Abstract
A member of the family Circoviridae, porcine circovirus type 2 (PCV2), is associated with postweaning multisystemic wasting syndrome (PMWS), a recent emerging disease worldwide. PCV2 is also found in clinically asymptomatic animals. This paradoxical finding makes the syndrome etiology challenging. We developed new assays to study PCV2 with links to syndrome etiology. For analysis, we used PCV2-infected tissues from subclinically infected and diseased piglets. We compared antigen- and PCV2 DNA-derived signals for tissue localization and intensity. Oligonucleotides were designed to the signature motif of the PCV2 capsid open reading frame to discriminate experimentally between PCV2 genotype groups by PCR, in situ hybridization (ISH), and fluorescence in situ hybridization (FISH). Unexpectedly, all PCV2-infected animals carried both PCV2a and PCV2b genotype group members. Using confocal microscopy, genotype single-cell infections and cell superinfections were visible. Additionally, we discriminated replicative DNA from total PCV2 DNA isoforms with FISH. This aided in our inquiry into cellular genotype-specific replication. Importantly, single-genotype-group replication was not observed. In infected cells with replicating virus, both genotype groups were equally present. These findings suggest PCV2 genotype group members relaxed replication regulation requirements and may even point to PCV2 replication cooperativity in vivo. These observations explain the readily seen PCV2 DNA recombinations and the high overall PCV2 genome plasticity. Hence, we suggest a novel mechanism of syndrome etiology that consists of a continuously changing PCV2 genome pool in hosts and pig herds, posing a constant challenge to the individual maturing immune system.
INTRODUCTION
Members of the family Circoviridae and, through interspecies recombination, related viruses cause diseases in vertebrates and plants (14, 20). Virus interspecies recombination has been linked to disease outbreak (43). The Circoviridae members porcine circovirus type 2 (PCV2) and its apathogenic PCV1 relative are the smallest autonomous replicating viruses known in eukaryotic cells. PCV2 and PCV1 genomic sequences elicit a little less than 80% sequence identity. They are nonenveloped viruses that possess a closed, circular, single-stranded DNA (ssDNA) genome (1). Upon infection, the ssDNA genome is converted into a double-stranded (ds) intermediate, the replicative virus DNA isoform (30) or late virus cycle form, which serves as a template for rolling-circle synthesis of the viral ssDNA (38). PCV replication occurs by the so-called melting-pot rolling-circle replication mechanism (11). Of note, one new PCV2 ssDNA is formed per PCV2 dsDNA molecule (11).
The small genome of PCV2 contains at least four open reading frames (ORFs) with known functions: ORF1 codes for two replicase proteins, ORF2 for the structural protein (cap gene), and ORF3 for a protein implicated in cellular apoptosis that overlaps with ORF1 (24, 28). The PCV2 genome is ambisense, i.e., the encapsidated viral DNA strand serves as a template for transcription of the capsid protein gene (ORF2) while the cDNA strand of the replicase functions as a transcription template for the replicase gene (ORF1) (28).
PCV2 emerged in pigs potentially as the result of a cross-species jump from birds into domestic pigs (13), most likely through the wild-boar intermediary host less than a couple of hundred years ago (13). PCV2 adaptation to new hosts is truly remarkable, and adaptation is not restricted to and does not end with pigs, as we (unpublished data) and other research groups (19, 39) found PCV2 in bovines. Also, other studies describe PCV2 infections in mice and humans (3, 23, 25). This adaptive virus characteristic is possibly due to the viral genomic plasticity at a mutation rate almost as high as that described for RNA viruses (13).
Indeed, PCV2 seems to be the primary etiological agent for postweaning multisystemic wasting syndrome (PMWS). Nevertheless, infections are more prevalent than disease, and in animal infection experiments, severe clinical signs are rarely manifested. In PCV2-infected animals, a transient lymphopenia, and in neonates, a general depression of the immune system are often observed (7, 40). PMWS is an excellent example of a disease caused by Circoviridae. It is a comparatively recent disease, as the first cases occurred in the early 1990s in western Canada (10). Although PMWS clinical symptoms are well described, with wasting and enlarged lymph nodes (35) and microscopic lesions in lymphoid organs due to lymphocyte depletion and histocytic infiltrations, the syndrome appears to be multifactorially triggered with a poorly defined disease etiology (36). Hence, researchers suggested that cofactors, such as other bacterial or viral infections, overstimulation or immune suppression, a general genetic shift in the pig population, or pig management changes, were essential for PMWS development (27). However, none of these cofactors were evident for the Swiss epizootic (41).
Phylogenetic studies divided PCV2 into two major genotype groups, namely, PCV2a and PCV2b (37). A simple distinction between the PCV2a and PCV2b genotypes was achieved by comparing a distinct stretch of amino acids in the viral capsid protein, the so-called signature motif (6). By this criterion, PCV2c (9), a third genotype group, may also be included within the PCV2b genotype group. A genetic shift from PCV2a to a newly emerging PCV2b genotype group was detected in Canadian and U.S. PMWS outbreaks (4, 6). By analogy, we found a dominance of PCV2b genotype group members during the epizootic, although PCV2b genotype group members were also found before the epizootic with the occurrence of dominant PCV2a genotype group members (41). Additionally, we found, besides the PCV2b genotype group dominance, a subgenotype shift (41) correlated with the PMWS epizootic. Interestingly, various research groups found further evidence for different virulence levels of PCV2 variants in vitro and in animal infection models (12, 17, 33). Although substantial efforts were made, neither genotype group members nor a single member was found to be directly correlated with PMWS disease (2, 32).
To complicate the mechanism of disease onset, it was seen that animals with PMWS disease might carry multiple PCV2 genotype group members (15, 18). Furthermore, several studies presented sequence evidence for PCV2 genotype group members' recombination (18, 21, 26). Recombination events were observed by amplicate sequencing analysis in both major PCV2 ORFs (5, 32). One may surmise that the contribution of recombination to PCV2 genomic evolution may be the critical factor in initiating PMWS in an animal's immune system already weakened by lymphopenia caused by PCV2. As different studies also showed, recombination in other viruses may be linked to zoonoses and severe disease outbreaks (14, 43).
In order for virus recombination to take place, at least two different noncompetitive viruses' DNAs need to intracellularly coreplicate. We studied the presence of major genotype groups in piglets at the organ and cellular levels by PCR, in situ hybridization (ISH), and fluorescence in situ hybridization (FISH) technologies. In this work, we consistently noticed the presence of both major PCV2 genotype groups in PCV2 preepizootic- and postepizootic-infected piglets irrespective of the virus concentration within the animal. We confirmed our previous observations that the PCV2a genotype group dominated preepizootically and PCV2b, more specifically PCV2b-CH, during the epizootic. Interestingly, we found superinfection and coreplication of members of both major PCV2 genotype groups. Surprisingly, however, the occurrence of PCV2 in vivo replication was exclusive to cells with both genotype group members, although virus genotype group single-cell infections were also seen.
MATERIALS AND METHODS
Ethics statement.
The tissue samples were taken from routine necropsy operations by the Institute of Veterinary Pathology, University of Zurich, and adhered to standards and regulations of ISO/IEC 17025 (number STS 255). This study was carried out in strict accordance with the recommendations of the Swiss Federal Veterinary Office (FVO) and according to the Ethical Principles and Guidelines for Experiments on Animals as formulated jointly by the Swiss Academy of Medical Sciences (SAMS) and the Swiss Academy of Sciences (SCNAT).
Samples, plasmids, and recombinant PCV2.
Paraffin-embedded archived necropsy tissues from piglets from the years 1981 through 2009 were selected randomly and arranged in different tissue microarray (TMA) blocks. Mostly, secondary lymphoid tissues, including the spleen, tonsils, lymph nodes, and Peyer's patches of the ileum, were used. The tissues were from piglets from 2 to 4 months of age. We selected and investigated 42 piglet cases and 6 subclinical immunohistochemically (IHC) negative cases. PMWS cases are defined by IHC (29) as containing medium to high levels of PCV2 antigen and showing histological lesions and clinical disease symptoms. As previously described, the presence of PCV2 antigen was determined by IHC (41). DNA was extracted from the same samples using the DNeasy blood tissue kit (Qiagen, Inc.) according to the manufacturer's instructions.
Plasmids for PCV2- and genotype-specific PCR controls were constructed by ligation of PCV2a (AF109398 or AY754018) or PCV2b (DQ923523) PCR fragments (137 bp excluding oligonucleotide sequences) (41) encoding the signature motif into the pCR2.1-TOPO vector (Invitrogen). Correct inserts were confirmed by EcoRI restriction endonuclease (New England BioLabs Inc.) digestion and sequencing of the constructs.
The recombinant PCV2a genotype of the AY754016 subgenotype and PCV2b DQ923523 subgenotype were constructed by PCR amplification from PCV2-infected fixed and paraffin-embedded pig tissue. We used the oligonucleotide pairs PCV2forP (5′-GCA AAT GGG CTG CTA ATT TTG CAG-3′) and PCV2revE (5′-GAA TAA GAA AGG TTA AGG TTG AAT TCT GG-3′), and PCV2forE (5′-CCA GAA TTC AAC CTT AAC CTT TCT TAT TC-3′) and PCV2revP (5′-CAC TTC TTC ACC ATG GTA ACC ATC C-3′) for PCR amplification. PCR amplificates were digested with EcoRI restriction endonuclease (New England BioLabs Inc.) and PflMI restriction endonuclease (New England BioLabs Inc.) and ligated into EcoRI (New England BioLabs Inc.)-digested calf intestinal alkaline phosphatase (CIP) (New England BioLabs Inc.)-treated pCR2.1-TOPO vector (Invitrogen). Whole PCV2 genotype members were excised by EcoRI and religated before introduction into the porcine kidney 15 (PK15) cell line with Lipofectamine 2000 reagent (Invitrogen). PK15 cells contained neither PCV1 nor PCV2 before transfection.
DNA preparation and genotype group-specific PCRs.
Total DNA was extracted from 6-μm slices of fixed and paraffin-embedded piglet tissues using the DNeasy blood tissue kit (Qiagen, Inc.) according to the manufacturer's instructions. PCR amplification of dominant PCV2 sequence from tissue sections was previously described (41). PCR amplification of PCV2a genotype group-specific sequences was achieved using the forward and reverse primers PCV2F (5′-CGY TGG AGA AGG AAA AAY GGC-3′) and PCV2R-A (5′-GTA GTA TTC AAA GGG TAY AGA GAT-3′). For PCR amplification of PCV2b genotype group-specific sequences, we utilized PCV2F oligonucleotide and a PCV2b-specific primer, PCV2R-B (5′-GTA TTC AAA GGG CAC AGA GMG G-3′). We used the PCV2a or PCV2b primer set for genotype group-specific sequence amplification, for semiquantitative analysis, and for sequencing. PCRs were performed with TGradient (Biometra) and FastStart Taq polymerase (Roche and Molecular Biochemicals). The PCR products were separated on an agarose gel by electrophoresis and visualized with UV light after staining with ethidium bromide (Fluka and Sigma-Aldrich) or GelRed (Chemie Brunschwig AG,Basel).
Oligonucleotides used for ISH and FISH analysis.
Oligonucleotides were either biotinylated or fluorescently labeled with Atto 565 or Dyomics 630 (Dy 630) (Microsynth AG, Switzerland) on both ends, 5′ and 3′, depending on usage. All oligonucleotide probes were designed to recognize sequences of PCV2 ORF2, excluding PCV1 sequence recognition. The AB (5′-CCA TCT TGG CCA GAT CCT CCG CCG-3′) and ABr (5′-CGG CGG AGG ATC TGG CCA AGA TGG-3′) oligonucleotides are both 24-mers. The sequence of ABr is exactly the reverse complement to the AB oligonucleotide sequence. Both oligonucleotides recognize all described PCV2a, PCV2b, and PCV2c genotype group members. They differ in that the AB oligonucleotide (the AB sequence is identical to the sense sequence) recognizes, in the absence of RNA, PCV2-specific ssDNA and dsDNA while ABr recognizes exclusively the PCV2 dsDNA isoform. Also, the oligonucleotides A (5′-GGG GAC CAA CAA AAT CTC TRT ACC-3′) and B (5′-GGC TCA AAC CCC CKC TCT GTG C-3′) distinguish between the PCV2a and PCV2b genotype groups, respectively. Since the B oligonucleotide is more GC rich, the A oligonucleotide is 2 bp longer to allow parallel hybridization conditions. Both genotype-specific oligonucleotides were designed over the PCV2 ORF2 signature motif; thus, the critical nucleotides are located mostly toward the middle of the hybridization sequence (31). The oligonucleotides Ar (5′-GGT AYA GAG ATT TTG TTG GTC CCC-3′) and Br (5′-GCA CAG AGM GGG GGT TTG AGC C-3′) were designed to distinguish the PCV2a and PCV2b genotype groups in PCV2 ORF2. Additionally, both are from the complement and reverse sequences of A and B oligonucleotides; hence, in the absence of RNA target, they would recognize solely the dsDNA isoform of the virus.
ISH with 5′ and 3′ biotinylated DNA oligonucleotide probes.
Before ISH commencement, we manually deparaffinized mounted tissue sections. We used the Discovery instrument (Ventana Medical Systems) to establish PCV2-specific ISHs. Experimental protocols were run with the help of DISCOVERY software. We standardized pretreatment on formalin-fixed, paraffin-embedded tissue sections by using the following Ribomap reagents: we treated the slices for 28 min with Riboprep and 8 min with Riboclear at room temperature (RT) before Protease I (Ventana Medical Systems) incubation for 4 min at 37°C. Next, the tissue sections were overlaid with corresponding 5′ and 3′ biotinylated oligonucleotides (Microsynth AG, Switzerland) and hybridization solution consisting of 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 5× Denhardt solution, 12% dextran sulfate sodium salt, and distilled water. The probes were diluted to a final concentration between 2 and 10 pmol in hybridization solution, depending on the negative controls and signal intensity. Afterward, the overlaid slides were subjected to a 4-min denaturing step at 90°C, followed by hybridization for 2 h at 37°C. Slides with mounted tissue were washed in two stringency washes of 2× Ribowash at 47°C for 4 min each. Next, samples were treated with Ribofix reagent for 24 min to fix stable remaining oligonucleotide probe to the target DNA. A washing step with 1× reaction buffer (Ventana Medical Systems) automatically followed. For signal detection, tissue sections were incubated for 2 h with a Bluemap detection kit (Ventana Medical Systems) and Pab-Block (Ventana Medical Systems), which reduced unnecessary background staining. The slides were counterstained using ISH-Red (Ventana Medical Systems), washed, dehydrated, and mounted.
FISH with 5′ and 3′ fluorochrome Atto 565- or Dy 630-labeled oligonucleotide probes for laser confocal microscopy.
Mycrosynth AG (Switzerland) synthesized 5′ and 3′ chromophore-coupled oligonucleotides with either Atto 565 or Dy 630. The ISH protocol on the Discovery instrument (Ventana Medical Systems) provided the groundwork for FISH experimental steps: the two protocol procedures were identical to the step with postfixation with Ribofix reagent. Separately for FISH, we washed the slides twice with distilled water, followed by nuclear staining with 1 μg/ml diamidino-2-phenylindole (DAPI) (AppliChem GmbH, Germany) in methanol for 20 min. Then, the slides were washed again in distilled water and air dried prior to the application of an aqueous mounting medium, Immu-Mount (Thermo, Pittsburgh, PA), and coverslips. We compared oligonucleotide hybridization reactivity to low-level or non-PCV2-infected pig tissue and those of other species, including dog, cat, and snake organ sections, as controls.
For the detection of signals, we used a Leica SP5 laser confocal scanning microscope with three lasers and four confocal fluorescence detectors at the Center for Microscopy and Image Analysis (University of Zurich, Switzerland). We used the diode laser excitation at 405 nm for DAPI signal visualization and the helium neon laser excitations at 561 nm or 633 nm for Atto 565 or Dy 630 detection, respectively.
Confocal pictures were analyzed with Imaris 6.3.0 (Bitplane Scientific Software), a multicolor and 3-dimensional/4-dimensional (3D/4D) image-processing software program.
Mounted tissue sections were digested with either DNase or RNase enzyme.
The ISH protocol on the Discovery instrument (Ventana Medical Systems) was adjusted for digestion of mounted tissue sections with either DNase or RNase enzyme, and slide-mounted tissue sections were subjected to either DNase I or RNase digestion before oligonucleotide hybridization. Consecutive tissue section slides were preferentially used. In brief, for DNA or RNA digestion, tissue sections were overlaid with 300 μl enzyme aqueous buffer solution and incubated at 37°C in a humidifying chamber for the times indicated. We overlaid concentrations of 1 to 100 U DNase I (Fermentas) per tissue section, which were incubated for 1 h at 37°C. Other tissue sections were incubated with RNase cocktail for 1 h at 37°C. We used a combination of 83 μg/ml RNase A (Fermentas) and 83 U/ml endoribonuclease RNase H (Biolabs) to digest tissue sections with possible target RNA presence. These enzyme concentrations were chosen from the higher end of the concentration recommendations of the manufacturer. After the tissue slices were washed twice with reaction buffer (Ventana Medical Systems) and introduced into the Discovery instrument (Ventana Medical Systems), we continued the experiment immediately with the step of oligonucleotide hybridization.
RESULTS
Analysis of PCV2 genotype group distribution.
The genetic shift to PCV2b genotype members was obvious; nevertheless, no particular PCV2b genotype group member was found to cause disease. Hence, we wondered whether PCV2a genotype group members disappeared in the epizootic or whether double-genotype-group infections were common and possibly correlated with disease. Therefore, we first optimized PCV2a and PCV2b template-specific PCR methods by construction of the respective PCV2 genotype sequence-containing vectors. We titrated individual constructs and optimized PCR conditions to detect as few as 10 templates per reaction (Fig. 1). Genotype group-specific oligonucleotides were designed within the signature motif to specifically distinguish all known PCV2a subgenotypes from PCV2b subgenotypes. These oligonucleotides differ by 2 bp at the 3′ end that prevent cross-reaction amplification products even in template concentrations as high as 107 to 108 templates per reaction (Fig. 1). These template concentrations compare to the highest we found from DNA extractions from animals with PMWS disease.
Fig. 1.
Genotypic PCR specificity and sensitivity. Shown are genotypic PCR amplificate signals separated by agarose gel electrophoresis. From left to right, the gels are further divided by DNA ladders into PCRs against plasmids containing PCV2a or PCV2b genotype sequence. The numbers at the bottom indicate serial template dilutions (100 to 107) from corresponding plasmids. On the right, the sizes of DNA ladder single bands are indicated in base pairs.
From our tissue block collection, we selected 48 cases by two criteria (Table 1): we analyzed cases from the time periods before and during the Swiss PMWS epizootic; no, low, or medium to high antigen concentrations were identified immunohistochemically (Table 1). These tissue blocks were further reevaluated by three different PCR methods. A PCV2-specific oligonucleotide set from our recent study allowed us to amplify the dominant PCV2 subgenotype irrespective of the genotype group affiliation for each case and tissue. PCV1 was not detected by this PCV2-specific PCR amplification. Of note, the data indicate that the optimized PCR method is more sensitive than IHC (Table 1). From real-time PCR data, we estimated that a PCR amplification signal was at least 2 log units of virus genome more sensitive than PCV2 antigen-antibody signals (unpublished data). By amplificate sequencing, we found PCV2b genotype group infections predominately occurred during the epizootic, reminiscent of our previous findings: 27% before and 97% during the Swiss epizootic were of the PCV2b genotype group. In fact, all 30 PCV2b group infections from the epizootic harbored the PCV2b-CH subgenotype. Separately, for four randomly selected PCV2-infected cases, we analyzed different organs, including lung, spleen, kidney, and lymph node, by PCR amplification and sequencing. The same virus variant dominantly infected all PCV2-infected organs of individual animals. By genotype group-specific PCR amplification we searched for the presence of PCV2a or PCV2b genotype group member infections. We found that all animals were harboring both genotype groups, including the 6 cases that were IHC negative, indiscriminately from the time of case occurrence. Actually, many PCV2b-specific PCR amplificate agarose gel electrophoresis bands appeared more intense than the counterpart PCV2a genotype group signals from the same animal epizootic, and the opposite was true preepizootically. It was puzzling to find both genotype groups in all infected tissue sections, especially as no molecular controls indicated any contamination. However, it has yet to be shown whether there was coincidental contamination.
Table 1.
Comparison of fixed and paraffin-embedded piglet tissue sections before and during the epizootic by PCV2-specific IHC, ISH, and PCRa
| Time of tissue sampling | Clinical diagnosisd | Signal intensitye,g |
Dominant PCV2 genotypef,g | |
|---|---|---|---|---|
| IHC | ISH | |||
| Before PMWS epizooticb | Subcl. infec. | 5 neg. | 5 + | a/b (n = 2)/(n = 3) |
| 2 + | 2 + | a (n = 2) | ||
| PMWS | 2 ++ | 2 ++ | a (n = 2) | |
| 6 +++ | 6 +++ | a/b (n = 5)/(n = 1) | ||
| During PMWS epizooticc | Subcl. infec. | 1 neg. | 1 + | a (n = 1) |
| 4 + | 4 + | b (n = 4) | ||
| PMWS | 6 ++ | 2 ++/4 + | b (n = 6) | |
| 22 +++ | 5 +++/9 ++/8 + | b (n = 22) | ||
PMWS clinical diagnosis, IHC, and the Swiss epizootic were described previously (41).
Fifteen piglet cases.
Thirty-three piglet cases.
Piglets subclinically infected (Subcl. infec.) with PCV2 were clinically inconspicuous.
ISH signal intensity comparisons were achieved with the aid of oligonucleotide AB tissue section labeling. The pluses indicate the staining strength, with +++ indicating the strongest label; neg., negative [no cell was visibly labeled]).
The dominant genotype group (a or b) was determined by PCV2-specific PCR amplification and product sequencing.
Numerals or (n = x) indicates the number of piglets investigated.
ISH specificity and sensitivity support PCR-derived data on the presence of both major genotype group members in PCV2-infected animals.
We decided to further analyze these 48 cases by ISH (Table 1). Its advantage over PCR is the signal-defined localization. Moreover, ISH-derived signal localization was compared to well-defined antigen staining (Fig. 2). Coincidental contamination from the organ environment was readily distinguished by ISH. For optimization of the signal intensity, we first titrated probes on low to high PCV2 antigen content-containing tissue sections. We compared oligonucleotide hybridization reactivity to low-level- or non-PCV2-infected pig tissue and those of other species, including dog, cat, and snake organ sections (Fig. 3). These negative controls included tissue sections with high concentrations of hyaluronic acid, amyloids (Fig. 3g), and necrotic (Fig. 3h) and apoptotic (Fig. 3e) tissue. Even with an oligonucleotide concentration 5-fold higher than that we finally applied in hybridization reactions, we did not find any nonspecific reaction signals on tissue sections (Fig. 3a, c, e, g, and h). We found, reminiscent of antigen tissue localization, PCV2 DNA-derived signals that were centrofollicular and interspersed in surrounding tissue, probably caused by infected immigrating macrophages (Fig. 4a). Apart from PCR-positive IHC-negative tissue sections, signal intensities from low- to high-level PCV2-infected tissues were correlated between IHC and ISH (Fig. 2b and c and Table 1). However, PCV2 DNA-dependent signals were not comparable to antigen-derived signals in a few tissues that were strongly positive for PCV2 antigen in PMWS cases with questionable cellular structural integrity.
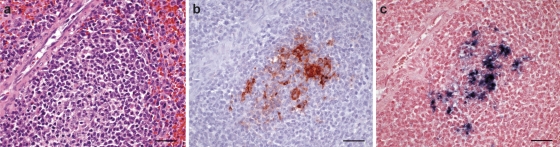
Fig. 2.
Comparison of hematoxylin staining, IHC, and ISH of a moderately PCV2-infected spleen section. (a) Hematoxylin-stained section (HE). (b) Anti-PCV2 IHC (F217 monoclonal anti-PCV2 antibody clone [29]). (c) ISH with AB oligonucleotide hybridization against PCV2 DNA. Bars, 20 μm.
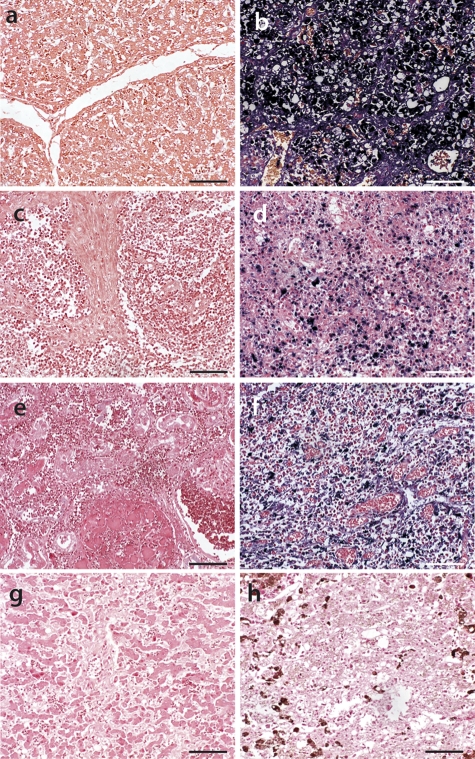
Fig. 3.
Comparison of highly PCV2-infected and noninfected tissues by PCV2-specific ISH oligonucleotide hybridization. We used an AB oligonucleotide probe (blue) on previously PCV2 antigen grade (IHC)-defined tissue sections from pig liver (a and b), pig lymph node (c and d), dog kidney with leptospirosis diagnosis (e), pig tonsil (d), cat liver with amyloidosis diagnosis (g), and snake liver with hemosiderosis diagnosis (h) (reptile pigmentation is dark brown, and iron deposits are light brown). (b, d, and f) Highly PCV2-infected tissue samples. (a, c, e, g, and h) ISH-negative tissue samples. All tissues were compiled in one TMA block and exposed together to AB oligonucleotide probe hybridization and signal (blue) development. Bars, 50 μm.
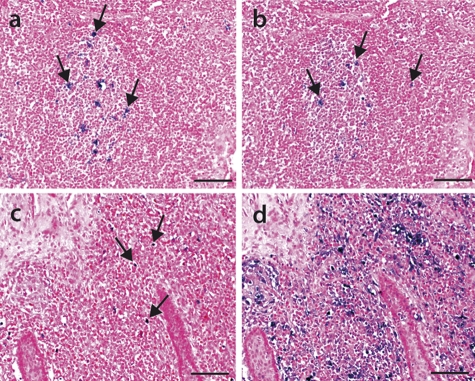
Fig. 4.
Oligonucleotide hybridization target identification on spleen sections from piglets with PMWS disease. Oligonucleotide ISH was performed on consecutive formalin-fixed paraffin-embedded spleen sections. The tissue sections were either untreated (a) or incubated for 1 h with digestion buffer only (b), digested for 1 h with 50 U DNase I (c), treated for 5 min with 50 U DNase I (d), digested for 1 h with 20 U DNase I (e), or incubated for 1 h with an effective RNase A/RNase H cocktail followed by ISH with oligonucleotide AB (f). The arrows indicate PCV2 target presence (blue signal) in the periarteriolar lymphatic sheath and probably in histocytes/macrophages distributed throughout the tissue sections. The tissue sections were counterstained with ISH-Red. Bars, 50 μm.
Template specificity of signals detected by ISH.
For detailed analysis, we identified the type of nucleic acids recognized by oligonucleotides and whether these ISH signals were the result of the oligonucleotide hybridization to DNA, RNA, or both targets. We applied serial dilutions of digests with corresponding activities to remove DNA or RNA targets. Notably, the degree of infection varies among a pig's primary and secondary lymph organs. As a consequence, we selected spleen tissue with a medium antigen concentration from an animal with PMWS disease. At this PCV2 load, the splenic tissue ultrastructure was fully conserved. ISH-derived blue staining signals were located mostly in histocytes (Fig. 4).
We observed increased ISH signal intensity in the DNase I, as well as RNase, buffer controls throughout the different experiments (Fig. 4b). One hour of digestion with 50 U DNase I enzyme obliterated signals completely (Fig. 4c). With less time or a lower concentration of DNase I, about 10% of the blue signals remained (Fig. 4d and e). Tissue sections treated with 1 U DNase I did not reduce the signal intensity (data not shown). The reduction of DNA-dependent signals was in contrast to tissue sections treated with a combination of RNase A and RNase H (Fig. 4f). We did not observe any signal intensity reduction in sections digested with the RNase cocktail over buffer control alone, although this RNase cocktail was sufficient in other experiments to completely eliminate RNA-derived signals. Therefore, we concluded that the visualized signals originated solely from DNA homodimers, namely, oligonucleotide probe and PCV2 DNA. Also, hybridization of other PCV2-specific oligonucleotides was dependent on the presence of viral DNA template (Fig. 4 and data not shown). Additionally, we verified the absence of RNA in tissue sections by two other methods: by reverse transcriptase PCR from RNA virus-infected tissue sections (unpublished data) and with an RNA positive control from Ventana Medical Systems for the detection of mRNA in tissue sections.
Cellular colocalization of PCV2a and PCV2b genotype group members.
Previously, we determined PCV2a and PCV2b genotype group ratios by PCR amplificate sequencing. Also, ISH hybridization in preepizootic pig cases revealed PCV2a genotype group signal dominance over PCV2b genotype group infections and epizootic PCV2b genotype group infection-governed signals. We show such an example of tonsil tissue from prior to (Fig. 5a and b) and during (Fig. 5c and d) the epizootic.
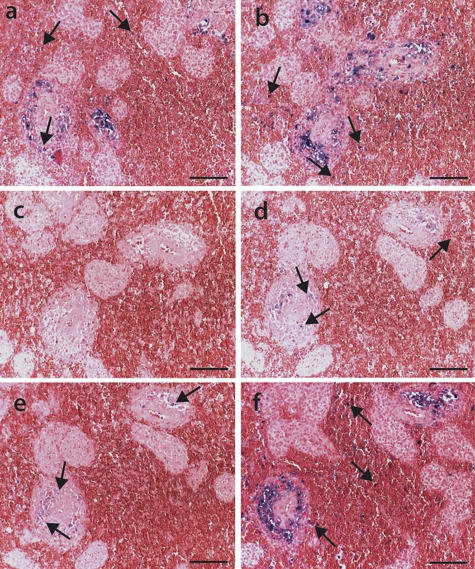
Fig. 5.
PCV2 genotype group distributions before and during the epizootic. The PCV2a and PCV2b genotype groups showed differential signals for fixed and paraffin-embedded tonsil tissue sections prior to (a and b) and during (c and d) the Swiss epizootic. ISH was performed with oligonucleotide A (a and c) and oligonucleotide B (b and d) probes that recognize PCV2a and PCV2b viruses, respectively. Note the presence of PCV2 genotype group signals (blue stain), indicated by the arrows. The tissue sections were counterstained with ISH-Red. Bars, 50 μm.
Additionally, we noticed on consecutive tissue sections that often ISH signals from both genotype groups overlapped. We questioned whether comparing consecutive tissue sections' signals might perturb the results. We performed FISH to compare genotype group distributions directly on the same tissue section. For FISH, we utilized oligonucleotide sequence and hybridization conditions reminiscent of ISH reactions. Specific oligonucleotides were coupled with either the Atto 565 or Dy 630 fluorochrome. The fluorescent colors are heat stable and clearly distinguishable in laser confocal microscopy. In contrast, the combination of Atto 594 and Atto 565 fluorochromes produced mutual interferance, depending on the PCV2 DNA contents in the sections. Additionally, we did not use green channels for laser confocal microscopy, as we found insurmountable interference with tissue section autofluorescence. Finally, we used a 3-color staining protocol for confocal microscopy: we utilized DAPI blue fluorescence to visualize nuclei and Atto-coupled oligonucleotide B and Dy-coupled oligonucleotide A for specific PCV2 genotype group DNA labeling. To expedite analysis, we compared sections with the help of TMAs. The genotype-specific oligonucleotides were designed over the signature motif in ORF2 to discriminate between all PCV2a and PCV2b genotype group members. The highest sequence variability was chosen around the middle of the oligonucleotide hybridization sequence to achieve the highest template specificity. Additionally, we confirmed oligonucleotide specificity on fixed and paraffin-embedded cell pellets transfected with either the recombinant PCV2a (Fig. 6a and b) or recombinant PCV2b (Fig. 6c and d) genotype. PCV2 infections in general were visualized with oligonucleotide AB in red (Fig. 6). Overlap with genotypic FISH appears yellow (Fig. 6a and d). Fixed cell pellet sections (Fig. 6a and c) were hybridized with genotypic oligonucleotide A, and panels (Fig. 6b and d) were hybridized with oligonucleotide B. No cross-hybridization was seen with genotypic oligonucleotides (Fig. 6b and c). In the TMA block sections, we included PCV1-infected and noninfected cell pellets, which were not recognized by any of the genotypic oligonucleotides.
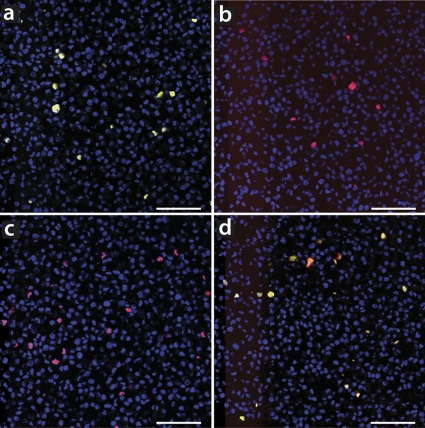
Fig. 6.
Specificities of genotypic oligonucleotides scrutinized on recombinant PCV2a- or recombinant PCV2b-infected cell layers in laser confocal microscopy. Shown are selected images from TMA block sections of formalin-fixed and paraffin-embedded genotype-specific transfected PK15 cell layers. PCV2a or PCV2b genotype-infected tissues were either stained with a combination of oligonucleotides AB (red) and A (green) as single colors (a and b) or separately with oligonucleotides AB (red) and B (green) as a single colors (c and d). The overlap of both colors, oligonucleotides AB and the genotypic oligonucleotides, appears yellow. Nuclei were counterstained with DAPI and thus appear blue. Bars, 80 μm.
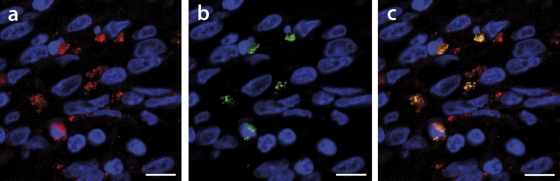
Also in tissue sections, both genotype groups, PCV2a and PCV2b, were found concurrently in the same cell, with FISH analysis indicated as yellow signal (Fig. 7c). PCV2 was present mostly in the cell cytoplasm and sometimes intranuclearly. Nevertheless, genotype group superinfections were found in variable ratios, and few cells were singly infected by one genotype group member. Often, a slight labeling of the PCV2b or PCV2a genotype group was found in the background of the other dominant genotype group. The presence of both genotype groups was particularly interesting in tissues from all six piglets with low-level PCV2 infections (Table 1). Cells with double infections always appeared equal or stronger in fluorescence intensity in either channel compared to genotype group single-cell infections.
Fig. 7.
Laser confocal microscopy analysis revealed PCV2a and PCV2b genotype group infections in the same cell. FISH was performed on fixed and paraffin-embedded lymph node tissue sections from an epizootic PMWS diseased animal. (a) Red signals derived from the PCV2b-specific oligonucleotide B. (b) Green signals achieved by oligonucleotide A target hybridization. (c) Results from overlay of panels a and b. In all panels, nuclei are counterstained with DAPI and thus appear blue. Bars, 10 μm.
Unexpected intracellular coreplication of the two major PCV2 genotype groups.
To identify the presence of a replicative PCV2 DNA isoform, we designed the complementary reverse oligonucleotide to the AB oligonucleotide, which we named the ABr oligonucleotide. The signals observed were nuclear and cytoplasmic and were comparable to AB oligonucleotide hybridization signals (Fig. 8; the control sections in Fig. 2 are rotated 90° clockwise in relation to the confocal microscopy images in Fig. 8).
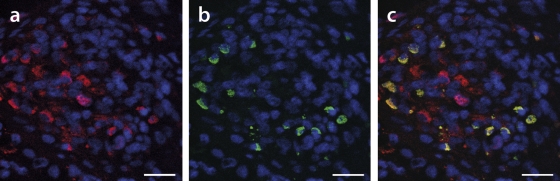
Fig. 8.
Detection of PCV2 replication by laser confocal microscopy. FISH was used specifically for identification of PCV2 dsDNA in PCV2-infected formalin-fixed and paraffin-embedded spleen tissue. (a) Red signals derived from oligonucleotide AB hybridization identify all PCV2 infections. (b) Green labeling was derived from oligonucleotide ABr hybridization. (c) Overlay of panels a and b. Nuclei were counterstained with DAPI and thus appear blue. Bars, 20 μm.
In all tissue sections, we found more AB oligonucleotide-labeled cells than ABr-based signals, and all cells labeled with ABr oligonucleotide were also strongly stained by AB oligonucleotide-specific probe. In cells with both fluorescence markers, labeling overlapped almost completely (Fig. 8c). Cells with a replicative isoform mostly appeared compacted and round (Fig. 8b and 9) compared to single AB oligonucleotide-stained cells (Fig. 8a and c). We estimated the ratio of tissue from PCV2 general infection to the PCV2 dsDNA replicative isoform at 1 to 1 to about 50 to 1. In low-level-infected tissues, we hardly detected any PCV2 replicative-isoform-specific signals. The PCV2 replicative isoform was readily detected in sections with moderate to very high viral antigen loads. Additionally, we observed this isoform in lymphoid organs, such as tonsil, Peyer's patches of the ileum, lymph nodes, and spleen, and to a lesser extent in the lung and liver (data not shown).
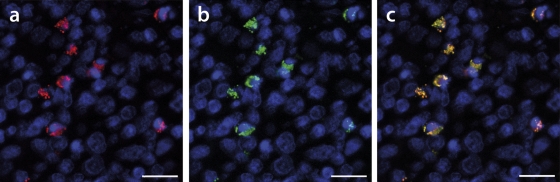
Fig. 9.
PCV2 genotype group colocalization and coreplication visualized in a confocal micrograph. Genotype group superinfection and coreplication were visualized by FISH of a formalin-fixed and paraffin-embedded tonsil tissue section. (a) Red labeling achieved by hybridization of PCV2a dsDNA-specific oligonucleotide Ar. (b) Oligonucleotide Br target hybridization specific for PCV2b dsDNA appears green. (c) Overlay of panels a and b. In all panels, nuclei are counterstained with DAPI and thus appear blue. Bars, 15 μm.
As we found many infected cells carrying both major genotype groups, albeit at different ratios, it was of particular interest to understand whether these genotypes might coreplicate intracellularly. Hence, we designed a FISH experiment with reverse complement sequence to A and B oligonucleotides, namely, with oligonucleotides Ar and Br, which specifically recognize genotype group-specific replicative isoforms. Labeling was observed in moderate- to high-level antigen-containing tissue sections. Notably, cells with the PCV2 replicative DNA isoform were always labeled with both Ar and Br oligonucleotides (Fig. 9). We observed only minor signal intensity differences, if any, between the specific PCV2 genotype groups' cell-internal replication (Fig. 9c). These colocalized signals rarely varied, in contrast to genotype-specific A and B oligonucleotide labeling. This pattern was supported in different tissue sections.
DISCUSSION
We demonstrated double-genotype-group infections in all investigated PCV2-infected pigs and organs. Furthermore, we found in vivo cell superinfections and coreplication of the two major PCV2 genotype groups, PCV2a and PCV2b. This explains the recent findings about the PCV2 nature in general and in particular the heightened recombination efficiency of these two major PCV2 genotype groups. To our knowledge, this is the first report directly showing superinfections of two so closely related viruses and suggests genotype group cooperation for efficient coreplication in vivo. These findings give a new twist to our understanding of syndrome etiology. A major leap of thought is required to connect PCV2 with PMWS, and consequently, cofactors are assumed to be essential to trigger disease. In our suggested model, no known cofactor is required to initiate PMWS. Instead, we suggest that the presence, coevolution, and autogenic selection of members of both major genotype groups is essential and is possibly driven by the host's maturing immune system.
We report that 100% of investigated PCV2-infected pigs and their organs carry both genotype groups. These surprising results were confirmed by three unrelated methods: genotype-specific PCR, ISH, and FISH. Even IHC-negative tissue samples with low virus concentrations were found to be infected with both genotype groups. Although other research had already reported double infections in pigs (6, 8, 15, 18), the prevalence was no higher than 25% of all infected animals (18). Until our current study, there was no remote suggestion of cell superinfections by both genotype group members. Retrospectively, we found double infections in tissue samples dating back to 1981. These data indicate a genotype colocalization requirement for PCV2 replication in vivo and also support the surprising theoretical notion that coevolutionary PCV2a and PCV2b genotype group members were transferred from birds to pigs (13).
The model needed further evaluation of the specificities and sensitivities of the applied methods. Research reports of PCV2 recombination have relied heavily on sequences derived by the PCR method, and thus, possible artifacts could not be excluded. We checked in depth the genotype group-specific PCR amplification sensitivity and specificity. The sensitivity limits were about 10 to 100 templates per reaction, and no cross-reactivity of genotype group-specific PCRs was found. Also, ISH or FISH signals were dependent on the DNA template. Moreover, on single-genotype-group-infected porcine kidney cells, we found only oligonucleotide hybridization to corresponding genotype DNA. We wondered whether double-genotype infection would enhance PCV2 propagation in vitro. Preliminary data indicated that this might not be the case. In this line of thought, we were not able to find any PCV2 dsDNA isoform in the single-subgenotype-infected cell culture. The isoform may be transient or, alternatively, present in low concentrations in PCV2-infected cell culture. This observation correlates with the generally low propagation efficiency of PCV2 in vitro.
Previously, we identified 42% of all IHC-negative cases by PCR as PCV2 infected (41). We randomly chose six of them for genotypic PCR, ISH, and FISH analysis. The signals were confined to histocytes, a cell type generally known to contain virus antigen when infected with PCV2. Also, we observed a genetic shift to the PCV2b genotype group. Nevertheless, the data indicate that PCV2b genotype group members were always present and did not newly occur, even though their occurrence dominates the present epizootic in Switzerland, as well as in other countries, including the United States and Canada. Even as this genetic shift was observed, every PCV2-infected animal and organ was found to carry both PCV2a and PCV2b genotype group numbers. In fact, by PCR amplification and sequencing, we and others identified several members of a particular genotype group in the same animal (15, 41).
We demonstrated the presence of both genotype groups in individual cells. Double-genotype-group infections per cell were not seen sporadically; on the contrary, they were found even in tissue with very few infected cells. Indeed, superinfections were more prominent in these tissues. These observations were counter to expectations, as the general assumption is that one phylogenetically closely related virus has a slight replication advantage over the other, and consequently, one genotype will become diluted and disappear unless there is a special requirement for the presence of both genotypes. Alternatively, at least in secondary lymphoid organs, these PCV2-carrying cells might be immigrating phagocytes. In our observations, both PCV2 genotype groups were always seen replicating together, suggesting that the two genotype groups replicate cooperatively in vivo. It remains to be seen whether this replication cooperativity can be studied in in vitro experiments.
Although we found sporadic single-cell infections by the PCV2a or PCV2b genotype group, we observed more cells infected with both genotype group members at various infection ratios. This pattern was visible, not only in the secondary lymph organs, but also in lung and liver tissue. Efficient replication was observed only in cells containing almost equal amounts of the two genotype group members. Affected cells predominantly showed a round phenotype, which might be an indication of cellular stress. In neonates, only double-genotype infections caused severe disease (16). This supports our in vivo findings that effective pathogenicity needs both genotype groups. Some characteristics of PCV2 can also be seen in geminiviruses, where interspecies recombination of phylogenetically closely related viruses correlated with severe disease outbreak (43). However, not all phylogenetically closely related viruses allow superinfections (34). The relaxed replication regulation requirements were not previously observed for DNA viruses in general, and in particular with closely related viruses. This is truly a new characteristic for PCV2 and also needs to be addressed for other members of the family Circoviridae.
We identified PCV2a and PCV2b genotype group coreplication. It is easily imagined that within the genotype group coreplication is common and thus facilitates recombination events (22, 32). This also makes sense in light of virus replication, the ssDNA nature of the virus, and its propagation by a melting-pot rolling-cycle mechanism (11). Every new virus appears first as an ssDNA template, a replication system known to be exceptionally prone to point mutations. The recent accumulation of reports about PCV2 recombination supports the presence of active replicating genotype and subgenotype members in the cell. This new perspective on the virus allows a rapidly changing genetic PCV2 pool in infected cells and pigs, and with it the occurrence of different virulent PCV2 mutants. We suggest that the combination of all these factors, and particularly the relaxed replication regulation requirement of PCV2, allows the virus to effectively adapt and avoid the host defense system pressure. Additionally, this heightened mutation rate (13) gives the virus a real advantage in zoonosis, as in less than a couple of hundred years, it transferred from birds to the wild boar and into the domestic pig population.
Most PCV2 disease research has focused on additional cofactors that would be needed to trigger PMWS disease. However, none of these identified cofactors was important at the commencement of the Swiss PMWS epizootic (41). Therefore, we favor a model with a constantly evolving genetic PCV2 pool in cells, in individual animals, and in pig herds to explain the disease etiology. This would further explain the population dynamic of germination centers for PMWS spread and outbreaks in Switzerland and Great Britain, as if a new infectious agent coemerged with PCV2 (42). Under this assumption, the slow progression of the disease in individual animals during maturation involves harmful virus mutation and recombination that lead to increased lymphopenia, which finally triggers disease. Additional cofactors would accelerate the disease course in this model. We will test this hypothesis in further animal infection experiments.
ACKNOWLEDGMENTS
We are indebted to Urs Ziegler, Andreas Kaech, and the research team at the Center for Microscopy and Image Analysis (University of Zurich) for all their help. We thank Roseline Weilenmann at the Institute of Veterinary Pathology for her dedicated technical support and Jeanne Peter and Irene Schweizer in the Graphic Department for professional assistance. We greatly appreciate Gordon Allen's gifts of the PK15 cell line and the F217 monoclonal anti-PCV2 antibody.
This work was partly supported by the Julius-Klaus foundation, the Vetsuisse Faculty, and the University of Zurich.
Footnotes
Published ahead of print on 24 August 2011.
REFERENCES
- 1. Allan G. M., Ellis J. A. 2000. Porcine circoviruses: a review. J. Vet. Diagn. Invest. 12:3–14 [DOI] [PubMed] [Google Scholar]
- 2. Allan G. M., et al. 2007. Temporal distribution of porcine circovirus 2 genogroups recovered from postweaning multisystemic wasting syndrome affected and nonaffected farms in Ireland and Northern Ireland. J. Vet. Diagn. Invest. 19:668–673 [DOI] [PubMed] [Google Scholar]
- 3. Bernstein C. N., Nayar G., Hamel A., Blanchard J. F. 2003. Study of animal-borne infections in the mucosas of patients with inflammatory bowel disease and population-based controls. J. Clin. Microbiol. 41:4986–4990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carman S., et al. 2006. Porcine circovirus-2 associated disease in swine in Ontario (2004 to 2005). Can. Vet. J. 47:761–762 [PMC free article] [PubMed] [Google Scholar]
- 5. Cheung A. K. 2007. Homologous recombination plays minor role in excision of unit-length viral genomes from head-to-tail direct tandem repeats of porcine circovirus during DNA replication in Escherichia coli. Arch. Virol. 152:1531–1539 [DOI] [PubMed] [Google Scholar]
- 6. Cheung A. K., et al. 2007. Detection of two porcine circovirus type 2 genotypic groups in United States swine herds. Arch. Virol. 152:1035–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Darwich L., Segales J., Mateu E. 2004. Pathogenesis of postweaning multisystemic wasting syndrome caused by Porcine circovirus 2: an immune riddle. Arch. Virol. 149:857–874 [DOI] [PubMed] [Google Scholar]
- 8. de Boisséson C., et al. 2004. Molecular characterization of Porcine circovirus type 2 isolates from post-weaning multisystemic wasting syndrome-affected and non-affected pigs. J. Gen. Virol. 85:293–304 [DOI] [PubMed] [Google Scholar]
- 9. Dupont K., Nielsen E. O., Baekbo P., Larsen L. E. 2008. Genomic analysis of PCV2 isolates from Danish archives and a current PMWS case-control study supports a shift in genotypes with time. Vet. Microbiol. 128:56–64 [DOI] [PubMed] [Google Scholar]
- 10. Ellis J., et al. 1998. Isolation of circovirus from lesions of pigs with postweaning multisystemic wasting syndrome. Can. Vet. J. 39:44–51 [PMC free article] [PubMed] [Google Scholar]
- 11. Faurez F., Dory D., Grasland B., Jestin A. 2009. Replication of porcine circoviruses. Virol. J. 6:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fenaux M., Opriessnig T., Halbur P. G., Elvinger F., Meng X. J. 2004. Two amino acid mutations in the capsid protein of type 2 porcine circovirus (PCV2) enhanced PCV2 replication in vitro and attenuated the virus in vivo. J. Virol. 78:13440–13446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Firth C., Charleston M. A., Duffy S., Shapiro B., Holmes E. C. 2009. Insights into the evolutionary history of an emerging livestock pathogen: porcine circovirus 2. J. Virol. 83:12813–12821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gibbs M. J., Weiller G. F. 1999. Evidence that a plant virus switched hosts to infect a vertebrate and then recombined with a vertebrate-infecting virus. Proc. Natl. Acad. Sci. U. S. A. 96:8022–8027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grau-Roma L., et al. 2008. A proposal on porcine circovirus type 2 (PCV2) genotype definition and their relation with postweaning multisystemic wasting syndrome (PMWS) occurrence. Vet. Microbiol. 128:23–35 [DOI] [PubMed] [Google Scholar]
- 16. Harding J. C., Ellis J. A., McIntosh K. A., Krakowka S. 2010. Dual heterologous porcine circovirus genogroup 2a/2b infection induces severe disease in germ-free pigs. Vet. Microbiol. 145:209–219 [DOI] [PubMed] [Google Scholar]
- 17. Hasslung F., et al. 2005. Experimental reproduction of postweaning multisystemic wasting syndrome (PMWS) in pigs in Sweden and Denmark with a Swedish isolate of porcine circovirus type 2. Vet. Microbiol. 106:49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hesse R., Kerrigan M., Rowland R. R. 2008. Evidence for recombination between PCV2a and PCV2b in the field. Virus Res. 132:201–207 [DOI] [PubMed] [Google Scholar]
- 19. Kappe E. C., et al. 2010. Bone marrow depletion with haemorrhagic diathesis in calves in Germany: characterization of the disease and preliminary investigations on its aetiology. Berl. Munch. Tierarztl. Wochenschr. 123:31–41 [PubMed] [Google Scholar]
- 20. Katoh H., Ogawa H., Ohya K., Fukushi H. 2010. A review of DNA viral infections in psittacine birds. J. Vet. Med. Sci. 72:1099–1106 [DOI] [PubMed] [Google Scholar]
- 21. Kim H. K., et al. 2009. Phylogenetic and recombination analysis of genomic sequences of PCV2 isolated in Korea. Virus Genes 39:352–358 [DOI] [PubMed] [Google Scholar]
- 22. Lefebvre D. J., Van Doorsselaere J., Delputte P. L., Nauwynck H. J. 2009. Recombination of two porcine circovirus type 2 strains. Arch. Virol. 154:875–879 [DOI] [PubMed] [Google Scholar]
- 23. Li L., et al. 2010. Multiple diverse circoviruses infect farm animals and are commonly found in human and chimpanzee feces. J. Virol. 84:1674–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu J., Chen I., Kwang J. 2005. Characterization of a previously unidentified viral protein in porcine circovirus type 2-infected cells and its role in virus-induced apoptosis. J. Virol. 79:8262–8274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lorincz M., et al. 2010. Detection of porcine circovirus in rodents. Acta Vet. Hung. 58:265–268 [DOI] [PubMed] [Google Scholar]
- 26. Ma C. M., et al. 2007. Evidence for recombination in natural populations of porcine circovirus type 2 in Hong Kong and mainland China. J. Gen. Virol. 88:1733–1737 [DOI] [PubMed] [Google Scholar]
- 27. Madec F., Rose N., Grasland B., Cariolet R., Jestin A. 2008. Post-weaning multisystemic wasting syndrome and other PCV2-related problems in pigs: a 12-year experience. Transbound Emerg. Dis. 55:273–283 [DOI] [PubMed] [Google Scholar]
- 28. Mankertz A., et al. 2004. Molecular biology of Porcine circovirus: analyses of gene expression and viral replication. Vet. Microbiol. 98:81–88 [DOI] [PubMed] [Google Scholar]
- 29. McNeilly F., et al. 2001. Production, characterisation and applications of monoclonal antibodies to porcine circovirus 2. Arch. Virol. 146:909–922 [DOI] [PubMed] [Google Scholar]
- 30. Meehan B. M., Creelan J. L., McNulty M. S., Todd D. 1997. Sequence of porcine circovirus DNA: affinities with plant circoviruses. J. Gen. Virol. 78:221–227 [DOI] [PubMed] [Google Scholar]
- 31. Naiser T., et al. 2008. Impact of point-mutations on the hybridization affinity of surface-bound DNA/DNA and RNA/DNA oligonucleotide-duplexes: comparison of single base mismatches and base bulges. BMC Biotechnol. 8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Olvera A., Cortey M., Segales J. 2007. Molecular evolution of porcine circovirus type 2 genomes: phylogeny and clonality. Virology 357:175–185 [DOI] [PubMed] [Google Scholar]
- 33. Opriessnig T., McKeown N. E., Zhou E. M., Meng X. J., Halbur P. G. 2006. Genetic and experimental comparison of porcine circovirus type 2 (PCV2) isolates from cases with and without PCV2-associated lesions provides evidence for differences in virulence. J. Gen. Virol. 87:2923–2932 [DOI] [PubMed] [Google Scholar]
- 34. Ramírez S., et al. 2010. Hepatitis C virus superinfection of liver grafts: a detailed analysis of early exclusion of non-dominant virus strains. J. Gen. Virol. 91:1183–1188 [DOI] [PubMed] [Google Scholar]
- 35. Segalés J., Allan G. M., Domingo M. 2005. Porcine circovirus diseases. Anim. Health Res. Rev. 6:119–142 [DOI] [PubMed] [Google Scholar]
- 36. Segales J., Domingo M. 2002. Postweaning multisystemic wasting syndrome (PMWS) in pigs. A review. Vet. Q. 24:109–124 [DOI] [PubMed] [Google Scholar]
- 37. Segalés J., et al. 2008. PCV-2 genotype definition and nomenclature. Vet. Rec. 162:867–868 [DOI] [PubMed] [Google Scholar]
- 38. Steinfeldt T., Finsterbusch T., Mankertz A. 2006. Demonstration of nicking/joining activity at the origin of DNA replication associated with the rep and rep′ proteins of porcine circovirus type 1. J. Virol. 80:6225–6234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tischer I., et al. 1995. Presence of antibodies reacting with porcine circovirus in sera of humans, mice, and cattle. Arch. Virol. 140:1427–1439 [DOI] [PubMed] [Google Scholar]
- 40. Vincent I. E., et al. 2007. Silencing of natural interferon producing cell activation by porcine circovirus type 2 DNA. Immunology 120:47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wiederkehr D. D., et al. 2009. A new emerging genotype subgroup within PCV-2b dominates the PMWS epizooty in Switzerland. Vet. Microbiol. 136:27–35 [DOI] [PubMed] [Google Scholar]
- 42. Woodbine K. A., et al. 2007. Spatiotemporal patterns and risks of herd breakdowns in pigs with postweaning multisystemic wasting syndrome. Vet. Rec. 160:751–762 [DOI] [PubMed] [Google Scholar]
- 43. Zhou X., et al. 1997. Evidence that DNA-A of a geminivirus associated with severe cassava mosaic disease in Uganda has arisen by interspecific recombination. J. Gen. Virol. 78:2101–2111 [DOI] [PubMed] [Google Scholar]