within the cell envelope and that a decrease in pH is not sufficient to trigger Mpl autocatalysis.
The results from the present study indicated that the cell wall of the prsA2 mutant was modified as assessed by an increased sensitivity to lysozyme and a modified sensitivity to an L. monocytogenes-specific cell wall hydrolase. These results, along with the previous observation that the prsA2 mutant is more sensitive to penicillin than the wild type (3), suggest that the prsA2 mutant has a modified cell wall. It appears that biochemical differences exist in the glycan chains of peptidoglycan between the wild type and the prsA2 mutant, as both lysozyme and phage endolysin Ply118 recognize the sugar moieties of peptidoglycan (23, 29). The resistance of L. monocytogenes to lysozyme is conferred by PgdA, an enzyme that deacetylates N-acetylglucosamine residues of peptidoglycan (19). The absence of PrsA2 may alter the activity of PgdA, thus making peptidoglycan more sensitive to lysozyme (7). Alternatively, lysozyme activity may be affected by cell wall charges. The levels of d-alanylation of teichoic and lipoteichoic acids modulate the levels of negative charges in the cell wall (33). Lysozyme is positively charged, and bacteria unable to d-alanylate teichoic acid are more susceptible to lysozyme (16, 20). The absence of PrsA2 may affect the cell wall charge and the levels of cations bound to the cell wall, which could modulate the rate of folding and secretion of the Mpl zymogen. Similarly, these differences could influence the folding and secretion of PC-PLC. Alternatively, these changes in cell wall properties could influence other proteins that contribute to maintain the Mpl zymogen and the proform of PC-PLC associated with bacteria.
The mechanism that controls the compartmentalization of PC-PLC is unknown. The proform of PC-PLC is secreted across the cell wall at a rate that is lower than the rate of translocation across the cytoplasmic membrane, enabling the protein to accumulate at the membrane-cell wall interface (40). However, when PC-PLC is synthesized without its propeptide or in the absence of PrsA2, the protein is rapidly secreted across the cell wall. Synthesis of PC-PLC in the absence of its propeptide generates an active enzyme independent of PrsA2. Therefore, neither the propeptide of PC-PLC nor PrsA2 is required for native folding of the catalytic domain, but both influence secretion of the enzyme. However, the secreted proform of PC-PLC is more stable in the presence of PrsA2, suggesting a role for PrsA2 in folding of this enzyme. Recent work has shown that the N terminus of PrsA2 is necessary for PC-PLC activity (3). Perhaps the propeptide of PC-PLC serves to slow down protein folding and to interact with the N terminus of PrsA2. The interaction with PrsA2 would be released only once the proprotein has reached its native conformation, which would explain the low rate of protein secretion and the difference in protein stability observed between the slowly and quickly secreted forms of proPC-PLC.
In conclusion, during intracytosolic growth, L. monocytogenes requires PrsA2 to maintain the Mpl zymogen and the proform of PC-PLC in close proximity within the bacterial cell envelope. This condition is imperative to rapidly generate and deliver active PC-PLC in vacuoles formed upon cell-to-cell spread. We hypothesize that PrsA2 regulates the compartmentalization and activity of these proenzymes by influencing cell wall properties and by interacting with the PC-PLC propeptide. This mechanism of regulating in a spatial and temporal manner the secretion of active virulence factors is similar, in principle, to the mechanisms used by Gram-negative bacteria to deliver type III and IV secretion system effectors into eukaryotic cells.
ACKNOWLEDGMENTS
We thank Nancy Freitag for providing us with strain NF-L1166, David Drubin and Georjana Barnes for allowing us to use the Horiba FluoroMax-3 fluorometer, Ben Kline for assistance in constructing pJZ095, and Chelsea Witte for assistance with the bacterial lysis luciferase assay. We also thank Alan Pavinski Bitar, Bryant Blank, and Gabriela Wagner for critically reading the manuscript.
This research was supported by National Institutes of Health grants AI27655 and AI063302 (D.A.P.) and AI52154 (H.M.). D.A.P. has a consulting relationship with and financial interest in Aduro Biotech.
Footnotes
Published ahead of print on 9 September 2011.
REFERENCES
- 1. Alonzo F., III, Freitag N. E. 2010. Listeria monocytogenes PrsA2 is required for virulence factor secretion and bacterial viability within the host cell cytosol. Infect. Immun. 78:4944–4957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alonzo F., III, Port G. C., Cao M., Freitag N. E. 2009. The posttranslocation chaperone PrsA2 contributes to multiple facets of Listeria monocytogenes pathogenesis. Infect. Immun. 77:2612–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alonzo F., III, Xayarath B., Whisstock J. C., Freitag N. E. 2011. Functional analysis of the Listeria monocytogenes secretion chaperone PrsA2 and its multiple contributions to bacterial virulence. Mol. Microbiol. 80:1530–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bakardjiev A. I., Stacy B. A., Fisher S. J., Portnoy D. A. 2004. Listeriosis in the pregnant guinea pig: a model of vertical transmission. Infect. Immun. 72:489–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bishop D. K., Hinrichs D. J. 1987. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139:2005–2009 [PubMed] [Google Scholar]
- 6. Bitar A. P., Cao M., Marquis H. 2008. The metalloprotease of Listeria monocytogenes is activated by intramolecular autocatalysis. J. Bacteriol. 190:107–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boneca I. G., et al. 2007. A critical role for peptidoglycan N-deacetylation in Listeria evasion from the host innate immune system. Proc. Natl. Acad. Sci. U. S. A. 104:997–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Braun P., Tommassen J., Filloux A. 1996. Role of the propeptide in folding and secretion of elastase of Pseudomonas aeruginosa. Mol. Microbiol. 19:297–306 [DOI] [PubMed] [Google Scholar]
- 9. Camilli A., Tilney L. G., Portnoy D. A. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 8:143–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duguay A. R., Silhavy T. J. 2004. Quality control in the bacterial periplasm. Biochim. Biophys. Acta 1694:121–134 [DOI] [PubMed] [Google Scholar]
- 11. Ellis R. J., van der Vies S. M. 1991. Molecular chaperones. Annu. Rev. Biochem. 60:321–347 [DOI] [PubMed] [Google Scholar]
- 12. Forster B. M., et al. 2011. The metalloprotease of Listeria monocytogenes is regulated by pH. J. Bacteriol. 193:5090–5097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freitag N. E., Rong L., Portnoy D. A. 1993. Regulation of the prfA transcriptional activator of Listeria monocytogenes: multiple promoter elements contribute to intracellular growth and cell-to-cell spread. Infect. Immun. 61:2537–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gaillard J. L., Berche P., Mounier J., Richard S., Sansonetti P. 1987. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect. Immun. 55:2822–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goffin C., Ghuysen J. M. 1998. Multimodular penicillin-binding proteins: an enigmatic family of orthologs and paralogs. Microbiol. Mol. Biol. Rev. 62:1079–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herbert S., et al. 2007. Molecular basis of resistance to muramidase and cationic antimicrobial peptide activity of lysozyme in staphylococci. PLoS Pathog. 3:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hodgson D. A. 2000. Generalized transduction of serotype 1/2 and serotype 4b strains of Listeria monocytogenes. Mol. Microbiol. 35:312–323 [DOI] [PubMed] [Google Scholar]
- 18. Hyyrylainen H. L., et al. 2010. Penicillin-binding protein folding is dependent on the PrsA peptidyl-prolyl cis-trans isomerase in Bacillus subtilis. Mol. Microbiol. 77:108–127 [DOI] [PubMed] [Google Scholar]
- 19. Kamisango K., et al. 1982. Structures and biological activities of peptidoglycans of Listeria monocytogenes and Propionibacterium acnes. J. Biochem. 92:23–33 [DOI] [PubMed] [Google Scholar]
- 20. Kristian S. A., et al. 2005. d-Alanylation of teichoic acids promotes group A Streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J. Bacteriol. 187:6719–6725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laible N. J., Germaine G. R. 1985. Bactericidal activity of human lysozyme, muramidase-inactive lysozyme, and cationic polypeptides against Streptococcus sanguis and Streptococcus faecalis: inhibition by chitin oligosaccharides. Infect. Immun. 48:720–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lauer P., Chow M. Y., Loessner M. J., Portnoy D. A., Calendar R. 2002. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J. Bacteriol. 184:4177–4186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loessner M. J., Kramer K., Ebel F., Scherer S. 2002. C-terminal domains of Listeria monocytogenes bacteriophage murein hydrolases determine specific recognition and high-affinity binding to bacterial cell wall carbohydrates. Mol. Microbiol. 44:335–349 [DOI] [PubMed] [Google Scholar]
- 24. Loessner M. J., Schneider A., Scherer S. 1996. Modified Listeria bacteriophage lysin genes (ply) allow efficient overexpression and one-step purification of biochemically active fusion proteins. Appl. Environ. Microbiol. 62:3057–3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marie-Claire C., Ruffet E., Beaumont A., Roques B. P. 1999. The prosequence of thermolysin acts as an intramolecular chaperone when expressed in trans with the mature sequence in Escherichia coli. J. Mol. Biol. 285:1911–1915 [DOI] [PubMed] [Google Scholar]
- 26. Marquis H., Doshi V., Portnoy D. A. 1995. The broad-range phospholipase C and a metalloprotease mediate listeriolysin O-independent escape of Listeria monocytogenes from a primary vacuole in human epithelial cells. Infect. Immun. 63:4531–4534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marquis H., Goldfine H., Portnoy D. A. 1997. Proteolytic pathways of activation and degradation of a bacterial phospholipase C during intracellular infection by Listeria monocytogenes. J. Cell Biol. 137:1381–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marquis H., Hager E. J. 2000. pH-regulated activation and release of a bacteria-associated phospholipase C during intracellular infection by Listeria monocytogenes. Mol. Microbiol. 35:289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Masschalck B., Michiels C. W. 2003. Antimicrobial properties of lysozyme in relation to foodborne vegetative bacteria. Crit. Rev. Microbiol. 29:191–214 [DOI] [PubMed] [Google Scholar]
- 30. Miner M. D., Port G. C., Freitag N. E. 2008. Functional impact of mutational activation on the Listeria monocytogenes central virulence regulator PrfA. Microbiology 154:3579–3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O'Neil H. S., et al. 2009. The propeptide of the metalloprotease of Listeria monocytogenes controls compartmentalization of the zymogen during intracellular infection. J. Bacteriol. 191:3594–3603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O'Neil H. S., Marquis H. 2006. Listeria monocytogenes flagella are used for motility, not as adhesins, to increase host cell invasion. Infect. Immun. 74:6675–6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perego M., et al. 1995. Incorporation of D-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis. Identification of genes and regulation. J. Biol. Chem. 270:15598–15606 [DOI] [PubMed] [Google Scholar]
- 34. Sarvas M., Harwood C. R., Bron S., van Dijl J. M. 2004. Post-translocational folding of secretory proteins in Gram-positive bacteria. Biochim. Biophys. Acta 1694:311–327 [DOI] [PubMed] [Google Scholar]
- 35. Sauer J. D., et al. 2010. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe 7:412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shetron-Rama L. M., Marquis H., Bouwer H. G., Freitag N. E. 2002. Intracellular induction of Listeria monocytogenes actA expression. Infect. Immun. 70:1087–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shetron-Rama L. M., et al. 2003. Isolation of Listeria monocytogenes mutants with high-level in vitro expression of host cytosol-induced gene products. Mol. Microbiol. 48:1537–1551 [DOI] [PubMed] [Google Scholar]
- 38. Slepkov E. R., Pavinski Bitar A., Marquis H. 2010. Differentiation of propeptide residues regulating the compartmentalization, maturation and activity of the broad-range phospholipase C of Listeria monocytogenes. Biochem. J. 432:557–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smith G. A., et al. 1995. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect. Immun. 63:4231–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Snyder A., Marquis H. 2003. Restricted translocation across the cell wall regulates secretion of the broad-range phospholipase C of Listeria monocytogenes. J. Bacteriol. 185:5953–5958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sun A. N., Camilli A., Portnoy D. A. 1990. Isolation of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect. Immun. 58:3770–3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tilney L. G., Portnoy D. A. 1989. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J. Cell Biol. 109:1597–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vazquez-Boland J. A., et al. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vitikainen M., et al. 2004. Structure-function analysis of PrsA reveals roles for the parvulin-like and flanking N- and C-terminal domains in protein folding and secretion in Bacillus subtilis. J. Biol. Chem. 279:19302–19314 [DOI] [PubMed] [Google Scholar]
- 45. Yeung P. S., Na Y., Kreuder A. J., Marquis H. 2007. Compartmentalization of the broad-range phospholipase C activity to the spreading vacuole is critical for Listeria monocytogenes virulence. Infect. Immun. 75:44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yeung P. S., Zagorski N., Marquis H. 2005. The metalloprotease of Listeria monocytogenes controls cell wall translocation of the broad-range phospholipase C. J. Bacteriol. 187:2601–2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zemansky J., et al. 2009. Development of a mariner-based transposon and identification of Listeria monocytogenes determinants, including the peptidyl-prolyl isomerase PrsA2, that contribute to its hemolytic phenotype. J. Bacteriol. 191:3950–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]