Abstract
Ectromelia virus (ECTV) is a natural pathogen of mice that causes mousepox, and many of its genes have been implicated in the modulation of host immune responses. Serine protease inhibitor 2 (SPI-2) is one of these putative ECTV host response modifier proteins. SPI-2 is conserved across orthopoxviruses, but results defining its mechanism of action and in vivo function are lacking or contradictory. We studied the role of SPI-2 in mousepox by deleting the SPI-2 gene or its serine protease inhibitor reactive site. We found that SPI-2 does not affect viral replication or cell-intrinsic apoptosis pathways, since mutant viruses replicate in vitro as efficiently as wild-type virus. However, in the absence of SPI-2 protein, ECTV is attenuated in mousepox-susceptible mice, resulting in lower viral loads in the liver, decreased spleen pathology, and substantially improved host survival. This attenuation correlates with more effective immune responses in the absence of SPI-2, including an earlier serum gamma interferon (IFN-γ) response, raised serum interleukin 18 (IL-18), increased numbers of granzyme B+ CD8+ T cells, and, most notably, increased numbers and activation of NK cells. Both virus attenuation and the improved immune responses associated with SPI-2 deletion from ECTV are lost when mice are depleted of NK cells. Consequently, SPI-2 renders mousepox lethal in susceptible strains by preventing protective NK cell defenses.
INTRODUCTION
Ectromelia virus (ECTV) is a large DNA virus and the causative agent of mousepox. This poxvirus has been long established as a classical model to study acute viral pathogenesis. ECTV carries 175 genes, and approximately 25% of the gene products are thought to be mediators of host immune evasion by targeting diverse processes, such as cellular signaling, intrinsic and extrinsic cell death pathways, and components of the innate immune response (6).
Host immune response modulation by poxviruses is essential for virulence and progeny production, with gene deletions of many of the immune host response modifiers resulting in virus attenuation (1, 47–49, 54). The first orthopoxvirus gene product found to be associated with evasion of the host immune system was the cytokine response modifier A gene (crmA) from cowpox virus (CPXV) (41). CrmA is one of three serine proteinase inhibitors (serpins or SPI proteins) encoded by such viruses and is referred to here as SPI-2. SPI-2 is highly conserved among orthopoxviruses (∼90% amino acid sequence identity), such as ECTV, vaccinia virus (VACV), and variola virus (VARV) (51), and orthologues can also be found outside the genus, for example, in myxoma virus (MYXV). Despite this conservation, a single common role for SPI-2 in poxvirus biology has so far not been identified, given that SPI-2 deletions in CPXV and VACV have varying effects in vivo, depending on the virus, host, and mode of infection (25, 28, 30, 41, 49), and orthologues from CPXV and MYXV are not functionally interchangeable (39).
Different serpins inhibit distinct types of proteinases. Thus far, caspase 1 (24, 27, 45), caspase 8 (11, 24), and granzyme B (44) have been shown to be targets of CPXV and/or VACV SPI-2 protein. Still, ECTV SPI-2 was observed to inhibit in vitro caspase 1 and caspase 8 but not granzyme B, despite the high level of conservation among orthopoxvirus SPI-2 proteins (50). The amino acids responsible for this apparent difference in specificity remain to be identified, and the question of whether they contribute to the differences seen in vivo remains to be answered. Thus, despite extensive studies, the role of SPI-2 in poxvirus infection is still poorly understood. There is contradictory evidence as to whether SPI-2 affects virus replication in vivo (28, 30). In addition, the wide range of targets identified for SPI-2 in vitro suggests a number of possible roles, from interference with cytolytic lymphocyte-mediated killing of infected cells (37) to inhibition of cleavage activation of proinflammatory cytokines, such as interleukin 1β (IL-1β) (24, 27, 45, 50) and IL-18.
In the present work, we generated ECTV SPI-2 mutants and examined the in vivo function of this viral gene product in the pathogenesis of mousepox, i.e., in a natural host-pathogen relationship. We found that SPI-2 is an important virulence factor that mediates its effects primarily via prevention of NK cell responses.
MATERIALS AND METHODS
Ethics statement.
This study was carried out in strict accordance with the recommendations of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. The protocol was approved by the Animal Experimentation Ethics Committee (AEEC) of the Australian National University (protocol number J.IG.68.08). All efforts were made to minimize suffering.
Mice and cells.
Eight- to 10-week-old female C57BL/6 and BALB/c mice were obtained from the specific-pathogen-free facility at the John Curtin School of Medical Research (JCSMR) (Canberra, Australia) or the Animal Resources Centre (Perth, Australia) and used according to institutional experimentation approval. BS-C-1, a continuous African green monkey kidney cell line; L929, a continuous fibroblast line from the C3H mouse; and mouse embryonic fibroblasts (MEF) (26) were maintained in Eagle's minimal essential medium (EMEM) plus 5% fetal bovine serum and antibiotics at 37°C in a humidified atmosphere with 5% CO2.
Viruses.
Plaque-purified ECTV (Moscow strain) and recombinant viruses were propagated in murine L929 cells as previously described (7). Virus titers were determined by plaque assay on BS-C-1 monolayers. For virus multistep growth curves, L929 cell and MEF lines were infected at a multiplicity of infection (MOI) of 3 for 1 h. Unabsorbed virus was washed off, and fresh medium was added. At various times postinfection (p.i.), the cell culture and cell-associated material were harvested separately, and virus titers were determined by plaque assay using BS-C-1 monolayers.
Recombinant virus generation.
Two ECTV SPI-2-deficient viruses containing different genetic modifications in the SPI-2 gene (locus ECTVgp162 of ECTV Moscow; NCBI accession NC_004105.1; product also known as EVM161 and serpin C7L) were generated. ECTV SPI-2Δ was generated by a general transient-dominant selection method used for constructing poxvirus mutants based on β-galactosidase (β–Gal) gene expression and Escherichia coli gpt with mycophenolic acid selection (12, 13). The complete SPI-2 gene sequence was deleted. ECTV SPI-2 SADΔ and ECTV SPI-2 REV were generated by a novel transient-dominant selection method using green fluorescent protein (GFP) fluorescence and blasticidin resistance as selection markers (53). Nucleotides +271 to +698 were deleted in the open reading frame of ECTV SPI-2 SADΔ. The deletion process inserts the AATGACGGCGAT exogenous sequence into the gene, generating a frameshift and an early stop codon in the mutant predicted protein. As a consequence, only amino acids 1 to 91 of the wild-type (wt) SPI-2 protein are expressed in ECTV SPI-2 SADΔ. ECTV SPI-2 REV was generated from the ECTV SPI-2 SADΔ mutant. Blasticidin was purchased from Invivogen. Gene deletion/restoration was confirmed by PCR and sequencing.
Western blotting.
For analysis of SPI-2 expression, 1 × 107 MEF were infected at an MOI of 10 for 4 h at 35°C. Cell lysates were prepared by cell pellet resuspension with radioimmunoprecipitation assay lysing buffer (25 mM Tris-HCl, pH 8, 137 mM sodium chloride, 2 mM EDTA, 1% glycerol, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]). Lysates were cleared using QIAshredder columns (Qiagen), separated by SDS-polyacrylamide gel electrophoresis with 12% gels, and blotted against nitrocellulose membranes (Bio-Rad). SPI-2 protein was detected using a mouse anti-SPI-2 monoclonal antibody (BD-Pharmingen; catalog no. 65921A; dilution, 1/400) as the primary antibody, a secondary anti-mouse IgG1 peroxidase-coupled antibody (BD Pharmingen), and enhanced chemiluminescence substrate (Amersham). For actin detection, a rabbit anti-actin polyclonal antibody (Santa Cruz) was used, followed by anti-rabbit IgG peroxidase-coupled antibody (BD Pharmingen).
Infections.
For in vivo studies of ECTV pathogenesis, mice were infected with 103 PFU or 106 PFU subcutaneously (s.c.) in both hind legs in a total volume of 50 μl phosphate-buffered saline (PBS) (25 μl per leg). The mice were monitored daily by blinded examiners for signs of mousepox, such as coat condition, conjunctivitis, body paralysis, and limb swelling. Mice were euthanized if disease manifestations were extremely severe in one or more illness parameters based on a numeric score. Scores of 1 to 3 were assigned to each disease parameter, and mice with a score of 3 in one parameter or 5 in cumulative parameters were sacrificed. Coat condition scores were as follows: 1, slightly rough; 2, disheveled/wounds forming; 3, bleeding or irritated wounds/severe hair loss. Eye condition scores were as follows: 1, mild discharge; 2, severe conjunctivitis. Movement scores were as follows: 1, abnormal/uncoordinated; 2, walking on tiptoe/reluctant to move; 3, staggering/paralysis. Limb condition scores were as follows: 1, swelling; 2, abnormal limbs; 3, severe necrosis/loss of limbs.
Virus titration of organs, histological evaluation, and liver enzyme and cytokine levels in the serum.
Mice were infected s.c. with 103 PFU and sacrificed at the indicated times p.i. for organ and serum collection. Liver, spleen, and lung samples were homogenized in a medium volume proportional to the sample weight (grams × 9 = ml). Popliteal draining lymph node samples were homogenized in a fixed volume of medium. The viral load was quantified by plaque assay using BS-C-1 monolayers. Histology samples were fixed in 10% formalin, and paraffin-embedded tissue sections were stained with hematoxylin and eosin (H&E) or with DAPI (4′-6-diamidino-2-phenylindole) (Sigma) and the Dead End Fluorometric TUNEL System (Promega) according to the manufacturers' protocols. The percentages of apoptotic cells measured by terminal deoxynucleotidyltransferase dUTP nick end labeling (TUNEL) staining were manually counted as the green fluorescent (TUNEL+) cells colocalized with blue fluorescent (DAPI+) cells within the total DAPI+ cell population. For each mouse sample, >500 cells were analyzed in 5 different regions of the tissue section visualized in a Leica TCS SP5 confocal microscope. Liver enzyme (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) levels in the serum were determined at the ACT Pathology Unit of the Canberra Hospital, Australia. The levels of the cytokines mouse gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), IL-2, IL-4, IL-10, IL-6, and IL-17A in the serum were quantified with the BD CBA mouse Th1/Th2/Th17 cytokine kit (BD Biosciences). The levels of IL-18 and IL-1β in the sera of infected mice were determined by the mouse IL-18 ELISA (Bender MedSystems) and the BD OptEIA mouse IL-1β ELISA set (BD Biosciences), respectively. For IFN-β detection, rabbit polyclonal antibody against mouse IFN-β (PBL interferon source 32400-1) and rat monoclonal antibody against mouse IFN-β (RMMB-1) (PBL interferon source 22400-1) were used in a capture enzyme-linked immunosorbent assay (ELISA).
Flow cytometry.
Infected mice were sacrificed at the indicated times p.i., and spleens and blood were collected. Single-splenocyte suspensions were obtained by pressing organs through cell strainers, followed by hypotonic red blood cell lysis. Total splenocyte numbers were determined by counting, and 106 cells per sample were analyzed by fluorescence-activated cell sorter (FACS). Twenty-five microliters of each blood sample treated with heparin, followed by red blood cell lysis, was used for FACS staining. Splenocytes and blood cells were incubated in 7-amino actinomycin D (7AAD) and stained for CD8α allophycocyanin (APC)-conjugated clone 53-6.7 (BD Pharmingen), CD8α fluorescein isothiocyanate (FITC)-conjugated clone 53-6.7 (BD Pharmingen), CD4 FITC-conjugated clone H129.19 (BD Pharmingen), B220 phycoerythrin (PE)-conjugated clone RA3-6B2 (BD Pharmingen), F4-80 FITC-conjugated clone CI:A3-1 (AbD Serotec), and CD49b Alexa Fluor 647-conjugated clone DX5 (BioLegend). The cells were then fixed with fixation buffer (BioLegend), permeabilized with 0.5% saponin, and stained with polyclonal rabbit anti-granzyme B serum (a gift from Markus Simon, Max Planck Institute, Freiburg, Germany) or preimmune rabbit serum, followed by Alexa Fluor 647-conjugated (Molecular Probes) or PE-conjugated (Caltag Laboratories) rat anti-rabbit IgG. For apoptosis assays, MEF were infected as described above, and at the indicated hours p.i., the cells were stained using the Annexin V-PE Apoptosis Detection kit I (BD Pharmingen) or with 3,3′-dihexyloxacarbocyanine iodide (DiOC6; Molecular Probes). The cells were analyzed on a FACSCalibur flow cytometer (BD Biosciences) and with WEASEL FACS software (Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia) or FlowJo (Flow Cytometry Analysis Software).
NK cell cytotoxic assay.
Mice were infected s.c. with 103 PFU and sacrificed at 4 and 6 days p.i. Spleens were collected and tested for NK cytotoxicity in a standard 51Cr release assay (34), using uninfected YAC-1 cells as targets. Uninfected mice were used as controls. P values were calculated from specific lysis from a four-point logarithmic regression curve, interpolated at an effector-to-target (e/t) ratio of 30.
NK depletion.
For NK depletion, 20 μl of anti-asialo GM1 antiserum (Wako Pure Chemical Industries, Ltd.) or 100 μl of preimmune rabbit serum (Sigma), each diluted to 200 μl with PBS, was injected intraperitoneally (i.p.) 1 day before virus infection, 2 days p.i., and 5 days p.i. Three mice per group were injected in each of two independent experiments. In one experiment, PBS was injected as a negative control, and in the other experiment, preimmune rabbit serum was used as a negative control for NK cell depletion.
RESULTS
ECTV SPI-2 mutants and revertant.
We generated two ECTV SPI-2-deficient viruses containing different genetic modifications in the SPI-2 gene. ECTV SPI-2 SADΔ was produced by deletion of 427 bp of the coding sequence, resulting in the absence of the carboxy-terminal region in the SPI-2 protein. Most of the SPI-2 mRNA is not translated due to a frameshift mutation in the coding sequence; however, a truncated form of SPI-2 protein lacking the serpin reactive site (LVSD) could be expressed. The second mutant, referred to as ECTV SPI-2Δ, was generated by deletion of the complete gene. A revertant virus (ECTV SPI-2 REV) was generated by restoring the wt SPI-2 gene on the ECTV SPI-2 SADΔ background. The genetic profile of the recombinant viruses was confirmed by PCR and sequencing. All of the remaining SPI-2 gene coding sequence plus 39 upstream nucleotides and 101 downstream nucleotides was determined by sequencing of ECTV SPI-2 SADΔ. Two hundred forty upstream nucleotides and 410 downstream nucleotides were determined by sequencing of ECTV SPI-2Δ.
SPI-2 or a truncated form of the protein was not detected by Western blot analysis of uninfected cells or cells infected with ECTV SPI-2 SADΔ or ECTV SPI-2Δ, whereas lysates of cells infected with wt ECTV or ECTV SPI-2 REV presented a band of the expected size for the full-length SPI-2 protein (see Fig. S1 in the supplemental material). All samples expressed actin, including uninfected cells.
Deletion of SPI-2 does not compromise virus growth in vitro.
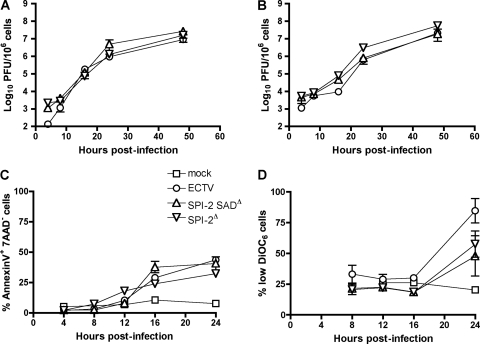
To determine whether SPI-2 is required for efficient replication in vitro, virus growth curves were established using two different types of mouse cell lines. The recombinant viruses tested had replication efficiencies similar to that of wt ECTV in L929 cells and MEF (Fig. 1A and B, respectively). In agreement with this observation, the appearances of apoptotic features triggered by infection were similar in wt virus and mutants as measured by phosphatidylserine exposure (Fig. 1C) and production of reactive oxygen species (data not shown). Both SPI-2 mutant viruses induced less disruption of the mitochondrial membrane potential than wt virus 24 h p.i., but the difference was not statistically significant (Fig. 1D).
Fig. 1.
In vitro replication and apoptosis induction of ECTV SPI-2 mutants is similar to that of wt virus. (A and B) L929 cells (A) and MEF (B) were infected at an MOI of 3 with ECTV or ECTV SPI-2 mutants. The cell-associated viral load was quantified by plaque assay. The graphs show means ± standard errors of the mean (SEM) of one representative of three independent experiments. (C and D) MEF were infected as for panel B. Infected-cell apoptosis (C) and mitochondrial potential disruption (D) were quantified by phosphatidylserine exposure (annexin+ 7AAD−) and DiOC6 staining of MEF, respectively, at the indicated times p.i.
ECTV SPI-2 protein is a potent virulence factor in susceptible mice.
To study the importance of SPI-2 in viral pathogenesis, C57BL/6 and BALB/c mice were infected s.c. with either wt ECTV or recombinant virus. Disease signs, such as disheveled coat, conjunctivitis, foot swelling, and movement impairment, were recorded daily and scored. It is well established that C57BL/6 mice are highly resistant (50% lethal dose [LD50] > 105 PFU) whereas BALB/c mice are susceptible (LD50 < 10 PFU) to s.c. ECTV infection (2). Therefore, C57BL/6 mice were infected with a high (106 PFU) or a low (103 PFU) dose of each virus. BALB/c mice were infected with 103 PFU, which results in 100% mortality when wt ECTV is used.
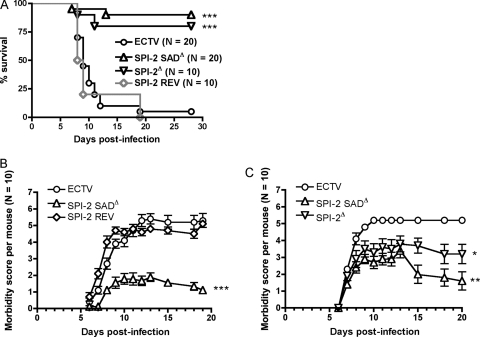
All C57BL/6 mice infected with ECTV SPI-2 SADΔ or wt virus survived for at least 4 weeks p.i. and showed few or no disease signs, regardless of the dose given (data not shown). As was expected, BALB/c mice infected with ECTV or ECTV SPI-2 REV were highly susceptible to classical mousepox disease, and most animals succumbed to infection, with a median survival time of 9 days p.i. Severe manifestations of disease were frequently observed before mice were euthanized. In contrast, 80 to 90% of mice infected with SPI-2-deficient viruses survived the infection (Fig. 2), although some signs of illness, including disheveled coat, conjunctivitis, and limb swelling were observed throughout the course of infection. About half of the mice that survived ECTV SPI-2 mutant infection still had detectable virus by 4 weeks p.i. (mean ± standard deviation [SD], 3.8 × 103 ± 1.6 × 103 in the spleen and 5.2 × 102 ± 1.9 × 102 in the liver), suggesting that clearance of the virus took longer than in mousepox infection of resistant strains. By 8 weeks p.i., mutant viruses were no longer detectable.
Fig. 2.
ECTV SPI-2 mutants are strongly attenuated in vivo. (A) Female BALB/c mice were infected s.c. with 103 PFU of either ECTV, ECTV SPI-2 SADΔ, ECTV SPI-2Δ, or ECTV SPI-2 REV. Illness signs were scored daily. “% survival” excludes dead and euthanized mice. The data from two independent experiments were combined. (B and C) Illness signs for the two experiments pooled in the survival graph in panel A. Mice that were euthanized or found dead were given the score on the day of euthanasia or 5, respectively, for the days following their death. *, P < 0.05; **, 0.001 ≤ P ≤ 0.01; ***, P < 0.0001 in relation to both ECTV and ECTV SPI-2 REV. The error bars indicate standard errors of the means (SEM).
Diminished viral loads in livers of mice infected with ECTV in the absence of SPI-2.
As the final cause of death in ECTV-infected mice has been described in the literature as acute hepatitis followed by multiorgan failure (18), it was of interest to know whether the virus loads in various target organs, especially the liver, were affected by deletion of SPI-2 from ECTV. Surprisingly, the viral loads in the spleen and popliteal draining lymph nodes were similar for all viruses (Table 1). A remarkably high viral load in the spleen was observed on day 4 and day 6 p.i. in mice infected with ECTV or ECTV SPI-2 mutants, and only a modest, but significant (P > 0.05), reduction in titer was seen for ECTV SPI-2 SADΔ at 4 days p.i. relative to wt virus (Table 1). On the other hand, the viral loads in the liver increased more slowly, and at both days 4 and 6 p.i., ECTV SPI-2 mutants had replicated to lower levels than ECTV (Table 1). The liver viral load decrease 6 days p.i. was about 100-fold in ECTV SPI-2 SADΔ-infected mice and 10-fold in ECTV SPI-2Δ-infected mice compared to wt virus. In another independent experiment, we found higher viral loads (10-fold) in the livers of mice infected with 103 PFU of ECTV SPI-2 REV than in the livers of mice infected with ECTV SPI-2 SADΔ (P = 0.0403) (data not shown). We also found lower viral loads in the lungs of ECTV SPI-2 SADΔ mutant-infected mice than in those of ECTV-infected mice (Table 1).
Table 1.
Viral loads from target organs of mice infected with wt or mutant virus
| Organ | Viral loada |
|||||
|---|---|---|---|---|---|---|
| Day 4 p.i. |
Day 6 p.i. |
|||||
| ECTV | SPI-2 SADΔ | SPI-2Δ | ECTV | SPI-2 SADΔ | SPI-2Δ | |
| Lymph node | 6.2 × 105 ± 1.9 × 105 | 1.7 × 105 ± 1.1 × 105 | 2.4 × 105 ± 1.7 × 105 | 2.8 × 106 ± 7.8 × 105 | 2.2 × 106 ± 4.6 × 105 | 2.2 × 106 ± 3.8 × 105 |
| Spleen | 4.3 × 107 ± 1.0 × 107 | 1.1 × 107* ± 5.8 × 106 | 1.5 × 107 ± 8.0 × 106 | 2.0 × 108 ± 7.2 × 107 | 1.15 × 108 ± 2.9 × 107 | 2.7 × 108 ± 9.4 × 107 |
| Liver | 2.8 × 105 ± 1.1 × 105 | 2.0 × 104* ± 6.3 × 103 | 6.0 × 104 ± 9.0 × 103 | 1.3 × 108 ± 3.3 × 107 | 9.9 × 105** ± 5.8 × 105 | 1.1 × 107** ± 1.0 × 107 |
| Lung | 3.5 × 103 ± 1.2 × 103 | 4.1 × 103 ± 2.5 × 103 | NT | 1.1 × 106 ± 2.8 × 105 | 6.7 × 104** ± 1.4 × 104 | NT |
BALB/c mice were infected s.c. with 103 PFU of ECTV or ECTV SPI-2 mutants. Viral loads in various organs at 4 and 6 days p.i. were determined by plaque assay. The viral loads are given in PFU/lymph node or PFU/g of tissue for spleen, liver and lung. The assay detection limit was ≤102 PFU/g. The data shown are means±standard errors of the mean (SEM) pooled from two independent experiments (n = 3 for each experiment). *, P < 0.05, and **, 0.001 ≤ P ≤ 0.01 in relation to ECTV. NT, not tested.
Liver and spleen pathology caused by mousepox is reduced in the absence of SPI-2 protein.
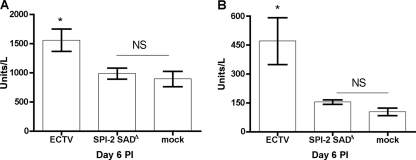
Next, we asked whether the higher viral loads seen in livers of ECTV-infected mice than in those of ECTV SPI-2 SADΔ-infected mice were associated with a difference in overt liver pathology. Examination of liver sections collected at days 6 and 9 p.i. revealed intense lymphocyte infiltration and several necrotic foci throughout the organ in both groups but no obvious differences between the two groups (see Fig. S2 in the supplemental material). In addition, the overall liver structure was preserved, unlike the pathology recorded after ECTV infection of highly susceptible perforin-deficient C57BL/6 mice (38). However, the levels of two serum markers of liver pathology, AST and ALT, were increased in ECTV- but not ECTV SPI-2 SADΔ-infected mice (Fig. 3A and B). The reduced viral loads in the liver and improved liver function in mice infected with an ECTV mutant lacking SPI-2 suggest that the increased survival of these mice is linked to an attenuation of the hepatitis caused by the wt ECTV infection in BALB/c mice (18).
Fig. 3.
Liver damage induced by mousepox is reduced in the absence of SPI-2. BALB/c mice were infected s.c. with 103 PFU of ECTV or ECTV SPI-2 SADΔ or mock infected. Serum was collected at 6 days p.i., and levels of the liver enzymes aspartate aminotransferase (A) and alanine aminotransferase (B) were determined. The graphs represent means ± SEM of data combined from three independent experiments. *, P < 0.05 in relation to mock-infected mice. NS, not statistically significant (P > 0.05).
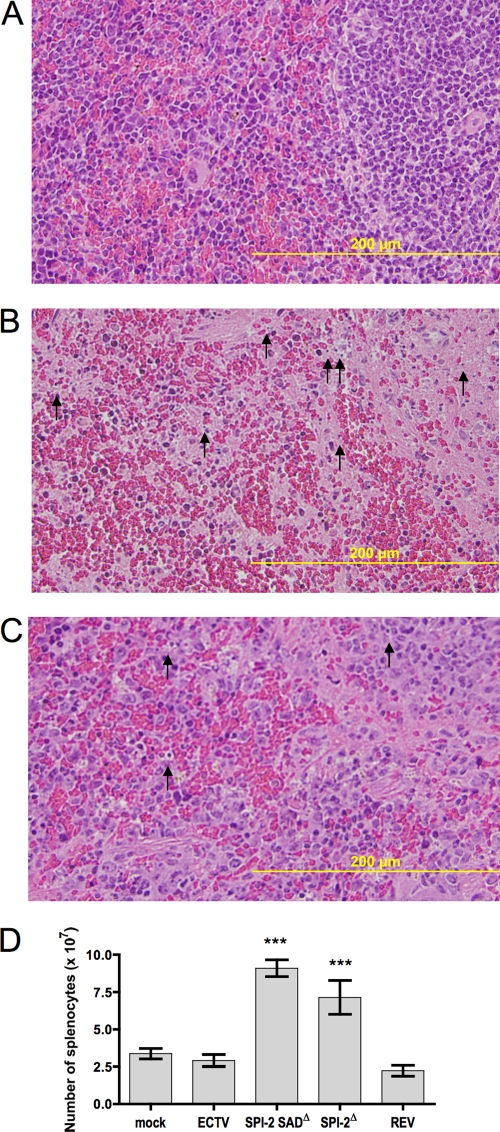
In contrast to the liver, a significant difference in histopathology was found between the spleens of mice infected with wt virus and those of mice infected with ECTV SPI-2 SADΔ. Wt ECTV-infected mice presented spleens with massive tissue destruction at day 6 p.i., whereas ECTV SPI-2 SADΔ-infected mice presented markedly less tissue damage (Fig. 4B and C, respectively). In accordance with this histological evidence of widespread necrotic damage, the sizes of spleens from mice infected with wt ECTV or ECTV SPI-2 REV were greatly reduced macroscopically, and total splenocyte numbers were at least 2-fold lower, compared to those from mice infected with ECTV SPI-2 SADΔ or ECTV SPI-2Δ (Fig. 4D).
Fig. 4.
Spleen pathology caused by mousepox is reduced in the absence of SPI-2 protein. (A to C) BALB/c mice were mock infected (A) or infected s.c. with 103 PFU of wt ECTV (B) or ECTV SPI-2 SADΔ (C). The animals were sacrificed at 6 days p.i., and the spleens were sectioned and stained with H&E. The arrows indicate the presence of pyknotic cells. (D) Total splenocyte numbers are increased in mice infected with ECTV lacking SPI-2. Mice were infected as in panels A to C with each virus. At day 6 p.i., the spleens were processed for cell counting. The graph represents means ± SEM of data combined from two independent experiments (n = 3 for each experiment). ***, P < 0.0001 in relation to both wt ECTV and ECTV SPI-2 REV.
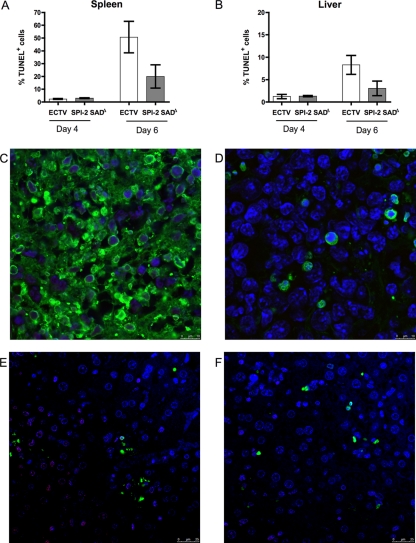
As a more direct measure of the damage to the liver and spleen caused by infection, tissue sections were analyzed for the presence of apoptotic cells presenting DNA fragmentation measured by TUNEL staining (Fig. 5). Similar levels of apoptosis were found in organs of mice infected with wt or mutant virus at day 4 p.i. In both liver and spleen tissues, wt ECTV induced higher proportions of TUNEL+ cells than ECTV SPI-2 SADΔ 6 days p.i., although the differences were not statistically significant.
Fig. 5.
ECTV lacking SPI-2 presents proportions of TUNEL+ cells in spleen and liver similar to those of wt ECTV. BALB/c mice were infected s.c. with 103 PFU of wt ECTV or ECTV SPI-2 SADΔ. Organs collected on the indicated days p.i. were sectioned, formalin fixed, and analyzed for DNA fragmentation with TUNEL staining. (A and B) Cells presenting green fluorescence (TUNEL+) colocalized with blue fluorescence (DAPI [4′,6-diamidino-2-phenylindole]) were counted in spleen (A) and liver (B) sections. The error bars indicate SEM. (C to F) Representative pictures of spleen (C and D) and liver (E and F) sections of mice infected with wt ECTV (C and E) or ECTV SPI-2 SADΔ (D and F) for 6 days and stained for TUNEL and with DAPI.
Thus, although similar viral loads were observed in the spleens of ECTV- and ECTV SPI-2 mutant-infected mice (Table 1), indicating that on a per volume basis virus titers are higher in wt virus-infected mice, the overall spleen destruction caused by mousepox is greatly exacerbated in the presence of the SPI-2 protein. In view of the comparable growth properties of the mutant and wt viruses, the spleen protection observed in mutant-infected mice suggests that the virulence factor SPI-2 thwarts immune-mediated control of ECTV infection.
IL-18 and IFN-γ production are enhanced in mice infected with ECTV SPI-2 mutants.
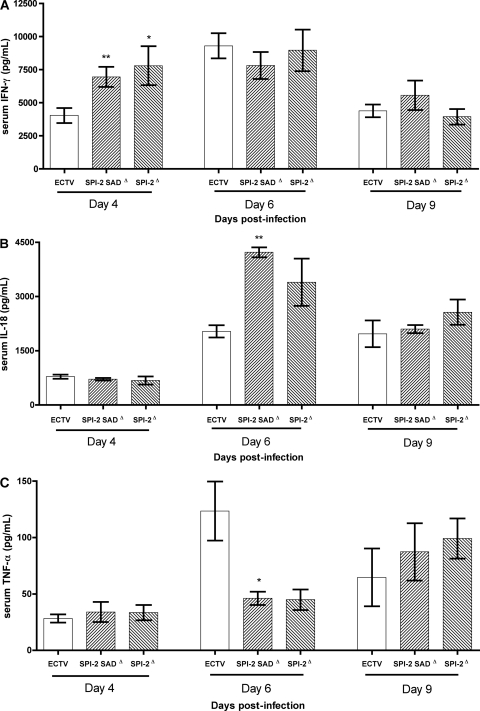
Host recovery from a primary mousepox infection depends on the induction of a Th1-type immune response and requires effective antiviral cytotoxic T and NK cell immunity (3, 5, 33, 43). To test if these immune responses were differentially elicited by ECTV in the presence or absence of SPI-2, the cytokine profile in the sera of infected mice was examined. The levels of IL-2, IL-4, IL-17A, IL-10, IL-6, IFN-γ, and TNF-α were assessed at days 2, 4, 6, and 9 p.i. Given their often rapid induction, IFN-β, IL-1β, and IL-18 were analyzed in addition at 0, 6, 12, and 24 h p.i. All infected mice generated a predominantly Th1 type of cytokine response, characterized by IFN-γ, IL-18, and TNF-α production. However, IL-10 was also detected in several mice throughout the infection (data not shown). Levels of IFN-β, IL-1β, IL-2, IL-4, and IL-17A were not markedly raised at any of the time points tested compared to uninfected mice.
Most notably, ECTV-infected mice had considerably lower levels of serum IFN-γ than mice infected with ECTV SPI-2 mutants at 4 days p.i., while 2 days later, all infected mice reached similar peak levels of IFN-γ, decreasing by 9 days p.i. (Fig. 6A). IL-18 release was detected in the serum only between 4 and 9 days p.i., and at 6 days p.i., the levels of the cytokine were higher in ECTV SPI-2 mutant-infected mice than in ECTV-infected mice (Fig. 6B). IL-18 was not detected in the blood at any time tested before day 4 p.i. In contrast, increased levels of TNF-α in the sera of mice infected with ECTV expressing the SPI-2 protein were found at 6 days p.i. (Fig. 6C). No difference in IL-10 and IL-6 levels in the serum was detected between wt virus- and mutant virus-infected mice. Thus, in the absence of SPI-2, the host Th1 cytokine responses, in particular IFN-γ and IL-18, are enhanced.
Fig. 6.
IFN-γ and IL-18 cytokine levels are increased in the sera of SPI-2 mutant-infected mice. BALB/c mice were infected s.c. with 103 PFU of ECTV, ECTV SPI-2 SADΔ, or ECTV SPI-2Δ. Sera were collected at the indicated time points. IFN-γ (A), IL-18 (B), and TNF-α (C) levels were determined by cytometric bead array or ELISA. The graphs show means ± SEM from combined data from three independent experiments (n = 3 for each experiment). *, P < 0.05, and **, 0.001 ≤ P ≤ 0.01 in relation to ECTV.
NK cell and CD8+ T cell responses are enhanced in the absence of SPI-2.
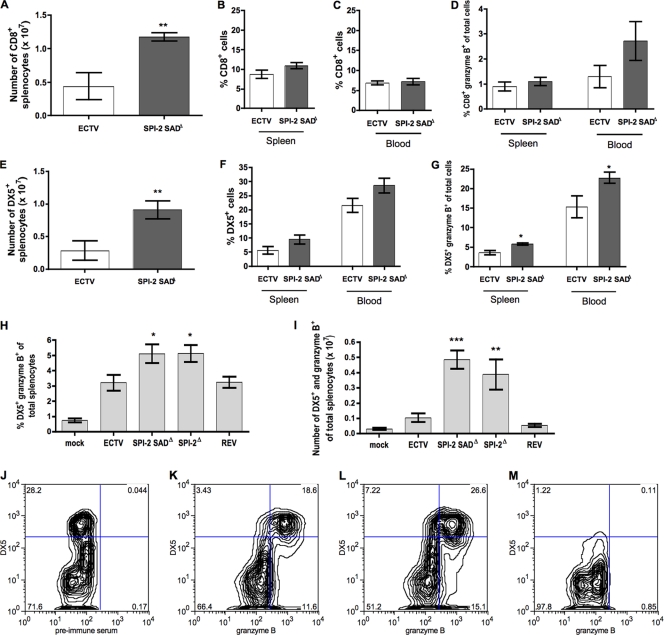
Given the increased protection of the splenic tissue and the Th1 cytokine response enhancement observed in mutant-infected mice, we investigated the development and activation of lymphocyte populations during ECTV infection with or without SPI-2. The relative percentages of B cells, CD4+ and CD8+ T cells, and NK cells in the spleen and circulating in the blood 6 days p.i. did not markedly differ between ECTV- and ECTV SPI-2 SADΔ-infected mice (Fig. 7B, C, and F and data not shown). However, because the total number of splenocytes in ECTV SPI-2 SADΔ-infected mice was around double that in wt virus-infected mice (Fig. 4D), the absolute number of each lymphocyte population was markedly reduced following wt virus infection (Fig. 7A and E and data not shown).
Fig. 7.
SPI-2 protein reduces the size of cytotoxic lymphocyte populations in mousepox infection. BALB/c mice infected s.c. with 103 PFU of each virus were sacrificed at 6 days p.i. Spleens and blood were harvested for FACS staining. (A) Total numbers of CD8+ splenocytes. (B and C) Percentages of CD8+ cells found in the spleen and blood, respectively. (D) Percentages of granzyme B+ CD8+ cells. (E) Total numbers of DX5+ splenocytes. (F) Percentages of DX5+ cells in the spleen and blood. (G and H) Percentages of granzyme B+ DX5+ cells. (I) Total numbers of DX5+ and granzyme B+ splenocytes. The graphs are the means ± SEM of data pooled from three independent experiments (A, B, and D to G) or two independent experiments (C, H, and I) (n = 3 for each experiment). *, P < 0.05; **, 0.001 ≤ P ≤ 0.01; and ***, P < 0.0001 in relation to both wt ECTV and ECTV SPI-2 REV. (J to M) Representative flow cytometry plots of blood lymphocyte samples from mice infected with ECTV SPI-2 SADΔ (J, L, and M) or ECTV (K) shown in the bar graph in panel G. The background staining of preimmune rabbit serum was used for gating granzyme B+ populations (J), and NK cell-depleted blood lymphocytes were used for gating DX5+ populations (M).
Granzyme B is an essential effector molecule for recovery from mousepox (38, 42). Therefore, granzyme B expression in CD8+ T cells and NK cells from ECTV- and ECTV SPI-2 SADΔ-infected mice was assessed. At 6 days p.i., the percentages of CD8- and granzyme B-positive cells in the spleen were low (around 1%) in both ECTV- and ECTV SPI-2 SADΔ-infected mice. In blood, an ∼2-fold-greater percentage of cells were CD8+ and expressed granzyme B in ECTV SPI-2 SADΔ-infected mice than in ECTV-infected mice (Fig. 7D), although this difference did not reach statistical significance. Ex vivo cytotoxicity against virus-infected, major histocompatibility complex class I (MHC-I)-matched target cells was marginal for splenocytes from mice infected with either virus for 4 and 5 days (data not shown). In contrast, splenocytes from mice infected with ECTV SPI-2 SADΔ for 6 days were able to induce target cell lysis and were more cytotoxic than splenocytes from mice infected with the wt (P < 0.0077) or revertant (P < 0.0008) virus (see Fig. S3 in the supplemental material).
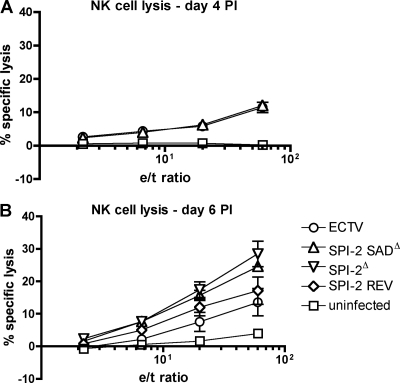
The proportion and total number of NK cells expressing granzyme B in the spleen and blood were higher in mice infected with ECTV SPI-2 SADΔ than in mice infected with wt virus at 6 days p.i. (Fig. 7G, H, and I). This increased granzyme B+ NK cell population accounted for approximately 25% of the total circulating leukocytes in the blood following mutant virus infection (Fig. 7G). The increased expression of granzyme B in NK cells of ECTV SPI-2 SADΔ-infected mice suggests an increase in the cytotoxic potential of these cells compared to NK cells of wt ECTV-infected mice. At day 4 p.i., the ex vivo cytotoxicities of splenocytes against YAC-1 cells were similar for ECTV- and ECTV SPI-2 SADΔ mutant-infected mice (Fig. 8A). However, at day 6 p.i., the ex vivo cytotoxicity of splenocytes against YAC-1 cells was higher for ECTV SPI-2Δ (P = 0.0097) and ECTV SPI-2 SADΔ (not statistically significant; P = 0.0526) mice than for mice infected with wt or revertant virus (Fig. 8B). Even though the increase in NK cytotoxicity induced by the ECTV SPI-2 SADΔ infection was not statistically significant with the sample size used, the two SPI-2 mutant viruses presented similar trends, and therefore, we believe that the increased levels of target cell lysis mediated by ex vivo splenocytes are representative for both mutant-infected mice compared to mice infected with ECTV expressing SPI-2. This result correlated with the lower granzyme B expression in NK cells and the lower levels of IL-18 in the blood of wt ECTV-infected mice at this time p.i. relative to ECTV SPI-2 SADΔ-infected mice, suggesting that SPI-2 may prevent optimal NK cell activation and cytotoxicity.
Fig. 8.
Increased NK cell cytotoxicity in the absence of SPI-2. Mice were infected s.c. with 103 PFU of each virus, spleens were harvested at 4 (A) and 6 (B) days p.i., and a standard 51Cr release assay against YAC cell targets was performed for 6 h. Splenocytes from uninfected mice were used as controls.
SPI-2 abrogates the protective NK cell response in mousepox infection.
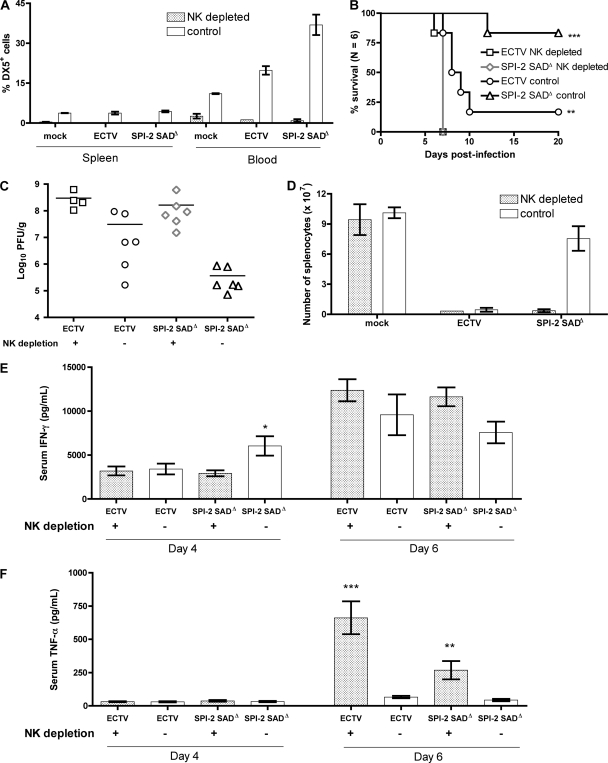
To test if the vigorous NK cell response is essential for resistance of BALB/c mice to infection with ECTV SPI-2 mutants, NK cells were depleted in vivo. Both CD4+ (data not shown) and CD8+ lymphocyte populations (see Fig. S4 in the supplemental material) were not markedly affected by anti-asialo GM1 treatment, whereas the DX5+ cell population was effectively depleted (Fig. 9A). Lack of NK cells rendered BALB/c mice as susceptible to ECTV SPI-2 SADΔ infection (all animals died by day 7 p.i.) as nondepleted mice were to wt ECTV (Fig. 9B). The liver viral loads were ∼10-fold and ∼1,000-fold increased in NK cell-depleted mice compared to control mice when infected with wt virus or mutant virus, respectively (Fig. 9C). Importantly, following NK cell depletion, mice infected with mutant and wt viruses presented liver viral loads that were similar in magnitude at 6 days p.i. Moreover, the depletion of NK cells in ECTV SPI-2 SADΔ-infected mice resulted in pronounced tissue destruction in the spleen and loss of splenocytes (Fig. 9D), similar to that seen in NK-sufficient mice infected with wt virus. The NK depletion treatment was not intrinsically cytotoxic because equivalent splenocyte numbers were obtained from NK cell-depleted and nondepleted, uninfected mice.
Fig. 9.
NK cells mediate survival of ECTV SPI-2 SADΔ-infected mice. BALB/c mice were treated with anti-asialo GM1 antibody or preimmune serum injected i.p. at days −1, 2, and 5 in relation to infection with 103 PFU of ECTV or ECTV SPI-2 SADΔ. (A) Percentage of DX5+ cells in the spleen and blood at 6 days p.i. The graph shows means ± SEM of one representative of two independent experiments. (B) Illness signs and survival were scored daily for 20 days. (C and D) At day 6 p.i., mice were sacrificed, liver samples were collected for plaque assay (C), and total splenocyte numbers were determined (D). The mice were bled for serum collection at days 4 and 6 p.i., and IFN-γ (E) and TNF-α (F) levels in the serum were determined by cytometric bead assay. Panels C, D, E, and F show combined data from two independent experiments (n = 3 for each experiment). The graphs show means ± SEM. *, P < 0.05; **, 0.001 ≤ P ≤ 0.01; ***, P < 0.001 in relation to the nondepleted group.
The levels of IFN-γ found in the sera of NK cell-depleted mice infected with the mutant virus were reduced to the levels found in NK-sufficient mice infected with the wt virus at 4 days p.i. (Fig. 9E). In contrast, the levels of TNF-α in the sera of NK-depleted mice were remarkably high compared to nondepleted mice at 6 days p.i. (Fig. 9F).
Collectively, the decreased survival, increased liver viral load, and reduced levels of serum IFN-γ of NK cell-depleted mice infected with ECTV SPI-2 SADΔ support the conclusion that NK cells are the key effector cells for protection against mousepox in mutant-virus-infected mice and that SPI-2 deletion counteracts this disease-ameliorating immune pathway. Furthermore, NK cells are the likely predominant source of early IFN-γ. Thus, the main downstream effect of SPI-2 expression is the prevention of NK cell responses in susceptible strains.
DISCUSSION
In the present study, we establish that the ECTV SPI-2 protein is a virulence factor that prevents the induction of a protective NK cell response in susceptible mouse strains. This conclusion is based on the striking similarity seen for multiple aspects of pathogenesis in NK cell-depleted mice infected with the SPI-2 mutant virus compared with NK-sufficient animals infected with wt ECTV. These similarities include viral loads in the liver, delayed IFN-γ response, spleen pathology, and levels of mortality (Fig. 9). In short, all the hallmarks of attenuation due to loss of SPI-2 activity from ECTV are absent in mice lacking NK cells.
We also found that the proportions of CD8+ T cells that express granzyme B and the ex vivo cytotoxicity of these cells were elevated in mutant-infected mice (Fig. 7D; see Fig. S3 in the supplemental material). However, it seems more likely that the improved CD8+ T cell responses observed in mice infected with the SPI-2 mutant virus are a consequence of the enhanced NK cell function rather than a direct effect of SPI-2 on CD8+ T cells. This is because previous work has shown that lack of an efficient NK response leads to uncontrolled virus replication and compromises the development of CD8+ T cells (14, 15). Our data support these findings, as we also found that NK cell-depleted ECTV-infected mice have severely lymphopenic spleens (Fig. 9D) with reduced numbers and proportions of CD8+ T cells expressing granzyme B (see Fig. S4 in the supplemental material), even though the proportion of all CD8+ T cells in the spleen is not affected by the absence of NK cells.
While virus attenuation was also found in other orthopoxvirus SPI-2 mutants (28, 30, 31, 41, 49), this is the first time that SPI-2 effects on NK cell function have been observed. The only previous work to suggest an inhibitory effect on in vivo cell-mediated immune responses by this viral protein was a report of an increased influx of CD3+ cells into the ear pinnae of C57BL/6 mice 8 days p.i. with a CPXV crmA mutant (30). In contrast, one study attributed the attenuation of the VACV SPI-2 mutant virus to in vivo replication deficiency and suggested that the mutant virus elicits humoral and cell-mediated responses as efficiently as the parental virus (28). Livers and spleens of mice infected with wt ECTV presented similar or slightly increased numbers of apoptotic foci compared to mice infected with mutant virus at 6 days p.i. (Fig. 5), suggesting that an anti-apoptotic function for SPI-2 is unlikely. These observations reinforce the notion that caution must be exercised when extrapolating findings across different infectious models even within closely related viruses (36).
There are several pathways by which SPI-2 might inhibit a protective NK cell response in the mousepox model. One possibility is the inhibition of killing of infected cells by blocking either the granule exocytosis pathway, proposed to be mediated mainly by granzyme B (16), or the death receptor pathway, mediated by caspase 8. However, in vitro studies have suggested that ECTV SPI-2 may only weakly inhibit mouse granzyme B (50), and this serine protease restricts ECTV replication even in the presence of SPI-2 (42). We have shown previously that the CPXV SPI-2 protein inhibits the death receptor pathway (37), and recently, we also found that ECTV SPI-2 blocks target cell killing by virus-immune splenocytes in the absence of perforin (C. R. Melo-Silva, J. Pardo, M. Lobigs, A. Koskinen, D. C. Tscharke, R. M. Buller, A. Müllbacher, and M. Regner, unpublished data). However, perforin and the granule exocytosis pathway are essential for mousepox recovery (35, 38), and mice deficient in Fas ligand or Fas molecule are not overtly susceptible to ECTV infection (33; Melo-Silva et al., unpublished), suggesting that effect of SPI-2 on NK cell responses is not only or not at all connected to inhibition of target cell lysis.
Interestingly, we found that at 6 days p.i., ECTV-infected mice present increased levels of TNF-α in the blood compared to ECTV SPI-2 mutant-infected mice (Fig. 6C). TNF-α is a cytokine classically associated with induction of apoptosis in TNF receptor (TNFR)-expressing infected cells (29, 33, 46). This cytokine binds to death receptors expressed in the plasma membranes of target cells, and the result is activation of proapoptotic caspases and cell death. However, the cytokine has also been implicated in immunopathology (8, 17, 55). Most ECTV-infected mice die by day 10 (Fig. 2), whereas ECTV SPI-2 mutant-infected mice survive. Moreover, ECTV-infected mice have higher viral loads in the liver than mutant-infected mice at 6 days p.i. (Table 1). Taken together, these data suggest that, even if the TNF-α circulating in the blood induces apoptosis in infected cells, it is not sufficient to protect ECTV-infected mice. Therefore, we speculated that the increased levels of TNF-α in the blood of ECTV-infected mice could contribute to disease by immunopathology. The liver and lungs exert specialized and essential functions, and excessive nonspecific tissue damage could contribute to multiorgan failure and death. In agreement with this notion, NK-depleted infected mice presented very high levels of the cytokine 6 days p.i., 1 day before their death (Fig. 9F).
Another putative mechanism of action is inhibition of caspase 1, a property that was the first function assigned to an orthopoxvirus-encoded SPI-2 protein (45). Caspase 1 converts IL-18 and IL-1β from their inactive precursors to the biologically active cytokines (10). IL-18 is an inducer of IFN-γ and a stimulator of NK cell activation and cytotoxicity (16, 19, 20, 40, 52). The SPI-2 inhibition of caspase 1 in, for instance, infected macrophages or dendritic cells (DC) might deprive developing NK cells of an environment containing IL-18, which could lead to reduced production of IFN-γ, a cytokine that is important for recovery from mousepox (23), or to reduced NK cell cytotoxicity (16). The increased levels of IL-18 in the blood of mice infected with the ECTV SPI-2 mutants (Fig. 6B) correlated with increased NK and CD8+ T cell numbers, increased granzyme B expression by these cells (Fig. 7), and higher NK cytotoxicity (Fig. 8B), suggesting that the SPI-2 inhibition of caspase 1 activity may indeed partially affect the NK cell response.
Similar in vivo responses were found in the present work and in studies using the ECTV p13 mutant. The p13 protein encoded by ECTV is an IL-18 binding protein (BP) capable of competing with the cytokine cellular receptor reducing IL-18-mediated NF-κB signaling (4). The ECTV p13 mutant does not seem to affect intrinsic virus replication, and the major consequence of reduced IL-18 signaling is a defect in NK cell responses. Taken together, these studies highlight the importance of IL-18 in the development of an efficient NK cell antiviral response.
Given that caspase 1 also activates pro-IL-1β, its inhibition may also lead to a reduction of the pyrogenic inflammatory response mediated by the cytokine. We could not detect systemic IL-1β at any time tested. In addition, we failed to detect any IL-1β secretion by mouse macrophages infected in vitro with ECTV SPI-2 mutants. For instance, IL-1β secretion induced by lipopolysaccharide (LPS) and ATP treatment of peritoneal exudate cells (32) was efficiently inhibited by ECTV infection, regardless of SPI-2 (see Fig. S5 in the supplemental material). We therefore believe that this is unlikely to be a dominant pathway for initiating a cascade ultimately leading to a protective NK cell response.
The finding that NK cells are important in recovery from mousepox in a susceptible strain reflects previous observations of their importance in resistant C57BL/6 mice (14, 15, 43). NK cells are essential to curb virus spread and allow the development of antiviral CD8+ T cell and B cell responses in resistant mice, and NK cell deficiency has been associated with susceptibility in DBA/2J mice (9, 21). The important difference between resistant and susceptible strains in an s.c. infection with ECTV is that, while C57BL/6 mice mount a strong NK cell response even in the presence of SPI-2, in the susceptible BALB/c strain, SPI-2 prevents NK cell development and/or induction. The resistant phenotype may be characterized by redundancy or stronger responses that are either inhibited or present at lower levels in susceptible mice, as has indeed been suggested for the inherent ability to produce IFN-γ (5, 22). The notion of redundancy is supported by the fact that BALB/c mice do not express mRNA for IL-12p40 (22), another activator of NK cells, suggesting that the NK cell response in BALB/c mice may be more dependent on IL-18 than are NK cells from C57BL/6 mice, and this is probably one of the reasons why the virulence mediated by SPI-2 is exacerbated in the BALB/c strain.
In conclusion, the data presented here demonstrate that the ECTV SPI-2 protein is a virulence factor in the infection of susceptible mice and that it plays a previously unexpected role in the prevention of NK cell responses. This adds to the repertoire of known biological functions of SPI-2 homologues and suggests that the genes may have been retained by different orthopoxviruses to counter different aspects of the host response to infection.
Supplementary Material
ACKNOWLEDGMENTS
We thank Julian Pardo and Markus M. Simmon for helpful ideas and discussions.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 17 August 2011.
REFERENCES
- 1. Bartlett N., Symons J. A., Tscharke D. C., Smith G. L. 2002. The vaccinia virus N1L protein is an intracellular homodimer that promotes virulence. J. Gen. Virol. 83:1965–1976 [DOI] [PubMed] [Google Scholar]
- 2. Blanden R. V. 1989. Immunology of virus diseases. Brolga Press, Curtin, Australia [Google Scholar]
- 3. Blanden R. V. 1974. T cell response to viral and bacterial infection. Transplant Rev. 19:56–88 [DOI] [PubMed] [Google Scholar]
- 4. Born T. L., et al. 2000. A poxvirus protein that binds to and inactivates IL-18, and inhibits NK cell response. J. Immunol. 164:3246–3254 [DOI] [PubMed] [Google Scholar]
- 5. Chaudhri G., et al. 2004. Polarized type 1 cytokine response and cell-mediated immunity determine genetic resistance to mousepox. Proc. Natl. Acad. Sci. U. S. A. 101:9057–9062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen N., et al. 2003. The genomic sequence of ectromelia virus, the causative agent of mousepox. Virology 317:165–186 [DOI] [PubMed] [Google Scholar]
- 7. Chen W., Drillien R., Spehner D., Buller R. M. 1992. Restricted replication of ectromelia virus in cell culture correlates with mutations in virus-encoded host range gene. Virology 187:433–442 [DOI] [PubMed] [Google Scholar]
- 8. Clark I. A. 2007. The advent of the cytokine storm. Immunol. Cell Biol. 85:271–273 [DOI] [PubMed] [Google Scholar]
- 9. Delano M. L., Brownstein D. G. 1995. Innate resistance to lethal mousepox is genetically linked to the NK gene complex on chromosome 6 and correlates with early restriction of virus replication by cells with an NK phenotype. J. Virol. 69:5875–5877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dinarello C. A. 2009. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27:519–550 [DOI] [PubMed] [Google Scholar]
- 11. Dobbelstein M., Shenk T. 1996. Protection against apoptosis by the vaccinia virus SPI-2 (B13R) gene product. J. Virol. 70:6479–6485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Falkner F. G., Moss B. 1988. Escherichia coli gpt gene provides dominant selection for vaccinia virus open reading frame expression vectors. J. Virol. 62:1849–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Falkner F. G., Moss B. 1990. Transient dominant selection of recombinant vaccinia viruses. J. Virol. 64:3108–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fang M., Lanier L. L., Sigal L. J. 2008. A role for NKG2D in NK cell-mediated resistance to poxvirus disease. PLoS Pathog. 4:e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fang M., Roscoe F., Sigal L. J. 2010. Age-dependent susceptibility to a viral disease due to decreased natural killer cell numbers and trafficking. J. Exp. Med. 207:2369–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fehniger T. A., et al. 2007. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity 26:798–811 [DOI] [PubMed] [Google Scholar]
- 17. Feldmann H., et al. 1996. Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J. Virol. 70:2208–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fenner F., Buller R. M. L. 1997. Mousepox. Lippincott-Raven Publishers, Philadelphia, PA [Google Scholar]
- 19. Haeberlein S., Sebald H., Bogdan C., Schleicher U. 2010. IL-18, but not IL-15, contributes to the IL-12-dependent induction of NK-cell effector functions by Leishmania infantum in vivo. Eur. J. Immunol. 40:1708–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Humann J., Lenz L. L. 2010. Activation of naive NK cells in response to Listeria monocytogenes requires IL-18 and contact with infected dendritic cells. J. Immunol. 184:5172–5178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jacoby R. O., Bhatt P. N., Brownstein D. G. 1989. Evidence that NK cells and interferon are required for genetic resistance to lethal infection with ectromelia virus. Arch. Virol. 108:49–58 [DOI] [PubMed] [Google Scholar]
- 22. Karupiah G. 1998. Type 1 and type 2 cytokines in antiviral defense. Vet. Immunol. Immunopathol. 63:105–109 [DOI] [PubMed] [Google Scholar]
- 23. Karupiah G., Fredrickson T. N., Holmes K. L., Khairallah L. H., Buller R. M. 1993. Importance of interferons in recovery from mousepox. J. Virol. 67:4214–4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kettle S., et al. 1997. Vaccinia virus serpin B13R (SPI-2) inhibits interleukin-1beta-converting enzyme and protects virus-infected cells from TNF- and Fas-mediated apoptosis, but does not prevent IL-1beta-induced fever. J. Gen. Virol. 78:677–685 [DOI] [PubMed] [Google Scholar]
- 25. Kettle S., Blake N. W., Law K. M., Smith G. L. 1995. Vaccinia virus serpins B13R (SPI-2) and B22R (SPI-1) encode M(r) 38.5 and 40K, intracellular polypeptides that do not affect virus virulence in a murine intranasal model. Virology 206:136–147 [DOI] [PubMed] [Google Scholar]
- 26. King N. J., Mullbacher A., Blanden R. V. 1986. Relationship between surface H-2 concentration, size of different target cells, and lysis by cytotoxic T cells. Cell Immunol. 98:525–532 [DOI] [PubMed] [Google Scholar]
- 27. Komiyama T., et al. 1994. Inhibition of interleukin-1 beta converting enzyme by the cowpox virus serpin CrmA. An example of cross-class inhibition. J. Biol. Chem. 269:19331–19337 [PubMed] [Google Scholar]
- 28. Legrand F. A., et al. 2004. Induction of potent humoral and cell-mediated immune responses by attenuated vaccinia virus vectors with deleted serpin genes. J. Virol. 78:2770–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Locksley R. M., Killeen N., Lenardo M. J. 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487–501 [DOI] [PubMed] [Google Scholar]
- 30. MacNeill A. L., Moldawer L. L., Moyer R. W. 2009. The role of the cowpox virus crmA gene during intratracheal and intradermal infection of C57BL/6 mice. Virology 384:151–160 [DOI] [PubMed] [Google Scholar]
- 31. Messud-Petit F., et al. 1998. Serp2, an inhibitor of the interleukin-1beta-converting enzyme, is critical in the pathobiology of myxoma virus. J. Virol. 72:7830–7839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Metkar S. S., et al. 2008. Human and mouse granzyme A induce a proinflammatory cytokine response. Immunity 29:720–733 [DOI] [PubMed] [Google Scholar]
- 33. Müllbacher A. 2003. Cell-mediated cytotoxicity in recovery from poxvirus infections. Rev. Med. Virol. 13:223–232 [DOI] [PubMed] [Google Scholar]
- 34. Müllbacher A., et al. 1991. Alloreactive cytotoxic T cells recognize MHC class I antigen without peptide specificity. J. Immunol. 147:1765–1772 [PubMed] [Google Scholar]
- 35. Müllbacher A., Hla R. T., Museteanu C., Simon M. M. 1999. Perforin is essential for control of ectromelia virus but not related poxviruses in mice. J. Virol. 73:1665–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Müllbacher A., et al. 2004. Can we really learn from model pathogens? Trends Immunol. 25:524–528 [DOI] [PubMed] [Google Scholar]
- 37. Müllbacher A., Wallich R., Moyer R. W., Simon M. M. 1999. Poxvirus-encoded serpins do not prevent cytolytic T cell-mediated recovery from primary infections. J. Immunol. 162:7315–7321 [PubMed] [Google Scholar]
- 38. Müllbacher A., et al. 1999. Granzymes are the essential downstream effector molecules for the control of primary virus infections by cytolytic leukocytes. Proc. Natl. Acad. Sci. U. S. A. 96:13950–13955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nathaniel R., MacNeill A. L., Wang Y. X., Turner P. C., Moyer R. W. 2004. Cowpox virus CrmA, Myxoma virus SERP2 and baculovirus P35 are not functionally interchangeable caspase inhibitors in poxvirus infections. J. Gen. Virol. 85:1267–1278 [DOI] [PubMed] [Google Scholar]
- 40. Okamura H., et al. 1995. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 378:88–91 [DOI] [PubMed] [Google Scholar]
- 41. Palumbo G. J., Pickup D. J., Fredrickson T. N., McIntyre L. J., Buller R. M. 1989. Inhibition of an inflammatory response is mediated by a 38-kDa protein of cowpox virus. Virology 172:262–273 [DOI] [PubMed] [Google Scholar]
- 42. Pardo J., et al. 2009. Caspase-dependent inhibition of mousepox replication by gzmB. PLoS One 4:e7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Parker A. K., Parker S., Yokoyama W. M., Corbett J. A., Buller R. M. 2007. Induction of natural killer cell responses by ectromelia virus controls infection. J. Virol. 81:4070–4079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Quan L. T., Caputo A., Bleackley R. C., Pickup D. J., Salvesen G. S. 1995. Granzyme B is inhibited by the cowpox virus serpin cytokine response modifier A. J. Biol. Chem. 270:10377–10379 [DOI] [PubMed] [Google Scholar]
- 45. Ray C. A., et al. 1992. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell 69:597–604 [DOI] [PubMed] [Google Scholar]
- 46. Ruby J., Bluethmann H., Peschon J. J. 1997. Antiviral activity of tumor necrosis factor (TNF) is mediated via p55 and p75 TNF receptors. J. Exp. Med. 186:1591–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Senkevich T. G., Koonin E. V., Buller R. M. 1994. A poxvirus protein with a RING zinc finger motif is of crucial importance for virulence. Virology 198:118–128 [DOI] [PubMed] [Google Scholar]
- 48. Smith V. P., Alcami A. 2000. Expression of secreted cytokine and chemokine inhibitors by ectromelia virus. J. Virol. 74:8460–8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tscharke D. C., Reading P. C., Smith G. L. 2002. Dermal infection with vaccinia virus reveals roles for virus proteins not seen using other inoculation routes. J. Gen. Virol. 83:1977–1986 [DOI] [PubMed] [Google Scholar]
- 50. Turner S. J., Silke J., Kenshole B., Ruby J. 2000. Characterization of the ectromelia virus serpin, SPI-2. J. Gen. Virol. 81:2425–2430 [DOI] [PubMed] [Google Scholar]
- 51. Wallich R., Simon M. M., Müllbacher A. 2001. Virulence of mousepox virus is independent of serpin-mediated control of cellular cytotoxicity. Viral Immunol. 14:71–81 [DOI] [PubMed] [Google Scholar]
- 52. Wang Y., Chaudhri G., Jackson R. J., Karupiah G. 2009. IL-12p40 and IL-18 play pivotal roles in orchestrating the cell-mediated immune response to a poxvirus infection. J. Immunol. 183:3324–3331 [DOI] [PubMed] [Google Scholar]
- 53. Wong Y. C., Lin L. C., Melo-Silva C. R., Smith S. A., Tscharke D. C. 2011. Engineering recombinant poxviruses using a compact GFP-blasticidin resistance fusion gene for selection. J. Virol. Methods 171:295–298 [DOI] [PubMed] [Google Scholar]
- 54. Xu R. H., et al. 2008. The orthopoxvirus type I IFN binding protein is essential for virulence and an effective target for vaccination. J. Exp. Med. 205:981–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yokota S. 2003. Influenza-associated encephalopathy—pathophysiology and disease mechanisms. Nippon Rinsho 61:1953–1958 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.