Abstract
Transmission of pathogenic avian influenza viruses (AIV) from wild birds to domestic poultry and humans is continuing in multiple countries around the world. In preparation for a potential AIV pandemic, multiple vaccine candidates are under development. In the case of H5N1 AIV, a clear shift in transmission from clade 1 to clade 2 viruses occurred in recent years. The virus-like particle (VLP) represents an economical approach to pandemic vaccine development. In the current study, we evaluated the humoral immune response in humans vaccinated with H5N1 A/Indonesia/05/2005 (clade 2.1) VLP vaccine manufactured in Sf9 insect cells. The VLPs were comprised of the influenza virus hemagglutinin (HA), neuraminidase (NA), and matrix 1 (M1) proteins. In an FDA-approved phase I/II human clinical study, two doses of H5N1 VLPs at 15, 45, or 90 μg HA/dose resulted in seroconversion and production of functional antibodies. Moreover, cross-reactivity against other clade 2 subtypes was demonstrated using virus neutralization assays. H5N1 whole-genome fragment phage display libraries (GFPDL) were used to elucidate the antibody epitope repertoire in postvaccination human sera. Diverse epitopes in HA1/HA2 and NA were recognized by postvaccination sera from the two high-dose groups, including large segments spanning the HA1 receptor binding domain. Importantly, the vaccine elicited sera that preferentially bound to an oligomeric form of recombinant HA1 compared with monomeric HA1. The oligomeric/monomeric HA1 binding ratios of the sera correlated with the virus neutralizing titers. Additionally, the two high-dose VLP vaccine groups generated NA-inhibiting antibodies that were associated with binding to a C-terminal epitope close to the sialic acid binding site. These findings represent the first report describing the quality of the antibody responses in humans following AIV VLP immunization and support further development of such vaccines against emerging influenza virus strains.
INTRODUCTION
The recent global spread of swine-origin H1N1 influenza viruses highlights the need for rapid development of effective vaccines against pandemic strains. Much of our recent knowledge about the potential for pandemic spread is derived from studies with the highly pathogenic (HP) H5N1 avian influenza A viruses (AIV) (12). H5N1 viruses cause severe human disease and may undergo adaptation permitting human-to-human transmission. As of 21 April 2011, there have been 552 human cases of H5N1, resulting in 322 deaths (fatality rate = 59%) (http://www.who.int/csr/disease/avian_influenza).
The production of hemagglutinin by using recombinant technology overcomes the constraints of traditional inactivated influenza virus vaccine manufacturing, which requires months to generate vaccine viruses using reassortant/reverse genetics and adaptation for high growth in eggs. Technologies that can be easily translated into a well-controlled large-scale manufacturing process will have a great advantage. Thus far, various vaccine prototypes produced in a baculovirus-insect cell expression system have undergone preclinical and clinical development (22, 49). Baculovirus-expressed recombinant hemagglutinin (HA) was shown to be immunogenic in humans (42, 44, 45) and protected against lethal infection in poultry challenged with avian H5 and H7 influenza virus subtypes (8).
Virus-like particles (VLPs) are multiprotein structures that mimic the organization and conformation of authentic viruses but lack the viral genome and can be rapidly produced in insect vector systems by coexpression of key viral membrane components (23, 25, 29, 32, 38). VLPs are predicted to elicit stronger protective immunity than recombinant monomeric proteins because they present numerous copies of oligomeric spike-like structures that efficiently cross-link antigen-specific receptors on B cells. Several VLP products have been evaluated in clinical trials. In addition to a hepatitis B virus VLP-based licensed vaccine, two human papillomavirus (HPV) VLP vaccines were recently licensed in the United States (6, 9, 15, 38).
To address the need for improved influenza virus vaccines and the limitation of egg-based manufacturing, VLPs based on diverse human and avian influenza virus strains using baculovirus vectors expressing the HA, neuraminidase (NA), and matrix 1 (M1) proteins were engineered (Novavax Inc.). In previous studies, these VLPs were shown to be immunogenic in mice and ferrets and to provide protection against respiratory challenge with homologous and heterologous influenza virus strains (4, 5, 37).
In the current study, we evaluated in depth the human antibody responses in a subset of serum samples from an FDA-approved clinical trial phase I/II study of an H5N1 (A/Indonesia/05/2005) VLP vaccine containing the HA, NA, and M1 proteins, produced in Sposoptera frugipertda (Sf9) insect cells. In addition to HA inhibition (HAI), NA inhibition (NAI), and virus neutralization assays, the immune sera were analyzed by use of H5N1 whole-genome fragment phage display libraries (GFPDL) expressing HA and NA inserts and by surface plasmon resonance to measure kinetics of antibody binding to oligomeric and monomeric recombinant HA (rHA) proteins. In our previous studies, influenza virus-specific GFPDL (FLU-GFPDL) expressing protein fragments from all the open reading frames of the avian influenza A/Vietnam/1203/2004 (H5N1) virus HA and NA genes were used for mapping of broadly neutralizing human monoclonal antibodies (MAbs) derived from these H5N1 survivors (18). These MAbs were found to recognize nonlinear conformational epitopes presented by large hemagglutinin fragments encompassing the receptor binding domain (RBD). FLU-GFPDL (H5N1) allowed the identification of large viral epitopes recognized by antibodies in sera of individuals who recovered from HP H5N1 infection (18). In a follow-up study we deciphered the role of oil-in-water adjuvant in augmenting the immune response following vaccinations against H5N1 (A/Vietnam) and H1N1pdm09 viruses (16, 19).
In the current study, we provide evidence that the H5N1 A/Indonesia/05/05 VLPs can elicit antibodies with preferential binding to oligomeric HA reminiscent of HA spikes on the influenza virus. The oligomeric/monomeric HA1 binding ratios correlated with neutralization titers and possibly explain the broad cross-reactivity of antibodies induced by VLP-based influenza virus vaccines.
MATERIALS AND METHODS
Vaccine product.
Influenza A/Indonesia/5/2005 (H5N1) virus VLPs were produced with recombinant baculovirus Autographa californica nuclear polyhedrosis virus (AcMNPV) expressing the HA, NA, and M1 genes. HA, NA, and M1 protein sequences were from A/Indonesia/05/2005 (H5N1), with GenBank accession numbers ABP51969, ABW06107, and ABI36004, respectively. All three genes were codon optimized for insect cells and biochemically synthesized by Geneart AG (Regensburg, Germany). The HA, NA, and M1 genes were cloned into pFastBac1 baculovirus transfer vector (Invitrogen, Carlsbad, CA) in a tandem fashion with NA/HA/M1 gene order through restriction enzyme digestion and ligation. Each gene was under the control of its own AcMNPV polyhedrin promoter and poly(A) termination signals. Recombinant baculoviruses expressing the HA, NA, and M1 genes were generated using a Bac-to-Bac baculovirus expression system (Invitrogen) as described previously (36). Briefly, the tandem pFastBac1 plasmid was transformed into Escherichia coli DH10Bac competent cells containing the AcMNPV baculovirus genome to produce a recombinant bacmid through site-specific homologous recombination. Bacmid DNA was purified from transformed E. coli and transfected into Spodoptera frugiperda (Lepidoptera) Sf9 insect cells. Recombinant baculovirus was recovered, plaque purified, and amplified to infect Sf9 cells for VLP production. A/Indonesia/5/2005 VLPs were purified through tangential filtration, sucrose density gradient centrifugation, and ion-exchange chromatography to ≥95% purity as measured by scanning densitometry of Coomassie blue-stained SDS-polyacrylamide gels. The dose of vaccine was based on HA content as measured using a single radial immunodiffusion (SRID) assay.
Clinical trial.
A phase I/IIa blinded, randomized, placebo-controlled study was conducted to evaluate the safety and immunogenicity of an A/Indonesia/05/2005 (H5N1) influenza virus VLP vaccine in subjects 18 to 40 years of age. The study was conducted in 2008 under informed consent (clinical trial number NCT 005 19389, approved by IRB ENC 1-07-324). The H5N1 VLP vaccine and placebo were packaged in 2-ml, single-dose glass vials, with each 0.5-ml dose of the vaccine formulated to contain 15, 45, or 90 μg of HA in sterile phosphate (25 mM HPO4)-buffered saline (0.5 M NaCl), pH 7.2, with calcium (10 μM) and polysorbate 80 (0.01%). The placebo injection (0.5 ml) contained the vehicle utilized for suspension of the VLPs.
The study was conducted in two stages. In stage A, 70 subjects received 15 or 45 μg of the H5N1 VLP vaccine or placebo (7:3 ratio) at days 0 and 28. Stage A safety and immunogenicity results were reviewed by a Data Safety Monitoring Board, which recommended that the study progress to stage B with a higher VLP vaccine dose. In stage B, 160 subjects received 15, 45, or 90 μg of H5N1 vaccine or placebo (1:1:1:1 ratio) at days 0 and 28. Subjects were assessed for all adverse events (AEs) through 28 days after receiving the second dose of vaccine, and serious AEs (SAEs) were recorded through 6 months after dose 2. For the 6-day period following each injection, subjects recorded the presence and severity of local symptoms (pain, tenderness, redness, and swelling) and systemic symptoms (fever, headache, fatigue, myalgia, nausea, vomiting, and diarrhea) as well as body temperature. Serum samples for evaluation of immunogenicity were obtained at days 0, 28, and 56. Day 0 and day 56 samples were used for the analyses performed in the studies described in this report.
All samples provided to CBER were deidentified except for dose number. The protocols were evaluated and approved by CBER Research Involving Human Subjects Committee (RIHSC) and were conducted under RIHSC exemption 03-118B.
Outcomes and statistical methods.
The stage A and stage B phases of the clinical trial were analyzed separately. The primary objective was to evaluate the safety of the H5N1 VLP vaccine. Secondary objectives were to assess the immunogenicity of the influenza virus H5N1 VLP vaccine as determined by hemagglutination inhibition (HAI) antibody responses as measured against a reassortant influenza A/Indonesia/05/2005(H5N1)/PR8-IBCDC-RG2 virus and microneutralization (MN) responses to the homologous influenza A/Indonesia/05/2005 virus. HAI and MN titers were measured as previously described (33), and the seroprotection rate (SPR) (the percentage of individuals with a postvaccination HAI or MN titer of ≥1:40) and seroconversion rate (SCR) (defined as either a prevaccination titer of <1:10 with a postvaccination titer of ≥1:40 or a prevaccination HAI titer of ≥1:10 with a minimum 4-fold rise in HAI titer in postimmunization serum relative to preimmunization serum) were determined.
Cross-clade microneutralization assay.
Cross-clade virus-neutralizing activity was analyzed in a subset of day 0 and day 56 serum samples in a virus microneutralization (MN) assay based on the method used in the pandemic influenza virus reference laboratories of the Centers for Disease Control and Prevention (CDC). The following low-pathogenicity and reassortant H5N1 viruses generated by reverse genetics were obtained from CDC: A/Indonesia/5/2005 (PR8-IBCDC-RG2; clade 2.1), A/Turkey/1/05 (NIBRG-23; clade 2.2), and A/Anhui/1/05 (IBCDC-RG5, clade 2.3.4). The experiments were conducted with three replicates for each serum sample and performed at least twice.
NA inhibition assay for serum immunized with H5N1 VLP vaccine.
NA inhibition was tested using H5N1 A/Indonesia/5/2005 VLPs as the source of active enzyme and with inhibition of NA activity assessed following incubation with serum samples. The NA activity was measured using a modified fluorometric assay with 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid sodium salt hydrate (MUNANA) as a substrate and fluorometric detection of the fluorescent reaction product, 4-methylumbellipherone (MU) (35). This assay was validated and performed at Novavax as previously described (13).
Briefly, VLP samples were diluted with assay buffer containing 32.5 mM MES (morpholineethanesulfonic acid) sodium salt (Sigma), 4 mM CaCl2 (Sigma), and 0.1% Tween 20 (Sigma) (pH 6.5) to provide final NA activity within the analytical range for MU measurement. For each pre- and postimmunization serum, six 3-fold serum dilutions in assay buffer starting from dilution at 1:2 were prepared. Diluted VLP sample (30 μl) was added into each well of 96-well black microplate (Greiner Bio-One), mixed with 30 μl of each serum dilution in duplicate or with 30 μl of assay buffer for the VLP control, and then incubated at room temperature with shaking for 40 min. Next, 30 μl of 300 μM MUNANA in assay buffer with 60 μg/ml bovine serum albumin BSA) was added to each well containing VLP-serum mixtures and to wells containing 60 μl of AB1 (substrate blank), and reaction mixtures were incubated at 37°C with shaking on a Jitterbug-4 incubator-shaker (Boekel) for 60 min. The reaction was stopped by adding 150 μM stop solution (0.1 M glycine in 25% ethanol at pH 10.7), and fluorescence signals were measured with a Modulus microplate multimode reader (Turner Biosystems) at an excitation wavelength of 365 nm and an emission wavelength of 410 to 460 nm.
Readings for samples were corrected on substrate blank fluorescence and were used to generate the inhibition curve as percent residual NA activity (NAA) relative to the initial NAA for the VLP control with no serum added versus log2 serum dilution. The NA inhibition titer (NIT) was defined as the inverse of the log2 serum dilution that inhibited neuraminidase activity ≥75% relative to the initial NAA.
The groups were compared using an analysis of covariance (ANCOVA) model with fixed effects for treatment group and baseline NAI titers (day 0 titer) as a covariate to adjust the variability seen at day 0. The data were log10 transformed prior to statistical analysis. The overall treatment difference was assessed using a global F test. Pairwise comparisons of vaccine groups were analyzed and estimated by least-squares mean (LSMEANS) at day 56.
Construction of H5N1 (A/Indonesia/5/2005) HA/NA GFPDL and panning of H5-GFPDL with polyclonal human vaccine sera.
The phage display libraries expressing inserts spanning the HA and NA genes of the H5N1 A/Indonesia/05/2005 strains were constructed as previously described for H5N1 A/Vietnam/1203/04 GFPDL (16, 18). For each round of panning, equal volumes of day 56 sera obtained from five individuals in each VLP vaccine dose group were pooled. Prior to panning of GFPDL, serum components that might nonspecifically interact with phage proteins were removed by incubation with UV-killed M13K07 phage-coated petri dishes. Each subsequent GFPDL selection was carried out in solution (with protein A/G-Sepharose), and inserts of bound phages were PCR amplified and sequenced as previously described (16, 18).
Generation of H5N1 HA1 recombinant proteins.
The DNA gene segments corresponding to the HA1(1-320) and HA(28-320) proteins of H5N1 A/Indonesia/05/2005 were cloned as NotI-PacI inserts into a T7 promoter-based pSK expression vector, in which the desired polypeptide can be expressed as a fusion protein with a His6 tag at the C terminus.
E. coli Rosetta Gami cells (Novagen) were used for expression of H5N1 A/Indonesia/05/2005 HA1 proteins. Following expression, inclusion bodies (IBs) were isolated by cell lysis and multiple washing steps with 1% Triton X-100. The final pellets containing IBs were resuspended in denaturation buffer containing 6 M guanidine hydrochloride and dithioerythritol (DTE) at a final protein concentration of 10 mg/ml and were centrifuged to remove residual debris. For refolding, supernatant was slowly diluted 100-fold in redox folding buffer, followed by dialysis against 20 mM Tris HCl (pH 8.0) to remove the denaturing agents (16, 18, 20, 21). The dialysate was filtered through a 0.45-μm filter and was subjected to purification by HisTrap fast-flow chromatography following the manufacturer's protocol.
Gel filtration chromatography.
HA1(1-320) and HA(28-320) proteins at a concentration of 5 mg/ml were analyzed on a Superdex S200 XK 16/60 column (GE Healthcare) preequilibrated with phosphate-buffered saline (PBS). Protein elution was monitored at 280 nm. Protein molecular weight marker standards (GE Healthcare) were used for column calibration and generation of standard curves to identify the molecular weights of the test protein sample.
Hemagglutination assay.
Human erythrocytes were separated from whole blood (Lampire Biologicals). After isolation and washing, 30 μl of 1% (vol/vol) human red blood cell (RBC) suspension in 1% BSA-PBS was added to 30-μl serial dilutions of purified HA1 proteins or influenza virus in 1% BSA-PBS in a U-bottom 96-well plate (total volume, 60 μl). Agglutination was read after incubation for 30 min at room temperature.
Binding to oligomeric and monomeric HA1 proteins and off-rate measurements by surface plasmon resonance.
Steady-state equilibrium binding of postimmunization H5N1 human vaccine sera, selected for highest HAI titers by group from day 56 sera (n = 5 per group and corresponding to the samples used in the GFPDL analysis), was monitored at 25°C using a ProteOn surface plasmon resonance biosensor (Bio-Rad) as previously described (19). The H5N1 rHA proteins were coupled to a GLC sensor chip with amine coupling with 500 resonance units (RU) in the test flow cells. Samples of 60 μl freshly prepared sera at 10-fold dilutions were injected at a flow rate of 30 μl/min (120-s contact time) for association, and dissociation was performed over a 600-s interval (at a flow rate of 30 μl/min). Responses from the protein surface were corrected for the response from a mock surface and for responses from a separate, buffer-only injection. MAb 2D7 (anti-CCR5) was used as a negative control in these experiments. Binding kinetics for the selected human vaccine sera and data analyses were calculated using Bio-Rad ProteOn manager software (version 2.0.1).
Antibody off-rate constants, which describe the stability of the complex, i.e., the fraction of complexes that decays per second, were determined directly from serum sample interaction with HA1 protein using surface plasmon resonance (as described above) and calculated using the Bio-Rad ProteOn manager software for the heterogeneous sample model. For all polyclonal sera, it was important to demonstrate that the dissociation rates were independent of total HA binding antibody titers. To that end, parallel dissociation curves for 10-fold and 100-fold dilutions for postvaccination human sera were established as previously described (19). To improve the measurements, the off-rate constants were determined from two independent surface plasmon resonance runs.
RESULTS
Vaccination with H5N1 A/Indonesia/05/2005 VLP elicits HAI and neutralizing antibodies against homologous and heterologous influenza virus strains.
Participants in the phase I/IIa clinical trial were immunized on days 0 and 28 with 15, 45, or 90 μg HA/dose of the A/Indonesia/5/2005 (H5N1) VLP vaccine. In the initial safety and immunogenicity arm (stage A), 70 subjects were given either placebo or H5N1 VLP at 15 μg and 45 μg HA/dose. The stage A study indicated that the vaccine was generally well tolerated, but the desired SCRs and SPRs were not reached. This was followed by a dose escalation study using 15, 45, and 90 μg of H5N1 VLP vaccine compared to placebo (stage B, n = 160).
Overall, 91% of subjects completed at least 4 weeks of safety follow-up after the second dose, and 87% completed 7 months of safety observation. Only a single subject was discontinued due to an adverse event, a clinically silent laboratory abnormality that reversed spontaneously. There were no serious adverse events. Transient injection site pain was approximately twice as frequently reported by active vaccinees (39 to 56%) as placebo recipients (19 to 23%) and was dose responsive in frequency in both stage A and stage B. Injection site erythema and swelling were less frequent, but also more common in active vaccinees than placebo recipients in stage B, although not in stage A. Systemic reactogenicity symptoms were not notably different among active vaccines than placebo recipients, although in stage B, myalgia was reported by 19% of vaccines versus 8% of placebo recipients. There was no apparent association of clinical laboratory abnormalities with active vaccine, and no significant association of any spontaneously offered adverse event with active vaccine when both stages were considered.
The sera were analyzed for HAI and MN titers as previously described (14, 32). HAI for the stage B subjects on day 0 was negative, and day 56 percent seroconversion and seroprotection are shown in Table 1. No seroconversion was observed after the first vaccination in any of the dose group. There was a dose-related trend toward increased seroconversion (4-fold increase in HAI titers) and seroprotection (HAI titer of ≥40) rates based on HAI and MN testing. There was a 61% seroconversion rate based on HAI and 76% seroconversion rate based on MN in the 90-μg dose group after two injections (Table 1). Unexpectedly, one placebo recipient had a positive titer on day 56.
Table 1.
Seroconversion and seroprotection rates after the second dose of H5N1 VLP vaccinea
| Assay and HA dose (μg) | No. of vaccinees | SCR (%)b | SPR (%)c |
|---|---|---|---|
| HAI | |||
| 15 | 39 | 40 (23, 59) | 40 (23, 59) |
| 45 | 36 | 57 (37, 76) | 54 (34, 73) |
| 90 | 35 | 61 (42, 78) | 55 (36, 73) |
| Placebo | 40 | 3.1 (0, 16) | 3.1 (0, 16) |
| MN | |||
| 15 | 33 | 39 (23, 58) | 39 (23, 58) |
| 45 | 33 | 52 (34, 69) | 52 (34, 69) |
| 90 | 33 | 76 (58, 89) | 76 (58, 89) |
| Placebo | 27 | 0 (0, 13) | 0 (0, 13) |
Data are for post-second-vaccination sera collected on day 56 as tested against A/Indonesia/05/2005 (clade 2.1) influenza virus by HAI and MN.
SCR, fraction of subjects demonstrating a 4-fold increase in HAI titers over the prevaccination titer. The 95% confidence interval values are shown in parentheses.
SPR, fraction of subjects demonstrating HAI titers of ≥1:40. The 95% confidence interval values are shown in parentheses.
A subset of samples representing each of the vaccine doses were selected for cross-clade analysis. Ten samples from subjects with low or undetectable HAI titers from the 15-μg immunization group and 10 samples each from the 45- and 90-μg dosing groups with high HAI titers were evaluated in the MN assay for cross-reactivity against other clade 2 viruses (data for the 45- and 90-μg dosing groups are shown in Table 2). In this subset, the neutralization titers against the vaccine H5N1 strain A/Indonesia/05/2005 (clade 2.1) ranged between 1:20 and 1:640, titers against A/Anhui/1/05 (clade 2.3.4) ranged between 1:10 and 1:160, and titers against A/Turkey/1/05 (clade 2.2) ranged between 1:10 and 1:160. Similar seroconversion rates were observed in the preclinical studies conducted in ferrets vaccinated with A/Indonesia/05/2005 (H5N1) VLPs (30).
Table 2.
Cross-clade MN serum titers in selected sera after the second dose of H5N1 VLP vaccinea
| VLP vaccine (n = 10 subjects per group) | 45 μg HA |
90 μg HA |
||
|---|---|---|---|---|
| SCR (%)b | GMT ± 95% CI (range) | SCR (%)b | GMT ± 95% CI (range) | |
| A/Indonesia/05/2005 (clade 2.1) | 90 | 166 ± 117 (20–320) | 100 | 208 ± 180 (40–640) |
| A/Turkey/1/05 (clade 2.2) | 70 | 48 ± 29 (10–80) | 80 | 75 ± 61 (10–160) |
| A/Anhui/1/05 (clade 2.3.4) | 80 | 62 ± 30 (10–80) | 90 | 90 ± 52 (20–160) |
Data are for post-second-vaccination sera collected on day 56 and selected for high HAI titers. GMT, geometric mean titer; CI, confidence interval.
SCR, fraction of subjects demonstrating a 4-fold increase in MN titer over the prevaccination titer.
GFPDL analyses of anti-HA and anti-NA antibodies following H5N1 VLP (A/Indonesia/5/2005) vaccination.
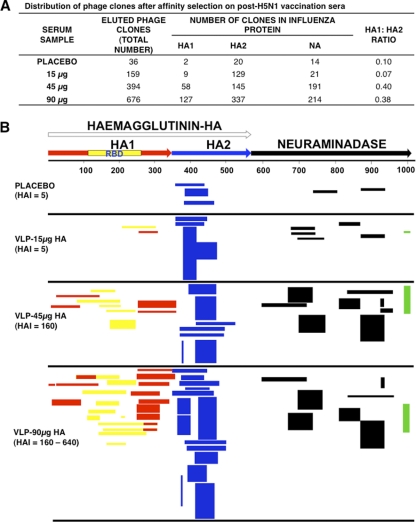
Five post-second-immunization samples (day 56 sera) from each vaccine dose group were used for panning of A/Indonesia/5/2005 GFPDL as previously described (16) (Fig. 1). The number of bound phages in the placebo group was low, and all bound to epitopes that mapped to the HA2 region, which is highly conserved with seasonal H1N1 strains. Similar findings were observed in the post-H5N1 subunit vaccination study with A/Vietnam/1194/2004 (16). In the 15-μg group with low or undetectable H5N1 HAI titers, a 5-fold increase in the number of bound phage clones was observed. However, the majority of the epitopes detected still mapped to the same HA2 region, with only a few epitopes mapping to HA1, resulting in an HA1/HA2 ratio of 0.07 (Fig. 1A and B; see Table S1 in the supplemental material). In contrast, pooled sera from both the 45- and 90-μg/dose groups exhibited a 10- to 20-fold increase in the number of bound phages. In addition to an increase in the frequency of HA2-specific phage clones detected, there was a significant expansion of HA1 fragments displaying phage clones observed using the day 56 (post-second-vaccination) immune sera (HA1/HA2 ratio = 0.4). Importantly, the HA1 epitopes captured included large segments encompassing the receptor binding domain (RBD) (shown in yellow in Fig. 1B) (Fig. 1A and B; see Table S1 in the supplemental material). Binding to neuraminidase epitopes also increased in the 45-μg and 90-μg vaccine groups (7- and 15-fold, respectively). This increase in NA binding was expected, since both HA and NA proteins are present in the VLP vaccine. Furthermore, at the two higher antigen doses (45 μg and 90 μg), the NA epitopes captured by the post-second-vaccination serum antibodies spanned the entire NA protein and included the C-terminal epitope predicted to border the sialic acid binding site (shown in green in Fig. 1B) (18).
Fig. 1.
Analysis of antibody repertoires elicited in adults following vaccination with VLP-based H5N1 A/Indonesia/5/2005 vaccine. (A) Distribution of phage clones after affinity selection with sera obtained from adults before and after two doses of H5N1 VLP vaccine. (B) Schematic alignment of the peptides recognized by post-dose 2 H5N1 sera as identified by panning with H5N1 GFPDL-A/Indonesia/5/2005. The amino acid designation is based on the H5N1 A/Indonesia/5/2005 HA protein sequence (see Fig. S1 in the supplemental material). Bars indicate identified inserts in HA1 (red bars), HA2 (blue bars), and NA (black bars). Phage displaying peptides from sequences within the HA1 receptor binding domain (RBD) are depicted with yellow bars. Peptides encompassing the C terminus of NA are shown in green. The thickness of each bar represent the frequency of repetitively isolated phage inserts (only clones with a frequency of ≥2 are shown; all sequenced clones are shown in Table S1 in the supplemental material).
H5N1 VLP (A/Indonesia/5/2005) vaccine elicits antibodies with a high capacity for binding to the functional oligomeric HA1 globular domain.
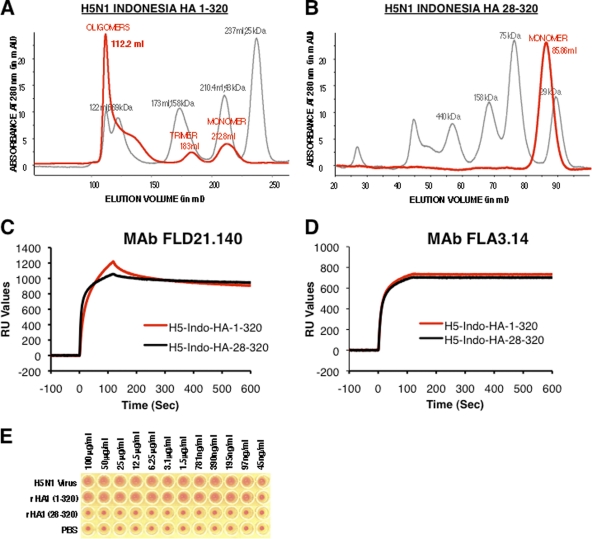
In previous studies, we demonstrated that the HA1 globular domains of multiple influenza virus strains can form functional trimers and oligomers in the absence of HA2 (17, 20, 21). The oligomerization was dependent on the first 8 amino acids in the N terminus of HA1 (20). Furthermore, HA1 globular domain proteins containing ≥80% oligomeric forms (mimicking the HA spikes) were shown to elicit broadly neutralizing antibodies and protected ferrets against challenge with pandemic influenza virus strains (20, 21). In the current study, oligomeric and monomeric HA1 recombinant proteins were used in surface plasmon resonance to capture the real-time kinetics of antibody association and dissociation rates, which reflects the overall antibody binding avidity (19). Both oligomeric rHA1(1-320) and monomeric rHA1(28-320) proteins from A/Indonesia bind to conformation-dependent H5N1-specific broadly neutralizing human monoclonal antibodies FLD21.140 and FLA3.14 (16, 18, 40) (Fig. 2 C and D). However, only the oligomeric rHA1(1-320) agglutinated human RBC (Fig. 2A and E), while the monomeric rHA1(28-320) did not (Fig. 2B and E).
Fig. 2.
Characterization of bacterially expressed and purified H5N1 HA proteins. (A and B) Characterization of purified H5N1 HA proteins from E. coli by Superdex S-200 gel filtration chromatography. Purified H5N1 HA1 proteins with an intact N terminus (positions 1 to 320) (A) and HA1 with an N-terminal deletion (28 to 320) (B) were subjected to gel filtration. The panels show superimposed elution profiles of purified HA1 proteins (red lines) overlaid with calibration standards (gray lines). The elution volumes of protein species are shown in parentheses. (C and D) Steady-state binding equilibrium analysis of conformation-dependent human H5N1 neutralizing MAbs FLD21.40 (C) and FLA3.14 (D) at 10 μg/ml to purified bacterially expressed H5N1 HA1 proteins immobilized on a sensor chip through the free amine group and on a blank flow cell, free of peptide. Binding was recorded using a ProteOn system surface plasmon resonance biosensor instrument (Bio-Rad Labs, Hercules, CA). (E) Agglutination of human RBCs by properly folded oligomeric H5N1 rHA1(1-320) protein and its monomeric H5N1 rHA1(28-320) counterpart along with rgH5N1 virus. Serial dilutions of purified HA1 proteins were mixed with washed RBCs, and hemagglutination was read after 30 min at room temperature. Reassorted virus rgH5N1xPR8 (2:6) A/Vietnam/1203/2004 (clade 1) was used as a positive control.
Binding of post-second-vaccination sera from the low-dose VLP (15 μg HA) to rHA1(1-320) was very low (≤300 RU) (Fig. 3 A). In contrast, binding of antibodies to the oligomeric rHA1(1-320) was much higher with sera obtained following administration of VLP vaccine containing either 45 μg or 90 μg of HA/dose, ranging from 500 to 1,500 RU and 1,000 to 2,000 RU, respectively (Fig. 3B and C). The total serum antibody binding to rHA1 correlated with the serum HAI titer trend within each vaccine group.
Fig. 3.
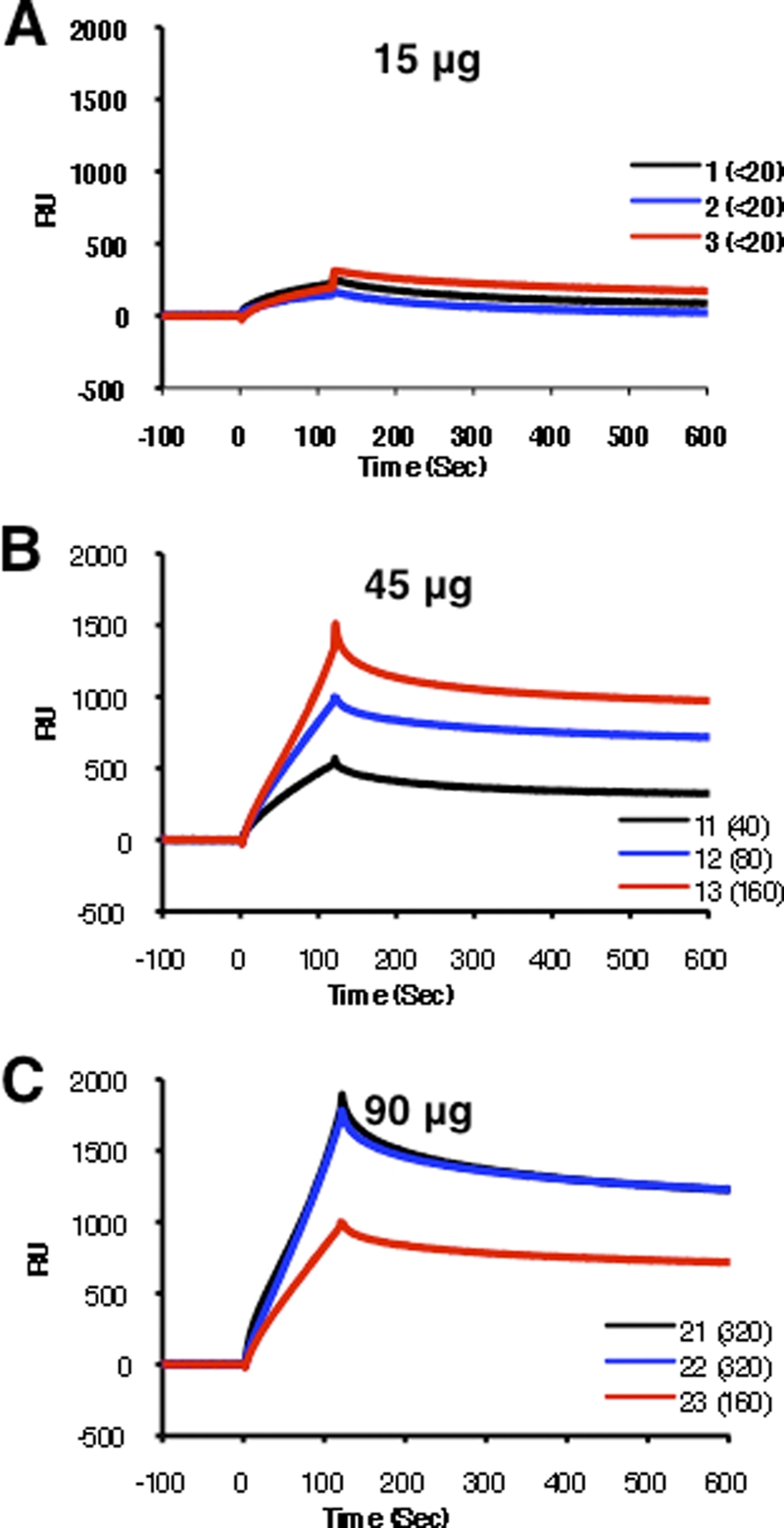
Kinetics of binding of post-H5N1 vaccination human serum to properly folded oligomeric H5N1 HA1 proteins using surface plasmon resonance. Steady-state equilibrium analysis of the binding of human postvaccine serum to properly folded functional HA1 oligomers was done using surface plasmon resonance and is shown for representative samples from each group. Ten-fold-diluted individual post-H5N1 vaccination sera from the three VLP dose vaccine groups (15 μg [A], 45 μg [B], and 90 μg [C]) after a second dose were injected simultaneously onto HA1 proteins immobilized on a sensor chip through the free amine group and onto a blank flow cell, free of peptide. Binding of the antibodies to the immobilized protein is shown as resonance units (RU). Data are shown for three representative samples for each dose group, and their HAI titers against H5N1 A/Indonesia/5/2005 are given in parentheses.
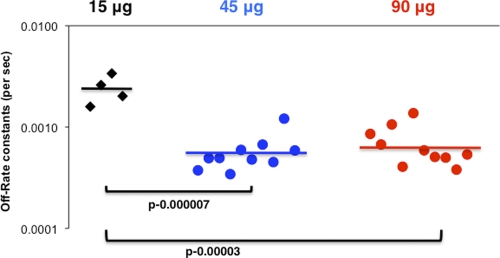
To further investigate if two vaccinations with H5N1 VLP (45 μg and 90 μg HA/dose) lead to an increase in antibody avidity compared to that with the low-dose vaccine (15 μg HA), we determined the off-rate constants of the post-second-vaccination human sera following binding to the functional oligomeric rHA1 using surface plasmon resonance. As depicted in Fig. 4, immune sera obtained from subjects given 15 μg/dose demonstrated low binding affinity with high dissociation rate constants (>10−3) (Fig. 4, black diamonds), while sera obtained from responders in the 45-μg and 90-μg dose groups showed around ∼1 log lower dissociation off-rate constants (≤10−4) than sera from subjects from the low-dose group (Fig. 4, blue and red circles).
Fig. 4.
Serum antibody affinity to hemagglutinin as measured by off-rate constants in post-H5N1 vaccination human sera in different VLP dose groups. Surface plasmon resonance analysis of human sera postvaccination with H5N1 VLP vaccine from three VLP dose groups of the vaccine trial was performed with properly folded functional oligomeric H5N1 HA1 (A/Indonesia/5/2005). Serum antibody off-rate constants were determined as described in Materials and Methods. Correlation statistics of the off-rate constants of the postvaccine human sera between different vaccine groups were highly significant for the 45-μg versus 15-μg and the 90-μg versus 15-μg dose groups (P < 0.005 by t test).
H5N1 A/Indonesia/5/2005 VLPs elicit antibodies with preferential binding to oligomeric rHA1.
The VLP platform was designed to express the recombinant HA proteins in a form that mimics native virus (5, 37). Therefore, we determined if the surface plasmon resonance assay can differentiate relative binding of postvaccination human sera to oligomeric versus monomeric rHA1 proteins. As shown in Fig. 5 A, the same serum samples from all the vaccine arms, which were analyzed by GFPDL (Fig. 1), bound preferentially to the oligomeric rHA1(1-320) compared to the monomeric rHA1(28-320) (Fig. 5A, red versus black bars). Furthermore, the ratios of maximum RU (oligomeric/monomeric) values from 10 individuals each from the 45-μg and 90-μg HA groups after the second vaccination correlated with the microneutralization (MN) titers against A/Indonesia/5/2005 (r = 0.83) (Fig. 5B). These findings suggest that HA oligomer binding antibodies are important in virus neutralization, as we previously demonstrated in ferrets (20, 21).
Fig. 5.
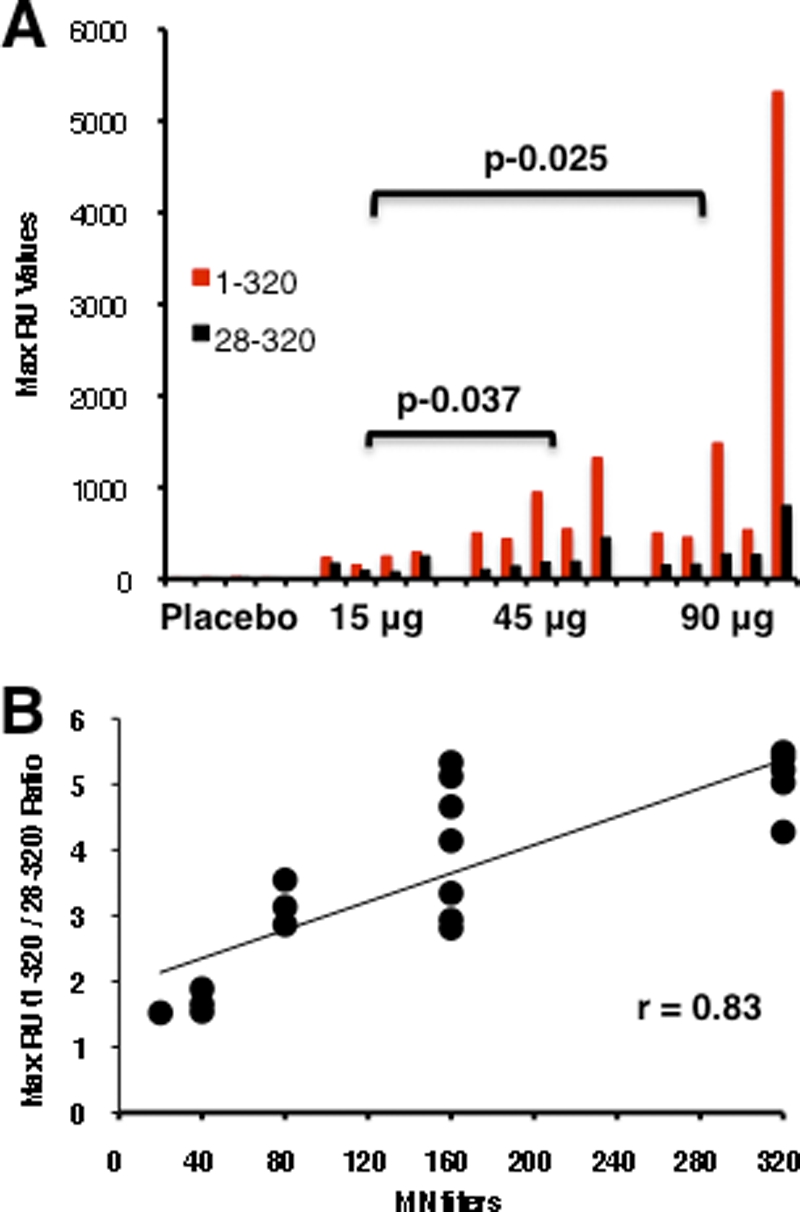
Binding of oligomeric versus monomeric HA1 proteins by human sera following immunization with H5N1 VLPs (A/Indonesia/5/2005). (A) Maximum RU values for oligomeric HA1(1-320) (red bars) versus monomeric HA1(28-320) (black bars) binding by serum antibodies from individuals after a second dose of A/Indonesia/5/2005 H5N1 VLP (placebo, 15 μg, 45 μg, or 90 μg) in surface plasmon resonance are shown for the same serum samples that were analyzed by GFPDL (Fig. 1). The differences in binding of post-H5N1 vaccination human sera to oligomeric HA1 between different VLP dose groups were statistically significant for the 45-μg versus 15-μg and the 90-μg versus 15-μg dose groups (P < 0.05 by t test). (B) Correlation between in vitro neutralizing titers and oligomeric/monomeric HA1 binding ratio in human sera following immunization with H5N1 VLP vaccine in different dose groups. Oligomeric/monomeric HA1 binding ratios [HA(1-320) versus HA(28-320), from panel A] correlated with microneutralization titers against A/Indonesia/5/2005 (r2 = 0.83).
High-dose H5N1 VLP (A/Indonesia/5/2005) vaccine elicits neuraminidase-inhibiting antibodies that differentially map to the sialic acid binding site.
Antineuraminidase antibodies were also detected in the stage B subjects (Fig. 6 A). The analysis of NAI responses indicated an overall treatment difference (global F test, P < 0.0001). The pair wise comparison (LSMEANS) of the vaccine groups revealed that the 45-μg and 90-μg groups were significantly different from the placebo group, with P values of <0.0001 and 0.0008, respectively. In addition, the 15-μg group was significantly different from the 45-μg group. However, no significant difference was observed between the 15-μg group and the placebo group.
Fig. 6.
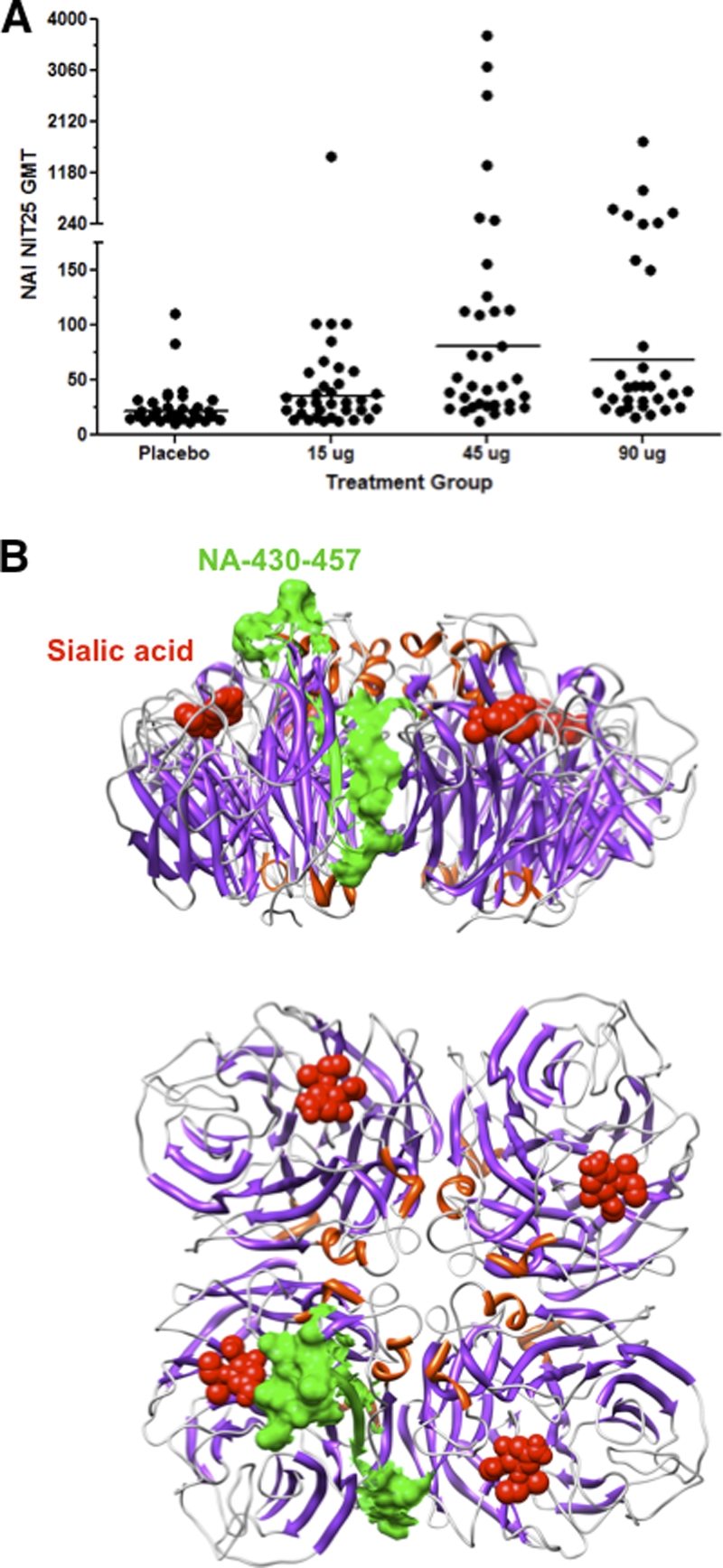
Antineuraminidase antibodies and antigenic mapping of neuraminidase-inhibiting antibodies elicited by human sera following immunization with H5N1 VLPs (A/Indonesia/5/2005). (A) Neuraminidase enzymatic activity inhibition (NAI) titers for prevaccine and post-second-vaccination human sera were measured as described in Materials and Methods. The pairwise comparison (LSMEANS) of the vaccine groups revealed that the 45-μg and 90-μg groups were significantly different from the placebo group, with P values of <0.0001 and 0.0008, respectively. In addition, the 15-μg group was significantly different from the 45-μg group. (B) The immunodominant epitope [NA(978-1004)] in NA is shown on the tetrameric NA structure (Protein Data Bank [PDB] identifier 2HTY) (corresponding to the green bars in Fig. 1B) and is aligned with the bound sialic acid shown in red. Side (top) and bird's-eye (bottom) views are shown.
By comparing the patterns of epitope recognition found in the sera from individuals receiving high and low vaccine doses, it is clear that only sera from subjects immunized with the high-dose vaccine contained antibodies that recognize segments encompassing the neuraminidase amino acid sequence positions 978 to 1004 (Fig. 1A and B; see Table S1 in the supplemental material). Antigenic mapping of this C-terminal epitope on the NA structure identified a region which juxtaposes the sialic acid binding site of neuraminidase, as previously observed in patients who survived H5N1 infection in Vietnam (Fig. 6B, shown in green) (18).
Together, these data suggest that the H5N1 VLPs generated functional antibodies against targets in both HA and NA, including epitopes dependent on the multimeric forms of these proteins.
DISCUSSION
Recombinant vaccine technology has the potential to provide a rapid response to emerging influenza virus infections with pandemic potential, since it does not rely on the derivation of vaccine viruses with high growth in eggs. The VLP platform is particularly attractive for vaccines against highly pathogenic zoonotic influenza virus strains, since it does not require the use of live virus in any stage of the manufacturing process. Furthermore, VLPs present the key antigenic targets of influenza virus, HA and NA, in a native-like conformation similar to the intact virion. Studies in animal models also provided evidence for generation of cross-clade protective antibodies following H5N1 VLP (but not rHA) immunization (4, 5, 30, 38).
The current study was conducted with immune sera from a subset of participants in the first human phase I/II trial of an A/Indonesia/05/2005 (H5N1) VLP vaccine candidate. The vaccine was generally well tolerated, and after two vaccinations, HAI seroconversion rates (SCRs) of 40%, 57%, and 61% and MN SCRs of 39%, 52%, and 76% were achieved in the 15-, 45-, and 90-μg groups, respectively. Cross-reactivity against other H5N1 clade 2 subtypes was also demonstrated with high-responder sera. GFPDL analyses (A/Indonesia/5/2005; HA/NA) revealed broad epitope repertoires recognized by immune sera from the two higher-antigen-dose groups, which included large segments spanning the RBD in the HA1 globular head and the NA C terminus. We identified an immunodominant epitope in the HA2 domain in preimmune sera that was significantly boosted following vaccination. A similar epitope was identified in our previous studies with H5N1 A/Vietnam/1203/2004 vaccine trials (16). This HA2 epitope sequence is highly conserved (98%) within H5N1 and seasonal H1N1 strains and therefore may be present due to preexisting immunity from either vaccination or exposure to seasonal H1N1 strains. Since reactivity to this HA2 epitope was also boosted with the 15-μg VLP dose, which had no significant virus-neutralizing activity, it is unlikely that antibodies against this HA2 epitope contribute to protection.
Binding of antibodies to properly folded oligomeric H5N1 rHA1(1-320) protein was significantly higher than that to monomeric rHA1(28-320) for all sera obtained following high-dose vaccinations. The oligomeric/monomeric HA1 binding ratio correlated with virus neutralization titers. Neuraminidase inhibition ratios of above 2 (post-second vaccination/prevaccination) were detected in sera from the high-dose groups and correlated with binding to an NA C-terminal epitope that is located close to the sialic acid binding site.
The HAI titers and cross-clade reactivity of sera in the human trial are in agreement with the preclinical studies involving ferrets and a similar VLP vaccine product (30). The H5N1 VLP vaccine was immunogenic, and the HAI titers were comparable to those reported with an inactivated egg-derived H5N1 (A/Vietnam/1203/04) vaccine previously licensed in the United States (1, 2, 43). Furthermore, we measured heterologous neutralization of other clade 2 subtypes not found after vaccination with the egg-based inactivated subunit A/Vietnam vaccine unless combined with oil in water adjuvants (16, 24, 28, 43). A plant-based H5N1 VLP vaccine was also reported to elicit cross-reactive neutralizing antibodies in animals and humans (23). This difference could also reflect a difference in the immunogenicities of A/Indonesia/05/2005 HA and A/Vietnam/1203/04, as previously observed in animal experiments (4). More recently, a VLP vaccine against the pandemic H1N1 strain was evaluated in the Mexican population and was found to be safe and immunogenic (27).
The role of oligomeric HA in eliciting protective antibodies is an important finding. There is growing evidence that oligomeric vaccines present epitopes that are specific to virion spikes and that are absent on monomeric proteins. Furthermore, antibodies that bind preferentially (with high avidity) to oligomeric compared with monomeric HA proteins were reported to have broader cross-reactivity (31, 34, 39, 48, 52). Therefore, several recombinant influenza virus and HIV vaccines were designed to include trimerization domains in order to elicit broadly neutralizing antibodies (3, 50, 51). Since the H5N1 VLP vaccine used in the current trial naturally presents multimeric HA and NA to the immune system, it was important to determine if the human immune sera contained antibodies that bind differentially to oligomeric versus monomeric structures. In earlier studies we have expressed recombinant HA1 proteins from multiple influenza virus strains that were enriched for oligomeric or monomeric HA structures. We previously demonstrated that oligomeric rHA1 proteins were highly immunogenic and induced superior protective immunity against homologous and heterologous strains compared with monomeric HA1 or HA0 recombinant proteins (references 20 and 21 and unpublished data). In the current study, we demonstrated that the H5N1 VLPs generated antibodies that preferentially bound to oligomeric A/Indonesia/5/2005 rHA1(1-320) compared with monomeric rHA1(28-320) protein (Fig. 5). Furthermore, a positive correlation between the oligomeric/monomeric HA1 binding ratios and the in vitro neutralization titers for individual postvaccination human sera was demonstrated. In concordance, we found that the oligomeric/monomeric binding ratios of human immune sera from egg-based H5N1 subunit vaccination (A/Vietnam/1203/04, SP) (1) were around 1, with no clear preference for oligomeric HA1 binding (data not shown).
Neuraminidase-neutralizing antibodies elicited by vaccines may provide added value to the protective immunity against both seasonal and avian influenza viruses. In the case of traditional inactivated subunit vaccines, the neuraminidase content is not monitored, and the fraction of functional protein may vary considerably with the manufacturing process (7, 10, 11, 26, 41, 46, 47). In contrast to the inactivated influenza virus vaccines, the VLP platform presents NA in its native tetrameric form, thereby increasing the opportunity to elicit protective antibodies against this important viral membrane protein. The reactivity of the antibodies against the NA C terminus, demonstrated in the current VLP vaccine trial, is likely to interfere with the binding of NA to sialic acid, which is required for its enzymatic activity and release of virions from infected cells.
In summary, our data provide the first insight into the quality of the epitope repertoire and avidity of the antibodies elicited by H5N1 VLP vaccine in humans. The increased binding to oligomeric HA and the C terminus of NA may contribute to the broader cross-reactivity of the immune sera seen in subjects administered VLP vaccines. However, no clear dose sparing was provided by the VLP platform compared with the inactivated H5N1 vaccine. Future trials should determine if a VLP-adjuvant combination could improve immunogenicity and dose response.
Supplementary Material
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 24 August 2011.
REFERENCES
- 1. Beigel J. H., Voell J., Huang C. Y., Burbelo P. D., Lane H. C. 2009. Safety and immunogenicity of multiple and higher doses of an inactivated influenza A/H5N1 vaccine. J. Infect. Dis. 200:501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bernstein D. I., et al. 2008. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J. Infect. Dis. 197:667–675 [DOI] [PubMed] [Google Scholar]
- 3. Bosch B. J., et al. 2010. Recombinant soluble, multimeric HA and NA exhibit distinctive types of protection against pandemic swine-origin 2009 A(H1N1) influenza virus infection in ferrets. J. Virol. 84:10366–10374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bright R. A., et al. 2008. Cross-clade protective immune responses to influenza viruses with H5N1 HA and NA elicited by an influenza virus-like particle. PLoS One 3:e1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bright R. A., et al. 2007. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine 25:3871–3878 [DOI] [PubMed] [Google Scholar]
- 6. Brown D. R., et al. 2011. The humoral response to Gardasil(R) over four years as defined by Total IgG and competitive Luminex immunoassay. Hum. Vaccin. 7:230–238 [DOI] [PubMed] [Google Scholar]
- 7. Colman P. M. 1992. Structural basis of antigenic variation: studies of influenza virus neuraminidase. Immunol. Cell Biol. 70:209–214 [DOI] [PubMed] [Google Scholar]
- 8. Crawford J., et al. 1999. Baculovirus-derived hemagglutinin vaccines protect against lethal influenza infections by avian H5 and H7 subtypes. Vaccine 17:2265–2274 [DOI] [PubMed] [Google Scholar]
- 9. Deschuyteneer M., et al. 2010. Molecular and structural characterization of the L1 virus-like particles that are used as vaccine antigens in Cervarix, the AS04-adjuvanted HPV-16 and -18 cervical cancer vaccine. Hum. Vaccin. 6:407–419 [DOI] [PubMed] [Google Scholar]
- 10. Eichelberger M., et al. 2008. FDA/NIH/WHO public workshop on immune correlates of protection against influenza A viruses in support of pandemic vaccine development, Bethesda, Maryland, US, December 10-11, 2007. Vaccine 26:4299–4303 [DOI] [PubMed] [Google Scholar]
- 11. Eichelberger M. C., Hassantoufighi A., Wu M., Li M. 2008. Neuraminidase activity provides a practical read-out for a high throughput influenza antiviral screening assay. Virol. J. 5:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gambotto A., Barratt-Boyes S. M., de Jong M. D., Neumann G., Kawaoka Y. 2008. Human infection with highly pathogenic H5N1 influenza virus. Lancet 371:1464–1475 [DOI] [PubMed] [Google Scholar]
- 13. Gavrilov V., et al. 2011. Influenza virus-like particles as a new tool for vaccine immunogenicity testing: validation of a neuraminidase neutralizing antibody assay. J. Virol. Methods 173:364–373 [DOI] [PubMed] [Google Scholar]
- 14. Katz J. M., et al. 1999. Antibody response in individuals infected with avian influenza A (H5N1) viruses and detection of anti-H5 antibody among household and social contacts. J. Infect. Dis. 180:1763–1770 [DOI] [PubMed] [Google Scholar]
- 15. Kemp T. J., et al. 2011. HPV16/18 L1 VLP vaccine induces cross-neutralizing antibodies that may mediate cross-protection. Vaccine 29:2011–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khurana S., et al. 2010. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci. Transl. Med. 2:15ra5. [DOI] [PubMed] [Google Scholar]
- 17. Khurana S., et al. 2011. Recombinant HA1 produced in E. coli forms functional oligomers and generates strain-specific SRID potency antibodies for pandemic influenza vaccines. Vaccine 29:5657–5665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khurana S., et al. 2009. Antigenic fingerprinting of H5N1 avian influenza using convalescent sera and monoclonal antibodies reveals potential vaccine and diagnostic targets. PLoS Med. 6:e1000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khurana S., et al. 2011. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci. Transl. Med. 3:85ra48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khurana S., et al. 2011. Bacterial HA1 vaccine against pandemic H5N1 influenza virus: evidence of oligomerization, hemagglutination, and cross-protective immunity in ferrets. J. Virol. 85:1246–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khurana S., et al. 2010. Properly folded bacterially expressed H1N1 hemagglutinin globular head and ectodomain vaccines protect ferrets against H1N1 pandemic influenza virus. PLoS One 5:e11548. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22. Lakey D. L., et al. 1996. Recombinant baculovirus influenza A hemagglutinin vaccines are well tolerated and immunogenic in healthy adults. J. Infect. Dis. 174:838–841 [DOI] [PubMed] [Google Scholar]
- 23. Landry N., et al. 2010. Preclinical and clinical development of plant-made virus-like particle vaccine against avian H5N1 influenza. PLoS One 5:e15559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Langley J. M., et al. 2010. Safety and cross-reactive immunogenicity of candidate AS03-adjuvanted prepandemic H5N1 influenza vaccines: a randomized controlled phase 1/2 trial in adults. J. Infect. Dis. 201:1644–1653 [DOI] [PubMed] [Google Scholar]
- 25. Latham T., Galarza J. M. 2001. Formation of wild-type and chimeric influenza virus-like particles following simultaneous expression of only four structural proteins. J. Virol. 75:6154–6165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li X., et al. 2006. Essential sequence of influenza neuraminidase DNA to provide protection against lethal viral infection. DNA Cell Biol. 25:197–205 [DOI] [PubMed] [Google Scholar]
- 27. Lopez-Macias C., et al. 2 August 2011. Safety and immunogenicity of a virus-like particle pandemic influenza A (H1N1) 2009 vaccine in a blinded, randomized, placebo-controlled trial of adults in Mexico. Vaccine. doi:10.1016/j.vaccine.2001.07.099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lu H., et al. 2011. A rapid Flp-In system for expression of secreted H5N1 influenza hemagglutinin vaccine immunogen in mammalian cells. PLoS One 6:e17297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maass D. R., Atkinson P. H. 1990. Rotavirus proteins VP7, NS28, and VP4 form oligomeric structures. J. Virol. 64:2632–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mahmood K., et al. 2008. H5N1 VLP vaccine induced protection in ferrets against lethal challenge with highly pathogenic H5N1 influenza viruses. Vaccine 26:5393–5399 [DOI] [PubMed] [Google Scholar]
- 31. Mikell I., et al. 2011. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog. 7:e1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Noad R., Roy P. 2003. Virus-like particles as immunogens. Trends Microbiol. 11:438–444 [DOI] [PubMed] [Google Scholar]
- 33. Noah D. L., Hill H., Hines D., White E. L., Wolff M. C. 2009. Qualification of the hemagglutination inhibition assay in support of pandemic influenza vaccine licensure. Clin. Vaccine Immunol. 16:558–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pancera M., et al. 2010. Crystal structure of PG16 and chimeric dissection with somatically related PG9: structure-function analysis of two quaternary-specific antibodies that effectively neutralize HIV-1. J. Virol. 84:8098–8110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Potier M., Mameli L., Belisle M., Dallaire L., Melancon S. B. 1979. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-alpha-d-N-acetylneuraminate) substrate. Anal. Biochem. 94:287–296 [DOI] [PubMed] [Google Scholar]
- 36. Pushko P., et al. 2010. Recombinant H1N1 virus-like particle vaccine elicits protective immunity in ferrets against the 2009 pandemic H1N1 influenza virus. Vaccine 28:4771–4776 [DOI] [PubMed] [Google Scholar]
- 37. Pushko P., et al. 2005. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine 23:5751–5759 [DOI] [PubMed] [Google Scholar]
- 38. Roldao A., Mellado M. C., Castilho L. R., Carrondo M. J., Alves P. M. 2010. Virus-like particles in vaccine development. Expert Rev. Vaccines 9:1149–1176 [DOI] [PubMed] [Google Scholar]
- 39. Scheid J. F., et al. 2009. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458:636–640 [DOI] [PubMed] [Google Scholar]
- 40. Simmons C. P., et al. 2007. Prophylactic and therapeutic efficacy of human monoclonal antibodies against H5N1 influenza. PLoS Med. 4:e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Straight T. M., Ottolini M. G., Prince G. A., Eichelberger M. C. 2008. Antibody contributes to heterosubtypic protection against influenza A-induced tachypnea in cotton rats. Virol. J. 5:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Treanor J. J., et al. 1996. Evaluation of a recombinant hemagglutinin expressed in insect cells as an influenza vaccine in young and elderly adults. J. Infect. Dis. 173:1467–1470 [DOI] [PubMed] [Google Scholar]
- 43. Treanor J. J., Campbell J. D., Zangwill K. M., Rowe T., Wolff M. 2006. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N. Engl. J. Med. 354:1343–1351 [DOI] [PubMed] [Google Scholar]
- 44. Treanor J. J., et al. 2006. Dose-related safety and immunogenicity of a trivalent baculovirus-expressed influenza-virus hemagglutinin vaccine in elderly adults. J. Infect. Dis. 193:1223–1228 [DOI] [PubMed] [Google Scholar]
- 45. Treanor J. J., et al. 2001. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine 19:1732–1737 [DOI] [PubMed] [Google Scholar]
- 46. Tulip W. R., Varghese J. N., Laver W. G., Webster R. G., Colman P. M. 1992. Refined crystal structure of the influenza virus N9 neuraminidase-NC41 Fab complex. J. Mol. Biol. 227:122–148 [DOI] [PubMed] [Google Scholar]
- 47. Tulip W. R., Varghese J. N., Webster R. G., Laver W. G., Colman P. M. 1992. Crystal structures of two mutant neuraminidase-antibody complexes with amino acid substitutions in the interface. J. Mol. Biol. 227:149–159 [DOI] [PubMed] [Google Scholar]
- 48. Walker L. M., et al. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang K., et al. 2006. Expression and purification of an influenza hemagglutinin—one step closer to a recombinant protein-based influenza vaccine. Vaccine 24:2176–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wei C. J., et al. 2008. Comparative efficacy of neutralizing antibodies elicited by recombinant hemagglutinin proteins from avian H5N1 influenza virus. J. Virol. 82:6200–6208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weldon W. C., et al. 2010. Enhanced immunogenicity of stabilized trimeric soluble influenza hemagglutinin. PLoS One 5:e12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wrammert J., et al. 2011. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 208:181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.