Abstract
The IE2 86 protein of human cytomegalovirus (HCMV) is essential for productive infection. The mutation of glutamine to arginine at position 548 of IE2 86 causes the virus to grow both slowly and to very low titers, making it difficult to study this mutant via infection. In this study, Q548R IE2 86 HCMV was produced on the complementing cell line 86F/40HA, which allowed faster and higher-titer production of mutant virus. The main defects observed in this mutant were greatly decreased expression of IE2 40, IE2 60, UL83, and UL84. Genome replication and the induction of cell cycle arrest were found to proceed at or near wild-type levels, and there was no defect in transitioning to early or late protein expression. Q548R IE2 86 was still able to interact with UL84. Furthermore, Q548R IE2 40 maintained the ability to enhance UL84 expression in a cotransfection assay. Microarray analysis of Q548R IE2 HCMV revealed that the US8, US9, and US29-32 transcripts were all significantly upregulated. These results further confirm the importance of IE2 in UL83 and UL84 expression as well as pointing to several previously unknown regions of the HCMV genome that may be regulated by IE2.
INTRODUCTION
Human cytomegalovirus (HCMV) is a large, double-stranded-DNA virus in the betaherpesvirus family. Primary infection in an immunocompetent individual can sometimes cause a mononucleosis-like syndrome, but acute infection is typically managed quickly by the immune system. In individuals with compromised immune systems (e.g., those who have undergone organ or hematopoietic stem cell transplantation or those with HIV/AIDS), HCMV is the cause of significant morbidity and mortality. HCMV is furthermore the number one viral cause of birth defects (22).
Lytic replication of HCMV occurs in three main stages of tightly controlled sequential events (22). The immediate early (IE) stage of infection entails creating a cellular environment that allows efficient viral replication. Viral DNA replication occurs during the early stage of infection, and production of IE proteins is required to initiate early protein synthesis. Finally, the late stage involves synthesis of mainly virion structural proteins.
The UL122-UL123 region of the HCMV genome is termed the major immediate early (MIE) gene locus (22). This region contains five exons and two polyadenylation signals. The MIE transcript is alternatively spliced to create the two major immediate early gene products, IE1 72 and IE2 86. Translation of each of these products starts at exon 2. The IE1 72 product consists of exons 2 to 4. The IE2 86 product shares exons 2 and 3 with IE1 72 but contains exon 5 rather than exon 4. Exon 5 of IE2 also produces two unspliced variants with late kinetics, termed IE2 40 and IE2 60, each of which has its own promoter (26). IE2 86 is an essential protein for productive infection, and when exon 5 is deleted in recombinant viruses, neither early nor late gene expression can occur (20, 30). The functions of IE2 86 include negative autoregulation of the MIE promoter and transactivation of viral early promoters (37).
It has been shown that HCMV halts the cell cycle at the G1/S transition, and it is thought that IE2 86 may play a role in this arrest (37). Because HCMV infects terminally differentiated cells that tend to be quiescent, the virus has developed multiple methods of pushing quiescent cells into a pseudo-G1/S state in order to take advantage of cellular DNA replication machinery (2, 4, 7, 13, 17, 27, 29, 43–45). In HCMV-infected cells, the tumor suppressor protein p53 is stabilized and the Rb family of proteins, which regulates transcription in complex with E2F in a cell cycle-dependent manner, becomes hyperphosphorylated and begins to accumulate (8, 13, 21, 23). It has been demonstrated that IE2 86 upregulates several E2F-responsive genes, which are responsible for pushing the cell into S phase (35). Furthermore, HCMV infection upregulates cyclin E expression, which is also essential for pushing the cell into S phase, and transfection studies have shown that IE2 86 can bind to and activate the cyclin E promoter (3).
The glutamine at position 548 of IE2 was found to be highly conserved in CMV IE2 homologs, and its mutation to arginine resulted in a severely debilitated virus that grew both slowly and to very low titers. This mutant, initially developed and characterized by Petrik et al. (25), appeared to maintain several of the known functions of IE2 86, including negative autoregulation of the MIE promoter, transactivation of viral early genes, and upregulation of E2F-responsive genes, and it was determined that the major defect of the Q548R IE2 virus was an apparent inability to halt the cell cycle. Given that all previous analyses of the role of IE2 86 in cell cycle regulation were transfection based, this study by Petrik et al. offered the first infection-based evidence that IE2 86 played a role in cell cycle regulation.
A thorough understanding of IE2 86 functions could be important for the development of antiviral therapies. However, as IE2 86 is an essential protein, it has been extremely difficult to create IE2 86 mutants capable of producing viral progeny that can then be studied in the context of infection. Thus, many of the studies of IE2 function have been conducted via transfection and in vitro methods. Importantly, in cases where the mutant virus is severely debilitated yet still capable of growth, there is the increased probability of developing compensatory secondary mutations, as there is selection for the most fit virus during preparation of viral stocks. In order to aid in the study of IE2 function during infection and reduce the probability of secondary mutations, the Spector lab has developed a cell line termed 86F/40HA that is capable of complementing mutant IE2 86 (30). This cell line generates wild-type (WT) IE2 86 and IE2 40 upon infection with a virus that expresses Cre and FLP recombinases. Thus, mutant IE2 viruses that are severely debilitated or fully incapable of viral production can be grown as viral stocks on these cells, and replication of the mutant virus can be studied following infection of noncomplementing cells.
The goal of this study was to further examine the Q548R IE2 86 recombinant virus because of its potential to elucidate the role of IE2 86 in cell cycle regulation. Although the Q548R IE2 mutant is capable of productive infection, its slow growth and reduced titers both severely limit the extent to which it can be studied via infection and greatly increase the probability of secondary mutations. By growing the Q548R IE2 virus in 86F/40HA cells, we were able to produce virus in high enough titers to be able to study it more thoroughly in the context of infection and further examine its potential role in cell cycle regulation.
MATERIALS AND METHODS
Cells.
Human foreskin fibroblasts (HFFs) were obtained from the University of California, San Diego, Medical Center. Medium supplements for both HFFs and the 86F/40HA cells are described elsewhere (30). 293FT cells were grown according to the manufacturer's instructions (Invitrogen), and medium supplements are described elsewhere (32). All cells were incubated at 37°C with 7% CO2.
BAC mutagenesis and reconstitution of recombinant viruses.
In order to function in the 86F/40HA-complementing cell line, any recombinant IE2 mutant bacterial artificial chromosomes (BACs) must also express Cre under the control of the mIE promoter and FLP under the control of the 1.2-kb promoter. To allow homologous recombination of IE-Cre and 1.2-FLP into the WT and Q548R IE2 Towne BACs (both generous donations from M. Stinski), a plasmid containing the Towne US14-15 regions was made. The following primers were designed to amplify the US14-15 regions from the Towne BAC with EcoRV restriction sites on the ends: CGATATCGGCGATGTGAAAGACCACTAGG and CGATATCTGCTCCTCTTCCAAATCTCCG. This fragment was then cloned into the EcoRV site of pACYC184 to make pACYC-US14-15. Mutagenesis with a QuikChange kit was then used to add SpeI, PmeI, and BglII restriction sites to the intergenic region between US14 and US15. The 1.2-FLP cassette was isolated from the previously described plasmid pFB1:1.2-FLP (30). The 1.2-FLP cassette was then subcloned into the BglII site of pACYC-US14-15 to make the vector pACYC-US14-1.2-FLP-US15. Next, the ampicillin resistance gene (Amp) and its promoter were PCR amplified from the vector pcDNA3 and subcloned into pACYC-US14-1.2-FLP-US15 using the PmeI site to make pACYC-US14-1.2-FLP/Amp-US15. Finally, the IE-Cre cassette was isolated from the previously described pFB1:IE-Cre plasmid (30), and SpeI sites were used to subclone the cassette into pACYC-US14-1.2-FLP/Amp-US15, yielding the final vector, pACYC-US14-1.2-FLP/Amp/IE-Cre-US15. The following primers were used to amplify a cassette from pACYC-US14-1.2-FLP/Amp/IE-Cre-US15 that contained the 1.2-FLP, Amp, and IE-Cre genes flanked by 200 bp homologous to US14 and US15 on either end: AAGCGGTTTCCAGCGTCAGCAATC and AATCGTGGTGGTCATCTTGAGGCG. This linear fragment was used for recombination into the WT Towne and Q548R IE2 Towne BACs in the DY380 cells as previously described (15). Resistance to chloramphenicol and ampicillin was used to select BACs. Restriction endonuclease digestion and field inversion gel electrophoresis (FIGE) were used to analyze the resulting BAC clones (data not shown). BACs that demonstrated the appropriate restriction digest patterns were amplified and purified as previously described (28).
In order to make the Q548R IE2 mutation in the Cre/FLP-containing HB5 background, we conducted a two-step recombination with the pENTR-1a system (Invitrogen). The pENTR-1a vector contains the ccdB bacterial toxin gene as well as the kanamycin resistance gene. The pENTR-1a vector was grown in DB3.1 cells (Invitrogen), which express ccdA, encoding the ccdB resistance protein. The ccdB and kanamycin genes were PCR amplified from pENTR-1a with a 72-mer and 74-mer primer that contained 50 bp homologous to the region of the IE2 gene surrounding the codon for amino acid 548 (nt 170340 to 170389 and nt 170401 to 170451 of the AD169 genome; accession no. BK000394.5). This linear 1.8-kb fragment was then used for the first recombination into the previously described WT C-F HB5 BAC (30) in DB3.1 cells that also contained the previously described recombinase expressing pBAD-RedGam plasmid (24). Resistance to chloramphenicol and kanamycin was used to select BACs. Restriction digestion and FIGE were used to analyze the resulting BAC clones. Several BACs were then electroporated into DH10B cells to check for the biological activity of the ccdB gene. A clone that exhibited no growth in DH10B cells was chosen for second-step recombination. For the second step, a 182-bp fragment containing the Q548R IE2 mutation was PCR amplified from the Q548R IE2 Towne BAC with the primers CAGGTCATGGTGCGCATCTTCTCC and CTGAGACTTGTTCCTCAGGTCCTG. This fragment was then used for the second recombination step in DB3.1 cells containing the IE2-ccdB-Kan HB5 BAC as well as the pBAD-RedGam plasmid. After recombination, DNA was isolated from the DB3.1 cells and transferred to DH10B cells. Colonies were selected for the ability to grow in DH10B cells as well as the absence of kanamycin resistance and analyzed via restriction digestion and FIGE. The resulting construct, Q548R IE2 C-F HB5 BAC, exhibited the appropriate restriction digest pattern, and the presence of the Q548R mutation was confirmed via sequencing.
Reconstitution of the Q54R IE2 C-F Towne and the Q548R IE2 C-F HB5 BACs was accomplished as previously described (30). Briefly, 6.25 μg of each BAC was electroporated into HFF or 86F/40HA cells along with 3.75 μg of pcDNA-pp71tag. Supernatant was harvested when the cultures reached 100% cytopathic effect (CPE). Virus titer was determined by plaque assay. The WT C-F Towne and Q548R IE2 C-F Towne BACs also contain a simian virus 40 (SV40) promoter-driven green fluorescent protein (GFP) that allowed the monitoring of cultures posttransfection via fluorescence microscopy.
In order to make a revertant virus from the Q548R IE2 C-F HB5 virus, an ∼1.5-kb fragment of WT IE2 was PCR amplified from the WT C-F HB5 BAC with the primers GCCCGATGAAGATAGTTCC and CGGGGTAACCAAAGAAATC. This fragment (5 μg) was then coelectroporated with Q548R IE2 C-F HB5 BAC (6.25 μg) and the pcDNA-pp71tag plasmid (3.75 μg) into HFFs. After plaques became apparent in the culture, supernatant was harvested, and a limiting-dilution assay was set up to isolate individual plaques. Extracellular virus from wells with individual plaques was harvested and further expanded, and viral DNA was isolated from the media (QIAamp DNA blood minikit; Qiagen). The viral DNA was then analyzed via PCR and sequencing. As a final test, viral DNA was isolated from cells infected with the revertant clones using the modified Hirt method. This DNA was transformed into bacteria, isolated, and analyzed by restriction digestion and FIGE to check for major rearrangements of the viral genome. This virus is referred to as Rev-Q548R IE2 C-F HB5 herein.
Cell synchronization and infection.
All experiments with HFFs were performed under G0 synchronization conditions with contact-inhibited cells released into G1 at the time of infection as previously described (27).
293FT cell transfection and construction of transfected plasmids.
The pcDNA3-86F and pcDNA3-40HA plasmids have been described (30). Mutagenesis with a QuikChange kit was used to create the pcDNA3-Q548R-86F and pcDNA3-Q548R-40HA plasmids. The pcDNA3-UL84HA plasmid has also been described (32). 293FT cells were transfected and harvested as previously described (32). Protein was isolated from cells with a Norgen RNA/DNA/protein purification kit.
Western blot analyses.
HFFs were harvested at various times postinfection (p.i.) as described for each experiment. Protein was isolated from cells using the Norgen DNA/RNA/protein purification kit. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot transfer conditions are described elsewhere (32). The geminin polyclonal antibody FL-209 was purchased from Santa Cruz Biotechnology. The monoclonal antibody (MAb) CH160 and MAbs for UL83, UL84, UL44, UL57, and UL99 were purchased from Virusys and the Goodwin Institute; the IE2 MAb 8140 was purchased from Chemicon; the β-actin MAb Ac15 was purchased from Sigma-Aldrich; UL32, UL85, and UL86 MAbs were kind gifts from B. Britt; UL82 MAb was a kind gift from T. Shenk.
BrdU assay.
HFFs were infected at a multiplicity of infection (MOI) of 1 with either WT C-F HB5 or Q548R IE2 C-F HB5 or were mock infected and were then seeded onto coverslips. At 24 h p.i., cells were treated with 10 μM bromodeoxyuridine (BrdU; Sigma). Cells were fixed at 48 h p.i. with 2% formaldehyde in phosphate-buffered saline (PBS) for 10 min. Next, cells were permeabilized with 0.2% Triton X-100 in PBS for 5 min and then washed in PBS. Cells were then blocked in 10% normal goat serum (Jackson ImmunoResearch) in PBS for 20 min. Next, cells were incubated with IE1 MAb (Virusys) for 20 min. Cells were washed in PBS and then incubated with a goat anti-mouse IgG2a antibody coupled to tetramethyl rhodamine isothiocyanate (Southern Biotech) for 20 min, after which cells were washed again in PBS. Next, in order to visualize the BrdU, cells were treated with 4 N HCl for 10 min to denature the DNA, washed in PBS, and incubated with the BrdU MAb (Sigma). Cells were then washed and incubated with a goat anti-mouse IgG1 antibody coupled to fluorescein isothiocyanate (Southern Biotech) along with Hoechst stain. Finally, cells were washed in PBS before being mounted over SlowFade Gold (Molecular Probes). Cells were analyzed using a DeltaVision deconvolution microscopy system (Applied Precision) on a Silicon Graphics O2 workstation. Images were taken with a Photometrics charge-coupled-device camera mounted on a fluorescence/differential interference contrast microscope. Images were then deconvoluted using DeltaVision SoftWoRx programs. Adobe Photoshop was used to prepare images for figures.
Immunoprecipitation assay.
HFFs were infected at an MOI of 1 with either WT C-F HB5 or Q548R IE2 C-F HB5 or mock infected and were then harvested via trypsinization at 48 and 96 h p.i. Immunoprecipitation (IP) was carried out as previously described (31). Briefly, 4 × 105 HFFs/sample were immunoprecipitated for CH160. Pre-IP, IP, and post-IP lysates were loaded on an 8% acrylamide gel and separated via SDS-PAGE. Proteins were then transferred onto a nitrocellulose membrane and probed for UL84 and CH160.
Microarray analysis.
HFFs (3 × 106 cells/sample) were infected at an MOI of 2 with either WT C-F HB5 or Q548R IE2 C-F HB5 or mock infected. WT-infected cells were harvested at 48 and 96 h p.i., Q548R-infected cells were harvested at 48, 96, and 168 h p.i., and mock-infected cells were harvested at 96 h p.i. This set of infections was carried out three times to create three biological replicates. RNA was extracted, amplified, and Cy5 labeled as previously described (11).
The 18 Cy5-labeled RNA samples (6 samples with 3 replicates of each) were hybridized to a Combimatrix custom microarray. The custom design for this array was generously donated by E. Fortunato and was described previously (11). Each Combimatrix slide contains four identical arrays with 2,240 features, of which 305 are control features, 1,265 are cellular gene features, and 670 are viral gene features. Each gene on the array is represented by at least three features. The 18 samples were assigned at random to slides to account for any possible slide-to-slide differences in handling. After hybridization and washing of slides according to Combimatrix instructions, slides were scanned in a Genepix 4000B microarray scanner (Molecular Devices). Raw fluorescence data were acquired from the scanned images using the Combimatrix microarray imager software.
Arrays were normalized to account for sample-to-sample differences in loading and dye incorporation by the BIOGEM core at the University of California, San Diego. The change (fold) in the same feature between two different samples was calculated, and the t value of a two-tailed Student t test with a 95% confidence interval was used to calculate the P value for each feature. A gene was counted as demonstrating a significant change between samples if it met two criteria: first, more than 50% of a gene's features needed to have P values of less than 0.05, and second, the fold change averaged across all of a gene's features needed to be greater than 2.
Quantitative real-time PCR and reverse transcription-PCR (RT-PCR) analyses.
DNA for quantitative real-time PCR was isolated with a Norgen DNA/RNA/protein purification kit, and samples were analyzed for levels of viral DNA using primers and probes specific to the UL77 gene as previously described (42). DNA levels were normalized to levels of the cellular glyceraldehyde 6-phosphate dehydrogenase promoter as previously described (42).
RNA was isolated with the Norgen DNA/RNA/protein purification kit, and samples were treated with Turbo DNase (Ambion) to ensure lack of DNA contamination. The quantitative real-time RT-PCR analysis of RNAs for IE1 72, IE2 86, UL83, UL84, and the cellular housekeeping gene for glucose 6-phosphate dehydrogenase (G6PD) was carried out as previously described (40). The primers and TaqMan probes for the C terminus of IE2 that probes for IE2 86, IE2 60, and IE2 40 have been described (32).
RNA for the analysis of US8, US9 and US29-32 was taken from the total RNA samples used for microarray analysis that had been isolated with an SV total RNA isolation kit (Promega). An 18-mer oligo(dT) was annealed to 1 μg of total RNA and was then used to make concentrated cDNA with ThermoScript reverse transcriptase (Invitrogen) according to the manufacturer's instructions. SYBR green PCR Master Mix (Applied Biosystems) was then used to set up the quantitative PCR according to the manufacturer's instructions. The primers used for this experiment are listed in Table 1.
Table 1.
qPCR primers for confirmation of significant microarray genes
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| US8 | GCCTCGGTACCATATACGTT | CACACTTTTGGGGTACACAG |
| US9 | GTACTCTCGAGCCGCTCCAA | GGCCGTATCGGAGAAGTTGC |
| US29 | ATACTCGTGAGCGGTTACGG | GGTGATAACCAGTTGCTCAG |
| US30 | CAATATCATCTGGTGGACCG | GCAGTTTGCCTTCAGAAACG |
| US31 | CGACTACTCGCACAACCTGT | ACCCGAGGAATACGCCGTAC |
| US32 | GAGAGCACCTCTATTGTGTG | ACCTGTTGCTCTGGCGCTTC |
RESULTS
Growth of Q548R IE2 86 virus is severely debilitated in HFFs but is effectively complemented in 86F/40HA cells.
The Q548R IE2 86 virus was characterized previously and was thought to exhibit defects in the ability of HCMV to halt the cell cycle (25). However, because this virus grows extremely slowly and only to very low titers, it is difficult to produce enough virus to study via infection. In order to further study this mutant and its possible effect on the cell cycle in the context of infection, the Q548R IE2 Towne BAC was modified so that virus could be propagated in the previously described complementing cell line 86F/40HA (30). Expression of Cre recombinase in this cell line allows the production of Flag-tagged IE2 86. Similarly, expression of FLP recombinase allows the production of hemagglutinin (HA)-tagged IE2 40, a protein with late kinetics that is encoded by exon 5 of the IE2 86 gene and shares the 337 C-terminal amino acids with IE2 86. This cell line is thus able to compensate for defects that are shared by IE2 86 and IE2 40, like Q548R. To modify the Q548R IE2 Towne BAC and the WT Towne BAC (both BACs were generously provided by M. Stinski), a cassette carrying the Cre gene under the direction of the major immediate early (MIE) promoter, the FLP gene under the direction of the early-late 1.2-kb promoter, and the ampicillin resistance gene was inserted between US14 and US15 of both the WT Towne BAC and the Q548R IE2 Towne BAC, yielding the WT C-F Towne BAC and the Q548R IE2 C-F Towne BAC.
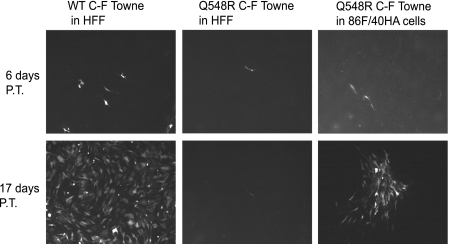
WT C-F Towne BAC was then transfected into HFF cells, and Q548R IE2 C-F Towne BAC was transfected into both HFF and 86F/40HA cells. The vector backbone of this Towne BAC contains an SV40 promoter-driven GFP gene, which was used to monitor the progression of the infection posttransfection. At 6 days posttransfection (p.t.), scattered GFP-positive cells were visible in the WT C-F Towne-transfected HFFs as well as in the Q548R IE2 C-F Towne-transfected 86F/40HA cells (Fig. 1). A few dimly GFP-expressing cells were visible in the Q548R IE2 C-F Towne-transfected HFFs. By 17 days p.t., the WT C-F Towne virus had spread throughout the culture and the Q548R IE2 C-F Towne-transfected 86F/40HA cells had developed multiple GFP-expressing plaques. In contrast, the Q548R IE2 C-F Towne-transfected HFFs still showed only scattered, dimly GFP-expressing cells with no evidence of plaque formation. The Q548R IE2 C-F Towne-transfected HFFs were maintained in culture for several weeks with weekly 1:1 passage to determine if the infection was capable of spreading in noncomplementing cells. Cytopathic effect (CPE) in the culture developed very slowly. Only at 12 weeks posttransfection did the majority of cells display CPE. In contrast, by 2 weeks p.t., WT-transfected cells exhibited 100% CPE, and by 4 weeks p.t., Q548R IE2-transfected 86F/40HA cells had 100% CPE. Thus, the severely debilitated growth of the Q548R IE2 mutation found by Petrik et al. (25) appeared to be reproduced here, and the 86F/40HA cell line was capable of complementing the Q548R IE2 mutation.
Fig. 1.
Growth of Q548R IE2 C-F Towne BAC is greatly debilitated in HFFs and is partially restored in 86F/40HA cells. WT C-F Towne BAC was transfected into HFFs, and Q548R IE2 C-F Towne BAC was transfected into both HFF and 86F/40HA cells. All BAC transfections were cotransfected with a plasmid expressing pp71 (UL82). Both BACs express an SV40 promoter-driven GFP, and this was used to monitor infection progress via fluorescence microscopy. Images were taken at 6 and 17 days posttransfection (P.T.).
Because the Q548R IE2 Towne virus showed barely any growth in HFFs, we wanted to further examine this mutant in the AD169 genome-containing pHB5 BAC background. Several other IE2 mutants have been characterized in this background (28, 30, 31, 40–42), thus allowing us to more easily compare the phenotype of the Q548R IE2 mutant to others. The Q548R IE2 mutation was inserted into the previously described Cre-FLP-containing HB5 BAC (30) via homologous recombination. A rescued virus of the Q548R IE2 C-F BAC was also made, termed Rev-Q548R IE2 C-F HB5.
In order to examine the ability of Q548R IE2 C-F HB5 to spread and produce infectious virus, a single-cycle infection was performed in which cells were infected and allowed to go through a single cycle of viral replication, after which extracellular virus was harvested and titers were determined. HFF and 86F/40HA cells were infected with Q548R C-F HB5 (MOI = 1), and extracellular virus was harvested at 5 days p.i. Extracellular virus titers were then determined via plaque assay on the complementing 86F/40HA cells. HFFs produced 100-fold less virus than 86F/40HA cells (2.05 × 102 ± 1.9 × 102 versus 5.02 × 104 ± 5.79 × 104), demonstrating that the Q548R IE2 mutation is also severely debilitated in the HB5 background. This study further demonstrated that Q548R IE2 recombinant virus grown on the 86F/40HA complementing cell line, and subsequently studied on noncomplementing HFFs, maintains its severely debilitated phenotype.
The Q548R IE2 mutant is not defective in genome replication or geminin stabilization.
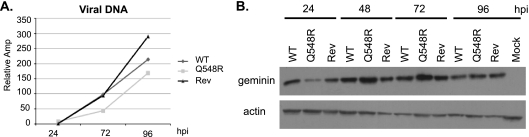
In order to maximize its ability to replicate its own genome, HCMV halts the cell cycle at the G1/S transition. In this pseudo-G1/S state, the cell expresses DNA replication factors without undergoing DNA replication itself. This allows HCMV to make use of these replication factors without needing to “share” them with the host cell. The previous characterization of the Q548R IE2 virus demonstrated an apparent inability of this mutant to halt the cell cycle at the G1/S transition. In order to determine whether viral genome replication was hindered in the supposed absence of cell cycle arrest, viral DNA levels were measured at various times postinfection. G0-synchronized HFFs were either mock infected or infected with WT, Q548R IE2, or Rev-Q548R IE2 C-F HB5 at an MOI of 1. Cells were then harvested at 24, 48, 72, and 96 h p.i. and the RNA, DNA, and protein were isolated. The UL77 gene was used to test viral genome levels of the 24, 72, and 96 h p.i. samples via quantitative PCR, and results were normalized to levels of the cellular glyceraldehyde 3-phosphate dehydrogenase promoter. Although there was a lag compared to WT infection, indicating about twofold less viral DNA present in the mutant at 48 h p.i., the Q548R virus was still able to replicate its genome to near WT levels by 96 h p.i. (Fig. 2A).
Fig. 2.
Q548R IE2 C-F HB5 virus is capable of genome replication and geminin stabilization. G0-synchronized HFFs were infected at the time of release into G1 with either WT C-F HB5, Q548R IE2 C-F HB5, or Rev-Q548R IE2 C-F HB5 (MOI = 1) or mock infected. Cells were harvested at the indicated times. (A) DNA from the 24-, 72-, and 96-h p.i. samples was isolated and analyzed via quantitative PCR of the UL77 gene. UL77 levels were normalized to levels of the cellular glyceraldehyde 3-phosphate dehydrogenase promoter in each sample. Values are relative amplification (Amp), with the lowest value set to 1. The experiment was carried out three times, and representative results are displayed. (B) Protein was isolated from the 24-, 48-, 72-, and 96-h p.i. samples. Equivalent amounts of protein were loaded on an 8% acrylamide gel and transferred to a nitrocellulose membrane. The membrane was then probed for geminin, and β-actin served as a loading control.
Next, we examined the cellular gene for geminin, which inhibits cellular DNA replication before the cell enters mitosis. Geminin is normally degraded by the anaphase-promoting complex and is stabilized by HCMV infection (2, 45). Protein from the 24, 48, 72, and 96 h p.i. samples was examined via Western blot and probed for geminin. Although there appears to be a slight lag of geminin levels in Q548R IE2-infected cells, the mutant has reached WT levels of geminin stabilization by 48 h p.i. (Fig. 2B). Geminin expression was also examined in infection with the Q548R IE2 C-F Towne BAC, and comparable stabilization was seen (data not shown).
Q548R IE2 is capable of halting the cell cycle.
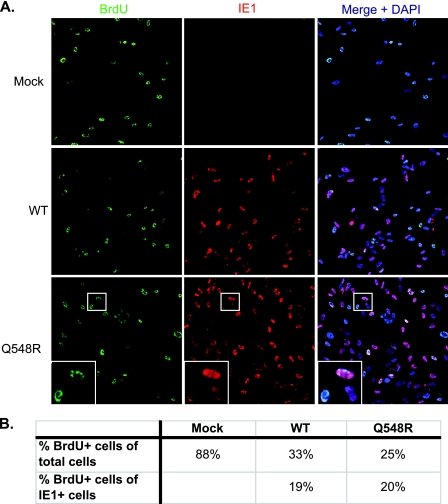
Given that the Q548R IE2 mutant was able to replicate its genome and stabilize geminin, a BrdU assay was set up to determine whether Q548R IE2 was halting the cell cycle. G0-synchronized HFFs were infected at the time of release into G1 with WT C-F HB5 or Q548R IE2 C-F HB5 at an MOI of 1 or were mock infected. BrdU (10 μM) was added to the cell medium at 24 h p.i., and cells were fixed at 48 h p.i. Cells were then costained for BrdU and IE1 and examined via immunofluorescent microscopy (Fig. 3A). BrdU-positive cells were counted, as well as cells that were positive for both IE1 and BrdU. Cells that contained BrdU organized in discrete nuclear compartments were assumed to be exhibiting viral DNA replication in the viral replication centers and were not counted as BrdU-positive cells. Cells were counted over two experiments, and the percentages in Fig. 3B are averages of the two. Cells that exhibited a diffuse nuclear BrdU labeling pattern were counted as BrdU-positive cells that had undergone cellular DNA replication. The insets in the Q548R row of Fig. 3 each show three cells: the upper cell is strongly IE1 positive and contains a pattern of discrete nuclear spots typical of viral replication center formation; the lower left cell has a diffuse nuclear BrdU stain and a very dim IE1 stain; the lower right cell is IE1 positive with no visible BrdU staining. Taken together, the results of two independent experiments showed that 88% of mock-infected HFFs were BrdU positive (containing diffuse nuclear staining), compared to 33% of WT-infected cells and 25% of Q548R IE2 infected cells. Of WT-infected cells that were IE1 positive, 19% were also BrdU positive, compared to 20% of Q548R-infected cells (Fig. 3B). Based on these results as well as the mutant's ability to undergo normal genome replication and geminin stabilization, we concluded that the Q548R IE2 mutant virus is in fact capable of halting the cell cycle.
Fig. 3.
Q548R IE2 C-F HB5 is capable of halting the cell cycle. (A) HFFs were mock infected or infected with WT C-F HB5 or Q548R IE2 C-F HB5 (MOI = 1). BrdU (10 μM) was added to the cell medium at 24 h p.i., and cells were fixed in 2% formaldehyde at 48 h p.i. Cells were then incubated with antibodies to BrdU and IE1 as described in Materials and Methods. Next, cells were incubated with appropriate TRITC (tetramethyl rhodamine isothiocyanate)- and FITC (fluorescein isothiocyanate)-conjugated secondary antibodies in addition to Hoechst stain (DAPI [4′,6-diamidino-2-phenylindole]) to visualize nuclei. Cells were then examined via immunofluorescent microscopy. (B) BrdU-positive cells were counted, as well as cells that were positive for both IE1 and BrdU. Cells were counted over two experiments, and at least 500 cells per sample per experiment were counted. Cells that exhibited a diffuse nuclear BrdU labeling pattern were counted as BrdU-positive cells that had undergone cellular DNA replication. Cells that contained BrdU organized in discrete nuclear compartments were assumed to be exhibiting viral DNA replication in the viral replication centers and were not counted as BrdU-positive cells.
Expression of IE2 40, IE2 60, UL83, UL84, and UL99 proteins is greatly downregulated in the Q548R IE2 mutant.
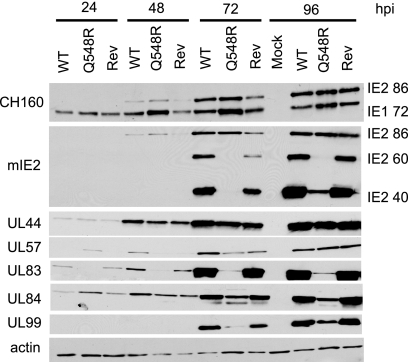
Since it was now apparent that the reason for this mutant's severely debilitated growth was not an inability to halt the cell cycle, the expression kinetics of several viral genes was examined to determine if a more obvious defect was present. Noncomplementing HFFs were mock infected or infected with WT C-F HB5, Q548R IE2 C-F HB5, or Rev-Q548R IE2 C-F HB5 virus at an MOI of 0.5. Cells were harvested at 24, 48, 72, and 96 h p.i., and cell lysates were examined via Western blotting (Fig. 4). Expression of IE2 86 remained relatively normal in the mutant, although it appeared to be at slightly higher levels at 48 h p.i. The levels of IE1 72 were also higher at all time points in the mutant-infected cells, indicating a possible defect in the ability of Q548R IE2 to interact with the cis repression signal (CRS). UL44 and UL57, early proteins involved in viral DNA replication, exhibited normal expression levels and kinetics, indicating that Q548R IE2 86 retains its ability to transactivate viral early gene expression. The early-late protein UL84, the only viral protein known to interact with IE2, shows evidence of significantly reduced protein expression starting at 72 h p.i. in the mutant. The viral proteins most affected by the mutant were the late proteins IE2 40, IE2 60, UL83, and UL99, all of which had greatly diminished expression in the Q548R IE2 mutant compared to WT.
Fig. 4.
IE2 60, IE2 40, UL83, and UL84 protein expression is greatly reduced in Q548R C-F IE2 HB5 infection. HFFs were mock infected or infected with either WT C-F HB5, Q548R IE2 C-F HB5, or Rev-Q548R IE2 C-F HB5 (MOI = 0.5). Cells were harvested at the indicated times. Equivalent amounts of protein were loaded onto an 8% acrylamide gel and then transferred to a nitrocellulose membrane. The membrane was probed with an antibody against both IE1 72 and IE2 86 (CH160), an antibody against IE2 86, IE2 60, and IE2 40 (mIE2), and antibodies against UL44, UL57, UL83 (pp65), UL84, and UL99 (pp28). β-Actin was used as a loading control.
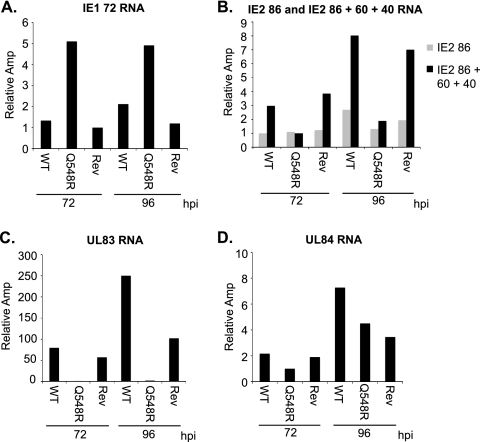
In order to determine whether the difference in IE1 72, IE2 40, IE2 60, UL83, and UL84 protein levels observed in the mutant was caused by a transcriptional or a posttranscriptional event, RNA levels at 72 and 96 h p.i. were measured via quantitative RT-PCR in cells infected at an MOI of 0.5 with WT, Q548R IE2, and Rev-Q548R IE2 C-F HB5 (Fig. 5A to D). IE1 72 RNA levels were higher in the mutant- than in WT-infected cells, confirming that the mutant's interaction with the CRS could be problematic (Fig. 5A). RNA for full-length IE2 86 (using a TaqMan probe specific to the exon 3-exon 5 junction of IE2 86) was compared to RNA for IE2 86, IE2 60, and IE2 40 combined (using a TaqMan probe targeting exon 5 of IE2). Expression of IE2 60 and IE2 40 increases greatly at late infection times in WT infection. However, a similar increase was not observed in infection with the mutant, indicating that IE2 60 and IE2 40 are reduced at the transcriptional level (Fig. 5B). Although UL84 did not appear to be severely affected at the transcriptional level, there was virtually no detectable UL83 RNA present (Fig. 5C and D).
Fig. 5.
IE1 72, IE2 60, IE2 40, and UL83 RNA levels are altered in Q548R IE2 infection. HFFs were mock infected or infected with either WT C-F HB5, Q548R IE2 C-F HB5, or Rev-Q548R IE2 C-F HB5 (MOI = 0.5). Cells were harvested at the indicated times. RNA was examined via quantitative RT-PCR using TaqMan primers and probes for IE1 72 (A), for IE2 86, IE2 60, and IE2 40 (B), for UL83 (C) and for UL84 (D). IE2 86 indicates a probe that measures only IE2 86 RNA, and IE2 86 + 60 + 40 indicates a probe that measures IE2 86, IE2 60, and IE2 40 RNA (B). RNA levels were normalized to levels of cellular glucose 6-phosphate dehydrogenase RNA. Values are relative amplification (Amp), with the lowest value set to 1. Experiments were carried out at least three times, and representative results are displayed.
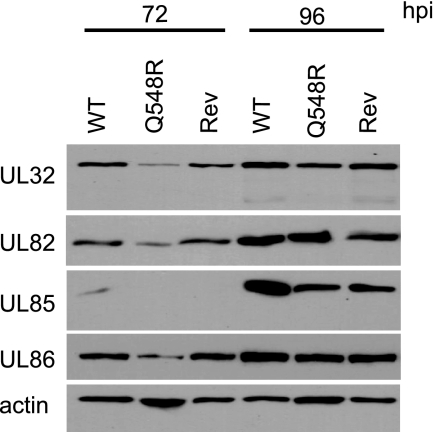
Figure 4 demonstrates that the expression of the late genes IE2 40, IE2 60, UL83, UL84, and UL99 was significantly altered in Q548R IE2 infection. To determine if there was a more general defect in transitioning to late protein expression, several other viral late proteins were examined via Western blotting. HFF cells were mock infected or infected with WT, Q548R, or Rev-Q548R IE2 C-F HB5 virus at an MOI of 0.5 and harvested at 72 and 96 h p.i. Lysates were examined via Western blotting and probed with antibodies to the late proteins UL32, UL82, UL85, and UL86 (Fig. 6). Although there appeared to be a lag in the appearance of the proteins in the mutant-infected cells, by 96 h p.i. the levels of all four of these proteins were comparable in WT-, Q548R-, and Rev-Q548R IE2 C-F HB5-infected cells. This confirms that there is a specific defect in IE2 40, IE2 60, UL83, UL84, and UL99 expression and not a global reduction in late protein expression.
Fig. 6.
Q548R IE2 C-F HB5 is not defective in transitioning to late gene expression. HFFs were mock infected or infected with either WT C-F HB5, Q548R IE2 C-F HB5, or Rev-Q548R IE2 C-F HB5 (MOI = 0.5). Cells were harvested at the indicated times. Equivalent amounts of protein were loaded onto an 8% acrylamide gel and then transferred to a nitrocellulose membrane. The membrane was probed with antibodies against UL32 (pp150), UL82 (pp71), UL85 (minor capsid protein), and UL86 (major capsid protein). β-Actin served as a loading control.
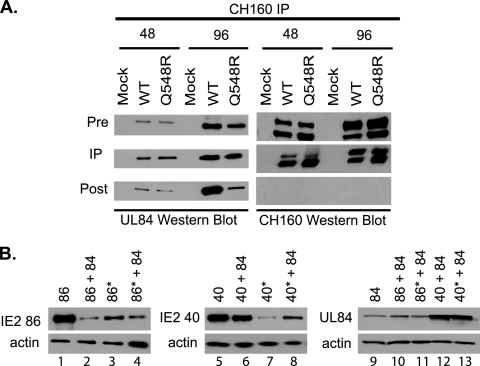
Q548R IE2 86 and Q548R IE2 40 are capable of interacting with UL84, and Q548R IE2 40 can enhance UL84 expression.
UL84 is an essential early-late protein that has been demonstrated to play a role in oriLyt-dependent viral genome replication (9). Furthermore, the majority of UL84 is in complex with IE2 86, IE2 40, and IE2 60 (31), and transfection studies have shown that IE2 86-mediated transactivation of early genes is inhibited by IE2 86 interaction with UL84 (6, 9, 46). In order to determine if Q548R IE2 86 was still capable of interacting with UL84 during the infection, an immunoprecipitation (IP) assay was performed. HFFs were mock infected or infected with WT or Q548R IE2 C-F HB5 (MOI = 1) and harvested at 48 and 96 h p.i. The CH160 antibody, which binds to the N terminus of both IE1 72 and IE2 86, was then used to immunoprecipitate the HFF lysate. A probe of the CH160 IP for UL84 demonstrated that full-length Q548R IE2 86 was still binding to UL84 (Fig. 7A) The post-IP lysate in the WT 96 h p.i. sample still contains a significant proportion of UL84. This is because much of UL84 is bound to IE2 60 and IE2 40 at this time point. The mutant expresses far less IE2 60 and IE2 40, and therefore there is a much smaller proportion of UL84 evident in the mutant post-IP 96 h p.i. sample.
Fig. 7.
Q548R IE2 86 interacts with UL84 and is not directly responsible for the decrease in UL84 expression. (A) HFFs were mock infected or infected with either WT C-F HB5 or Q548R IE2 C-F HB5 and were harvested at the indicated times. Cell lysates were then immunoprecipitated with the CH160 antibody. Protein from the pre-IP, IP, and post-IP samples were then examined via Western blotting with the UL84 antibody as well as the CH160 antibody. Pre-IP and post-IP samples represent 10% of the total IP sample. (B) 293FT cells were transfected with equivalent amounts of plasmids expressing the indicated proteins. 86*, Q548R IE2 86; 40*, Q548R IE2 40. Cells were harvested at 48 h p.t. and examined via Western blotting with the monoclonal IE2 antibody and the monoclonal UL84 antibody. β-Actin served as a loading control.
Previous work demonstrated that in cotransfection assays, IE2 40 greatly enhanced expression of UL84, whereas cotransfection of UL84 with IE2 86 resulted in only a modest increase in UL84 levels (32). As the UL84 gene is essential for viral growth, it is possible that the reduced protein levels of UL84 are partially responsible for the Q548R IE2 growth defect. In order to determine whether Q548R IE2 was defective in its ability to enhance UL84 expression, a cotransfection assay was performed. 293FT cells were transfected with plasmids encoding UL84, IE2 86, IE2 40, Q548R IE2 86, and Q548R IE2 40. IE2 plasmids were either transfected with an empty vector or cotransfected with UL84. The total amount of DNA transfected was normalized for all of the samples. Cells were then harvested at 48 h p.t. and their lysates were examined for DNA, RNA and protein expression. Q548R IE2 86 and Q548R IE2 40 proteins were generally expressed at lower levels than WT IE2 86 and IE2 40 (Fig. 7B, compare lanes 1 and 3 and lanes 5 and 7). Coexpression of UL84 with Q548R IE2 40 demonstrated a slight increase in Q548R IE2 40 expression compared to Q548R IE2 40 alone (compare lanes 5 and 6 and lanes 7 and 8). Probing for UL84 demonstrated that Q548R IE2 40 is still able to enhance UL84 expression (lanes 9 to 13). Based on these results, the decreased levels of UL84 observed in the mutant-infected cells is likely not due to a defect in the ability of Q548R IE2 40 to enhance UL84 expression but rather due to the significantly lower levels of Q548R IE2 40.
Several viral genes are upregulated at the transcriptional level in Q548R IE2 infection.
Thus far, the principal defects that have been detected in the Q548R IE2 virus are the decreases in UL84, UL83, IE2 60, and IE2 40. However, the mechanism that leads to the severely debilitated viral growth remains unclear. In order to gain a greater understanding of the Q548R IE2 mutant, we examined the expression levels of viral and cellular RNA corresponding to approximately 2,000 RNA oligonucleotides via a custom microarray. The design for this array, which contains 1,265 cellular features and 670 viral features, was generously donated by E. Fortunato (11). For this experiment, G0-synchronized HFFs were mock infected or infected with WT C-F HB5 or Q548R IE2 C-F HB5 virus (MOI = 2). Mock-infected cells were harvested at 96 h p.i., WT-infected cells were harvested at 48 and 96 h p.i., and Q548R-infected cells were harvested at 48, 96, and 168 h p.i. This set of infections was performed a total of three times in order to generate three biological replicates. RNA was isolated from each of these samples, and mRNA was amplified via the Invitrogen Superscript indirect amplification system. Amplified RNA was then labeled with Cy5 and 1 μg of Cy5-labeled RNA from each sample was then hybridized onto the 4-by-2,000 array from Combimatrix (now CustomArray, Inc.). Each chip contains four arrays, and the samples were randomly distributed across the chips in order to account for any possible chip-to-chip differences. After scanning the chips, the data were normalized to account for loading and dye incorporation differences between samples. A pairwise comparison of relevant samples was then performed via a two-tailed Student t test. Each gene was represented by at least three features on the array, and genes were considered significant if they met two criteria. First, the P value of at least half of a given gene's features needed to be less than 0.05. Second, at least a 2-fold difference between the two samples being compared needed to be present.
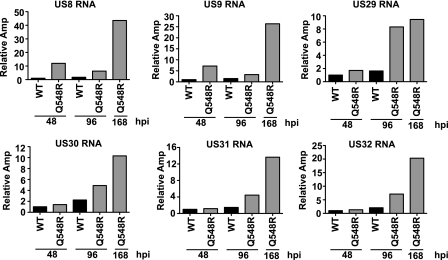
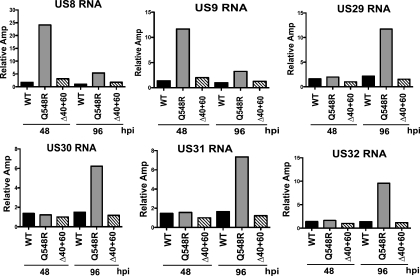
An evaluation of the viral genes generated several interesting differences between the WT- and Q548R-infected cells (Tables 2 and 3). The largest difference was the strong reduction in UL83 gene expression previously observed in the mutant (Table 2). UL82 is also listed as one of the most downregulated genes; however, it is bicistronic with UL83, and therefore it is not possible to determine via this analysis whether its transcription was affected. Given that its protein expression remains unchanged, it is unlikely that its transcription was drastically affected (Fig. 6). Interestingly, in cells infected with the mutant, there were several viral genes that exhibited a marked rise in RNA levels (Table 3). At 48 h p.i., the levels of IE1, US8, and US9 RNAs were significantly higher. The relative levels of US8 and US9 were even greater (up to 20-fold) as the infection progressed (96 and 168 h p.i.). At these later times, expression of the group of US29-32 genes also increased. To validate the results obtained from the microarray analysis, the increase in the levels of the US8, US9, and U29-32 were all confirmed via quantitative RT-PCR (Fig. 8). We also noted smaller increases (2.5- to 4-fold) for several other genes when the RNA levels in the mutant-infected cells at 168 h p.i. were compared to those in the WT-infected cells at 96 h p.i.
Table 2.
Viral genes significantly downregulated upon Q548R IE2 infection
| Gene | Relative downregulation at time (h) postinfectiona |
||
|---|---|---|---|
| WT 48 vs R 48 | WT 96 vs R 96 | WT 96 vs R 168 | |
| UL83 | 20.00 | 25.00 | |
| UL82 | 12.50 | 5.88 | |
| US27 | 3.33 | ||
| RL11 | 2.70 | ||
| US28 | 2.70 | ||
| TRL12 | 2.63 | ||
| IRL10 | 2.63 | ||
| RL10 | 2.56 | ||
| UL4 | 2.56 | 2.50 | |
| UL33 | 2.38 | 2.33 | |
| UL23 | 2.27 | ||
| UL111A | 2.50 | 2.22 | |
| UL34 | 2.50 | 2.17 | |
| RL13 | 2.13 | ||
| UL25 | 2.08 | 2.63 | |
| UL32 | 2.04 | ||
Significant changes (fold) between the indicated samples are shown. WT, wild type; R, Q548R IE2. The time (number of hours) postinfection is the time at which the sample was harvested; e.g., WT 48 vs R 48 indicates the ratio of WT 48 h p.i. to Q548R IE2 48 h p.i. for a given gene. Blank cells indicate that the change did not meet the criteria for significance.
Table 3.
Viral genes significantly upregulated upon Q548R IE2 infection
| Gene | Relative upregulation at time (h) postinfectiona |
||
|---|---|---|---|
| R 48 vs WT 48 | R 96 vs WT 96 | R 168 vs WT 96 | |
| US8 | 4.2 | 8.8 | 20.8 |
| US9 | 4.9 | 7 | 18.7 |
| US29 | 7.7 | 7.5 | |
| US31 | 3.5 | 5.7 | |
| US32 | 4.2 | 5.5 | |
| US32 | 4.2 | 5.5 | |
| US30 | 5.4 | 4.3 | |
| US10 | 4 | ||
| US7 | 3.5 | ||
| UL63 | 3.3 | ||
| UL111 | 2.5 | 3 | |
| UL27 | 2.5 | ||
| UL28 | 2.5 | ||
| UL74 | 2 | ||
| US11 | 2 | ||
| IE1 | 3.4 | ||
| TRS1 | 3.3 | ||
| UL11 | 2.1 | ||
| US12 | 2 | ||
| US18 | 2 | ||
| US19 | 2.1 | ||
Significant changes (fold) between the indicated samples are shown. WT, wild type; R, Q548R IE2. The time (number of hours) postinfection is the time at which the sample was harvested; e.g., R 48 vs WT 48 indicates the ratio of Q548R IE2 48 h p.i. to WT 48 h p.i. for a given gene. Blank cells indicate that the change did not meet the criteria for significance.
Fig. 8.
Quantitative RT-PCR confirmation of viral genes deemed significantly changed by microarray analysis. The US8-9 and US29-32 genes were examined via quantitative RT-PCR as described in Materials and Methods. RNA levels are normalized to the level of cellular glucose 6-phosphate dehydrogenase RNA. Values are relative amplification (Amp), with the lowest value set to 1.
A comparison of the cellular gene expression between samples yielded no apparent differences between the WT and the Q548R samples. However, several differences between mock-infected and virus-infected samples were noted (Tables 4 and 5). The largest differences were observed in beta-actin (5.5- to 14-fold reduced in viral samples), lamin A (4- to 6.6-fold reduced in viral samples), Timp3 (3.8- to 4.3-fold reduced in viral samples), cyclin D1 (3.6- to 4.2-fold reduced in viral samples), and c-FOS (3.6- to 3.7-fold reduced in viral samples).
Table 4.
Cellular genes significantly downregulated upon HCMV infection
| Gene | Relative downregulation at time (h) postinfectiona |
||||
|---|---|---|---|---|---|
| M 96 vs WT 48 | M 96 vs R 48 | M 96 vs WT 96 | M 96 vs R 96 | M 96 vs R 168 | |
| β-Actin | 6.67 | 5.56 | 10.00 | 6.67 | 14.29 |
| Lamin A | 4.76 | 4.00 | 6.67 | 6.67 | 6.67 |
| Cyclin D1 | 3.85 | 3.57 | 4.17 | 4.00 | 3.85 |
| TIMP3 | 4.35 | 4.35 | 4.00 | 4.00 | 3.85 |
| c-FOS | 3.57 | 3.57 | 3.70 | 3.70 | 3.70 |
| JAK1 | 3.23 | 2.13 | 3.57 | 2.94 | 3.57 |
| SHC | 2.33 | 3.03 | |||
| SP1 | 2.56 | 2.27 | 2.50 | 2.94 | |
| NEDD8 | 2.56 | 2.86 | |||
| PKCa | 2.63 | 2.33 | 2.63 | 2.27 | 2.70 |
| STAT2 | 2.27 | 2.27 | 2.63 | 2.44 | 2.44 |
| ROC1 | 2.44 | 2.27 | |||
| p50 | 2.27 | 2.17 | |||
| SHP2 | 2.17 | ||||
| PLCγ | 2.13 | ||||
| c-JUN | 2.00 | 2.00 | 2.00 | ||
| CDK4 | 2.00 | ||||
| DP1 | 2.08 | 2.00 | |||
| CAS | 2.17 | ||||
| DSH | 2.33 | 2.04 | 2.22 | ||
Significant changes (fold) between the indicated samples are shown. M, mock; WT, wild type; R, Q548R IE2. The time (number of hours) postinfection is the time at which the sample was harvested; e.g., M 96 vs WT 48 indicates the ratio of mock 96 h p.i. to WT 48 h p.i. for a given gene. Blank cells indicate that the change did not meet the criteria for significance.
Table 5.
Cellular genes significantly upregulated upon HCMV infection
| Gene | Relative upregulation at time (h) postinfectiona |
||||
|---|---|---|---|---|---|
| WT 48 vs M 96 | R 48 vs M 96 | WT 96 vs M 96 | R 96 vs M 96 | R 168 vs M 96 | |
| H2A | 2.2 | 2.4 | 2.5 | 3.6 | 2.7 |
| RAD9 | 2.03 | 2.14 | 2.37 | 2.42 | |
| CHOP | 2 | ||||
| HOX B2 | 2.5 | 2.4 | 2.3 | 2.2 | 2 |
Significant changes (fold) between the indicated samples are shown. M, mock; WT, wild type; R, Q548R IE2. The time (number of hours) postinfection is the time at which the sample was harvested; e.g., M 96 vs WT 48 indicates the ratio of mock 96 h p.i. to WT 48 h p.i. for a given gene. Blank cells indicate that the change did not meet the criteria for significance.
Viral genes significantly upregulated in Q548R IE2 infection are not upregulated in IE2Δ40 + 60 infection.
The Q548R IE2 mutant exhibits characteristics that are quite similar to those of several other IE2 mutants that have been examined by the Spector lab. IE2Δ136-290 deletes a region within exon 5 that includes the promoter regions for IE2 40 and IE2 60; thus, the recombinant virus expresses a truncated IE2 86 and does not express IE2 60 or IE2 40. IE2Δ40 + 60 contains mutations in the IE2 40 and IE2 60 promoter region, which allows production of full-length WT IE2 86 while preventing expression of IE2 40 and IE2 60. Similar to Q548R IE2, both IE2Δ136-290 and IE2Δ40 + 60 are viable mutants with severely debilitated growth characteristics. Furthermore, both IE2Δ136-290 and IE2Δ40 + 60 exhibit reduced UL84 at the posttranscriptional level and reduced UL83 expression at the transcriptional level (28, 41). In order to examine whether the reduced expression of IE2 40 and IE2 60 was responsible for the increased levels of US8-9 and US29-32 observed in Q548R IE2-infection, HFFs were infected with either WT, Q548R IE2, or IE2Δ40 + 60 C-F HB5 virus (MOI = 0.5). Cells were then harvested at 48 and 96 h p.i., and RNA was isolated. Expression levels of US8-9 and US29-32 were examined via quantitative RT-PCR (Fig. 9). Unlike Q548R IE2 infection, IE2Δ40 + 60 infection did not demonstrate increased expression of these genes, indicating that this increase was unlikely due to the reduced levels of IE2 40 and IE2 60.
Fig. 9.
Quantitative RT-PCR analysis of US8-9 and US29-32 in IE2Δ40 + 60 mutant infection. The US8-9 and US29-32 genes were examined via quantitative RT-PCR analysis as described in Materials and Methods. RNA levels are normalized to cellular glucose 6-phosphate dehydrogenase RNA. Values are relative amplification (Amp), with the lowest value set to 1.
DISCUSSION
HCMV IE2 86 is a complex and multifunctional protein. Much work has been done over the years attempting to elucidate its many roles. Until recently, most of the studies were accomplished via transfection assays and in vitro methods such as glutathione S-transferase pulldown assays. The advent of bacterial artificial chromosome (BAC) technology allowed easier manipulation of the viral genome and the subsequent development of various mutants that shed light on viral gene functions in the context of infection. HCMV halts the cell cycle in a pseudo-G1 state, which allows the virus to take advantage of cellular processes necessary for DNA replication (2, 4, 7, 13, 17, 27, 29, 43–45). Although there is some evidence that IE2 86 plays a role in this cell cycle arrest (36, 43), these initial studies were performed via transient transfection. The creation of the Q548R IE2 HCMV mutant BAC offered the first evidence that IE2 may play a key role in HCMV cell cycle regulation in the context of infection (25). In the paper by Petrik et al. (25), it was reported that the Q548R IE2 mutant virus was able to negatively autoregulate the MIE promoter, transactivate viral early genes, and upregulate E2F-dependent genes but was unable to cause cell cycle arrest. However, because Q548R IE2 HCMV grew so slowly and to such low titers, it was difficult to do extensive analyses in the context of infection. The initial aim of this study was to further characterize Q548R IE2 HCMV and determine why this mutant was defective in its ability to halt the cell cycle.
Despite the advent of BAC technology, IE2 mutants have remained difficult to study via infection, since the researcher is limited to mutants that maintain viral replication competency. The 86F/40HA cell line complements mutant IE2 86 and IE2 40 and allows more rapid production and higher titers of mutant IE2 viruses (30). All of the experiments for this study were performed with Q548R IE2 Towne or HB5 BAC-derived HCMV which was grown and whose titers were determined on 86F/40HA cells and which was subsequently studied on noncomplementing HFFs. Transfection of Q548R IE2 into both 86F/40HA cells and noncomplementing HFFs demonstrated the drastic difference in the ability of the mutant to grow in a complementing versus a noncomplementing background (Fig. 1). Complementation did not appear to be 100%, based on the more rapid spread of the WT virus through HFFs as well as the fact that the titers of Q548R IE2 virus grown on complementing cells were consistently about 10-fold lower than the titers of WT virus grown on HFFs (data not shown). The slightly slower growth and lower titers of Q548R IE2 virus on complementing cells are not unexpected, given that this system is not perfect and does not produce WT-level titers in IE2-ΔExon5 infection (30).
The mutant showed a slight decrease in viral genome replication at 48 h p.i. compared to WT and Rev-Q548R IE2 genome replication (Fig. 2A), which could possibly be explained by the recent transfection-based evidence that Q548R IE2 86 cannot bind to the chromatin assembly factor 1 protein p150 (16). However, the large increases in viral DNA levels between 24, 72, and 96 h p.i. in the mutant as well as the diminishing difference in viral DNA levels between the mutant and WT at 96 h p.i. indicate that viral DNA replication is not significantly affected and ultimately does not explain the severe nature of the growth defect in the mutant.
The ability of Q548R IE2 HCMV to replicate its genome (Fig. 2A), stabilize geminin (Fig. 2B), and block cellular DNA synthesis (Fig. 3) contributed to our conclusion that this mutant preserved the ability to halt the cell cycle. There are several possible explanations for these conflicting results compared to the initial characterization by Petrik et al. (25). One possibility lies in the time it takes for this mutant to grow in noncomplementing cells. It took many weeks for cells transfected with the Q548R IE2 mutant virus to reach 100% CPE, and this protracted growth time increases the possibility of secondary mutations. There is accumulating evidence that mutant viruses can develop a significant number of compensatory secondary mutations (Lynn Enquist, personal communication). Growing severely debilitated IE2 mutants in the complementing cell line 86F/40HA helps to alleviate the pressure on these mutants to develop secondary mutations that facilitate replication. On the other hand, growth in the complementing cell line does provide an alternative explanation for these conflicting results, since we cannot rule out the possibility that the composition of the virion that is produced by noncomplementing versus complementing cells is different. It is possible that differing levels of tegument proteins present in virus grown on complementing cells account for the differing phenotype that we observed in this mutant compared to the initial characterization.
The phenotype observed in the Q548R IE2 mutant was similar to that of several previously studied IE2 mutants. The deletion of amino acids 136 to 290 in exon 5 of IE2, termed IE2ΔSX, resulted in a viable but severely debilitated virus whose production of UL83 and UL84 was greatly diminished (28, 31). The IE2Δ40 + 60 virus, which expresses full-length IE2 86 but not IE2 40 or IE2 60, also exhibits severely debilitated growth, with diminished UL83 and UL84 production (31, 41). In both cases, UL83 expression was affected at the transcriptional level, while UL84 expression was affected at the posttranscriptional level. UL83 expression appeared to be most affected by the deletion of both IE2 40 and IE2 60, and it has been demonstrated that UL84 protein expression is tightly linked to the levels of IE2 40 present (32). It therefore seems likely that transcriptional repression of UL83 and the posttranscriptional reduction in UL84 are related to the greatly reduced levels of IE2 40 and IE2 60 observed in this mutant.
The results of the microarray analysis comparing the Q548R IE2 mutant to WT HCMV virus were unexpected in several ways. First, there were no cellular genes that showed a significant difference in expression between Q548R IE2 virus- and WT virus-infected cells. This could be due in part to the fact that the array contained only 1,265 cellular features, with each gene being represented by at least 3 features, which by no means approaches a full complement of cellular genes. Of course, one major limitation of this microarray is that it detects differences in gene expression only at the transcriptional level. Any posttranscriptional differences in protein levels are missed. Particularly surprising was the finding that there were very few viral genes that showed a significant decrease in Q548R IE2-infected cells. UL83 was by far the most downregulated viral gene in the mutant. Otherwise, the most significantly affected viral genes were upregulated in the mutant, particularly US8 and US9 as well as US29-32.
US8 and US9 are cytoplasmic glycoproteins that have been demonstrated to be dispensable for growth in tissue culture (5, 12, 14), but little is known about their function. There is some in vitro and transfection-based evidence that US8 binds to major histocompatibility complex class I heavy chains in the endoplasmic reticulum (ER) (38). A US9 deletion recombinant virus forms smaller plaques and appears to be replication deficient in human retinal pigment epithelial (ARPE-19) cells (12, 18). There is also transient-transfection evidence that US9 can localize both to the ER and to mitochondria (19). US8 and US9 are transcribed as a bicistronic mRNA with a single predicted polyadenylation site (10). The probable TATA box for the US8 and US9 transcript (nt 199706 to 199711; accession no. BK000394.5) is closely followed by two possible IE2-binding sites (nt 199635 to 199648 and nt 199661 to 199674). These sites, which contain 14 nucleotides framed by CG residues at both ends, share sequence similarities to the cis repression signal, and similar sequences have been shown to bind IE2 86 (1, 33, 34). The presence of these sequences in the potential promoter region of US8-9 could explain the ability of IE2 to affect transcription of these genes.
The US29-32 region remains largely uncharacterized. Each open reading frame in this region has its own predicted TATA box (39). There are multiple possible IE2-binding sites contained in this region: US29 has a possible site shortly after its predicted TATA box (nt 221300 to 221313); US31 has 3 possible sites, including two overlapping sites (nt 223615 to 223640) and a third (nt 223666 to 223679) all shortly after its predicted TATA box.
Analysis of US8-9 and US29-32 levels in cells infected with the IE2Δ40 + 60 mutant demonstrated that reduced levels of IE2 40 and IE2 60 alone are not sufficient to cause an increase in the expression of these genes. This indicates that the Q548R mutation in IE2 86, IE2 60, and IE2 40 may itself play a role in the altered expression of these genes. Further study will be required to determine whether IE2 86, IE2 60, or IE2 40 directly affects transcription from these regions.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant CA034729 to D.H.S. S.W.B. was supported by National Heart, Lung and Blood Institute training grant F30HL094041.
We thank Mark Stinski for providing the WT and Q548R IE2 Towne BACs, William Britt for providing the monoclonal antibodies to UL32, UL85, and UL86, Thomas Shenk for providing the UL82 monoclonal antibody, Elizabeth Fortunato for providing the microarray design, and Roman Sasik of the UCSD BIOGEM Core for normalization of microarray data. We appreciate the use of the DeltaVision microscope and SoftWoRx software at the UCSD Cancer Center Digital Imaging Shared Resource and the use of the Genepix 4000B Microarray Scanner at the GeneChip Microarray Core at the UCSD VA Hospital. We thank members of the Spector lab past and present for their help and advice in producing the manuscript.
Footnotes
Published ahead of print on 24 August 2011.
REFERENCES
- 1. Arlt H., Lang D., Gebert S., Stamminger T. 1994. Identification of binding sites for the 86-kilodalton IE2 protein of human cytomegalovirus within an IE2-responsive viral early promoter. J. Virol. 68:4117–4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Biswas N., Sanchez V., Spector D. H. 2003. Human cytomegalovirus infection leads to accumulation of geminin and inhibition of the licensing of cellular DNA replication. J. Virol. 77:2369–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bresnahan W. A., Albrecht T., Thompson E. A. 1998. The cyclin E promoter is activated by human cytomegalovirus 86-kDa immediate early protein. J. Biol. Chem. 273:22075–22082 [DOI] [PubMed] [Google Scholar]
- 4. Bresnahan W. A., Boldogh I., Thompson E. A., Albrecht T. 1996. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology 224:150–160 [DOI] [PubMed] [Google Scholar]
- 5. Chee M. S., et al. 1990. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 154:125–169 [DOI] [PubMed] [Google Scholar]
- 6. Colletti K. S., Xu Y., Cei S. A., Tarrant M., Pari G. S. 2004. Human cytomegalovirus UL84 oligomerization and heterodimerization domains act as transdominant inhibitors of oriLyt-dependent DNA replication: evidence that IE2-UL84 and UL84-UL84 interactions are required for lytic DNA replication. J. Virol. 78:9203–9214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dittmer D., Mocarski E. S. 1997. Human cytomegalovirus infection inhibits G1/S transition. J. Virol. 71:1629–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fortunato E. A., Spector D. H. 1998. p53 and RPA are sequestered in viral replication centers in the nuclei of cells infected with human cytomegalovirus. J. Virol. 72:2033–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gebert S., et al. 1997. The UL84 protein of human cytomegalovirus acts as a transdominant inhibitor of immediate-early-mediated transactivation that is able to prevent viral replication. J. Virol. 71:7048–7060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gretch D. R., Stinski M. F. 1990. Transcription of the human cytomegalovirus glycoprotein gene family in the short unique component of the viral genome. Virology 174:522–532 [DOI] [PubMed] [Google Scholar]
- 11. Hannemann H., Rosenke K., O'Dowd J. M., Fortunato E. A. 2009. The presence of p53 influences the expression of multiple human cytomegalovirus genes at early times postinfection. J. Virol. 83:4316–4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huber M. T., Tomazin R., Wisner T., Boname J., Johnson D. C. 2002. Human cytomegalovirus US7, US8, US9, and US10 are cytoplasmic glycoproteins, not found at cell surfaces, and US9 does not mediate cell-to-cell spread. J. Virol. 76:5748–5758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jault F. M., et al. 1995. Cytomegalovirus infection induces high levels of cyclins, phosphorylated Rb, and p53, leading to cell cycle arrest. J. Virol. 69:6697–6704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kollert-Jons A., Bogner E., Radsak K. 1991. A 15-kilobase-pair region of the human cytomegalovirus genome which includes US1 through US13 is dispensable for growth in cell culture. J. Virol. 65:5184–5189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee E. C., et al. 2001. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 73:56–65 [DOI] [PubMed] [Google Scholar]
- 16. Lee S. B., et al. 2011. Host-viral effects of chromatin assembly factor 1 interaction with HCMV IE2. Cell Res. 21:1230–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lu M., Shenk T. 1996. Human cytomegalovirus infection inhibits cell cycle progression at multiple points, including the transition from G1 to S. J. Virol. 70:8850–8857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maidji E., Tugizov S., Jones T., Zheng Z., Pereira L. 1996. Accessory human cytomegalovirus glycoprotein US9 in the unique short component of the viral genome promotes cell-to-cell transmission of virus in polarized epithelial cells. J. Virol. 70:8402–8410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mandic L., Miller M. S., Coulter C., Munshaw B., Hertel L. 2009. Human cytomegalovirus US9 protein contains an N-terminal signal sequence and a C-terminal mitochondrial localization domain, and does not alter cellular sensitivity to apoptosis. J. Gen. Virol. 90:1172–1182 [DOI] [PubMed] [Google Scholar]
- 20. Marchini A., Liu H., Zhu H. 2001. Human cytomegalovirus with IE-2 (UL122) deleted fails to express early lytic genes. J. Virol. 75:1870–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McElroy A. K., Dwarakanath R. S., Spector D. H. 2000. Dysregulation of cyclin E gene expression in human cytomegalovirus-infected cells requires viral early gene expression and is associated with changes in the Rb-related protein p130. J. Virol. 74:4192–4206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mocarski E. S., Shenk T., Pass R. F. 2007. Cytomegaloviruses, p. 2701–2772 In Knipe D. M., Howley P. M. (ed.), Fields virology, 5th ed., vol. 2 Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 23. Muganda P., Mendoza O., Hernandez J., Qian Q. 1994. Human cytomegalovirus elevates levels of the cellular protein p53 in infected fibroblasts. J. Virol. 68:8028–8034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muyrers J. P., et al. 2000. Point mutation of bacterial artificial chromosomes by ET recombination. EMBO Rep. 1:239–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petrik D. T., Schmitt K. P., Stinski M. F. 2006. Inhibition of cellular DNA synthesis by the human cytomegalovirus IE86 protein is necessary for efficient virus replication. J. Virol. 80:3872–3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Puchtler E., Stamminger T. 1991. An inducible promoter mediates abundant expression from the immediate-early 2 gene region of human cytomegalovirus at late times after infection. J. Virol. 65:6301–6306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Salvant B. S., Fortunato E. A., Spector D. H. 1998. Cell cycle dysregulation by human cytomegalovirus: influence of the cell cycle phase at the time of infection and effects on cyclin transcription. J. Virol. 72:3729–3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sanchez V., Clark C. L., Yen J. Y., Dwarakanath R., Spector D. H. 2002. Viable human cytomegalovirus recombinant virus with an internal deletion of the IE2 86 gene affects late stages of viral replication. J. Virol. 76:2973–2989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanchez V., McElroy A. K., Spector D. H. 2003. Mechanisms governing maintenance of Cdk1/cyclin B1 kinase activity in cells infected with human cytomegalovirus. J. Virol. 77:13214–13224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sanders R. L., Clark C. L., Morello C. S., Spector D. H. 2008. Development of cell lines that provide tightly controlled temporal translation of the human cytomegalovirus IE2 proteins for complementation and functional analyses of growth-impaired and nonviable IE2 mutant viruses. J. Virol. 82:7059–7077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sanders R. L., Del Rosario C. J., White E. A., Spector D. H. 2008. Internal deletions of IE2 86 and loss of the late IE2 60 and IE2 40 proteins encoded by human cytomegalovirus affect the levels of UL84 protein but not the amount of UL84 mRNA or the loading and distribution of the mRNA on polysomes. J. Virol. 82:11383–11397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sanders R. L., Spector D. H. 2010. Human cytomegalovirus IE2 86 and IE2 40 proteins differentially regulate UL84 protein expression posttranscriptionally in the absence of other viral gene products. J. Virol. 84:5158–5170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schwartz R., Sommer M. H., Scully A., Spector D. H. 1994. Site-specific binding of the human cytomegalovirus IE2 86-kilodalton protein to an early gene promoter. J. Virol. 68:5613–5622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scully A. L., Sommer M. H., Schwartz R., Spector D. H. 1995. The human cytomegalovirus IE2 86-kilodalton protein interacts with an early gene promoter via site-specific DNA binding and protein-protein associations. J. Virol. 69:6533–6540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Song Y. J., Stinski M. F. 2002. Effect of the human cytomegalovirus IE86 protein on expression of E2F-responsive genes: a DNA microarray analysis. Proc. Natl. Acad. Sci. U. S. A. 99:2836–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Song Y. J., Stinski M. F. 2005. Inhibition of cell division by the human cytomegalovirus IE86 protein: role of the p53 pathway or cyclin-dependent kinase 1/cyclin B1. J. Virol. 79:2597–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stinski M. F., Petrik D. T. 2008. Functional roles of the human cytomegalovirus essential IE86 protein. Curr. Top. Microbiol. Immunol. 325:133–152 [DOI] [PubMed] [Google Scholar]
- 38. Tirabassi R. S., Ploegh H. L. 2002. The human cytomegalovirus US8 glycoprotein binds to major histocompatibility complex class I products. J. Virol. 76:6832–6835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weston K., Barrell B. G. 1986. Sequence of the short unique region, short repeats, and part of the long repeats of human cytomegalovirus. J. Mol. Biol. 192:177–208 [DOI] [PubMed] [Google Scholar]
- 40. White E. A., Clark C. L., Sanchez V., Spector D. H. 2004. Small internal deletions in the human cytomegalovirus IE2 gene result in nonviable recombinant viruses with differential defects in viral gene expression. J. Virol. 78:1817–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. White E. A., Del Rosario C. J., Sanders R. L., Spector D. H. 2007. The IE2 60-kilodalton and 40-kilodalton proteins are dispensable for human cytomegalovirus replication but are required for efficient delayed early and late gene expression and production of infectious virus. J. Virol. 81:2573–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. White E. A., Spector D. H. 2005. Exon 3 of the human cytomegalovirus major immediate-early region is required for efficient viral gene expression and for cellular cyclin modulation. J. Virol. 79:7438–7452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wiebusch L., Hagemeier C. 1999. Human cytomegalovirus 86-kilodalton IE2 protein blocks cell cycle progression in G1. J. Virol. 73:9274–9283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wiebusch L., Hagemeier C. 2001. The human cytomegalovirus immediate early 2 protein dissociates cellular DNA synthesis from cyclin-dependent kinase activation. EMBO J. 20:1086–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wiebusch L., Uecker R., Hagemeier C. 2003. Human cytomegalovirus prevents replication licensing by inhibiting MCM loading onto chromatin. EMBO Rep. 4:42–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xu Y., Cei S. A., Rodriguez Huete A., Colletti K. S., Pari G. S. 2004. Human cytomegalovirus DNA replication requires transcriptional activation via an IE2- and UL84-responsive bidirectional promoter element within oriLyt. J. Virol. 78:11664–11677 [DOI] [PMC free article] [PubMed] [Google Scholar]