Abstract
The human papillomavirus type 16 (HPV-16) E5 oncoprotein is embedded in membranes of the endoplasmic reticulum and nuclear envelope with its C terminus exposed to the cytoplasm. Among other activities, E5 cooperates with the HPV E6 oncoprotein to induce koilocytosis in human cervical cells and keratinocytes in vitro. The effect of E5 on infected cells may rely on its interactions with various cellular proteins. In this study we identify calpactin I, a heterotetrameric, Ca2+- and phospholipid-binding protein complex that regulates membrane fusion, as a new cellular target for E5. Both the annexin A2 and p11 subunits of calpactin I coimmunoprecipitate with E5 in COS cells and in human epithelial cell lines, and an intact E5 C terminus is required for binding. Moreover, E5-expressing cells exhibit a perinuclear redistribution of annexin A2 and p11 and show increased fusion of perinuclear membrane vesicles. The C terminus of E5 is required for both the perinuclear redistribution of calpactin I and increased formation of perinuclear vacuoles. These results support the hypothesis that the E5-induced relocalization of calpactin I to the perinuclear region promotes perinuclear membrane fusion, which may underlie the development of koilocytotic vacuoles.
INTRODUCTION
E5 is one of three oncoproteins encoded by human papillomavirus type 16 (HPV-16) (11, 34), the most common causative agent of cervical and anogenital cancers (3, 58). E5 is a small, hydrophobic membrane protein, which is localized in the endoplasmic reticulum (ER) and nuclear envelope (7, 49). The N terminus of E5 is restricted to the ER lumen, while its C terminus is exposed to the cytoplasm (29) and mediates interactions with cytoplasmic and ER proteins, such as karyopherin β3 (27), Bap31 (43), EVER (30), and HLA class 1 (1). The C terminus of E5 is also essential for inducing koilocytosis, a long-recognized pathognomonic feature of papillomavirus infection (28). Other biological functions of E5 include the induction of epithelial tumors in transgenic mice (14), anchorage-independent growth (48), and altered epidermal growth factor endocytic trafficking (50). These functions rely upon the interactions of E5 with various cellular proteins (4, 6, 20, 30, 38, 45).
In this study we demonstrate that E5 binds calpactin I, potentially explaining its ability to induce perinuclear membrane fusion events. Calpactin I is a heterotetrameric protein complex that consists of two annexin A2 (ANXA2) subunits (also called lipocortin II) and two p11 subunits (also referred to as ANXA2 light chain or S100A10) (10, 17–19, 24, 41). Both components of calpactin I are important for its biological activities (41, 56). ANXA2, a 36-kDa protein that is a member of the annexin family of calcium-binding proteins, associates with phospholipids in a Ca2+-dependent manner (15, 16, 44) and regulates numerous cellular processes (12, 15, 22, 23, 36, 53), including endocytosis (13, 33, 35, 36, 56) and membrane fusion (9, 25, 32, 42). In addition to associating with p11 in the calpactin I complex, ANXA2 also can exist as a monomer (53), which is both distributed throughout the cell and loosely bound to the cytoplasmic surface of the plasma membrane. In contrast, p11 is tightly linked to the submembranous cytoskeleton (51, 57).
MATERIALS AND METHODS
Cells and viruses.
Retroviruses encoding HPV-16 E6 and E7 in the vector pBabePuro (37) or encoding codon-optimized E5 or C-terminal deletion mutants of codon-optimized E5 in the vector pLXSN were generated by using the Phoenix cell system (40). Codon-optimized E5 also was cloned into the pJS55 expression vector as described previously (7, 47). E5 proteins were N-terminally tagged with the AU1 epitope, DTYRYI (31).
Primary human ectocervical cells (HECs) were derived from cervical tissue after hysterectomy for benign uterine disease (2) and were immortalized by infection with HPV-16 E6/E7-encoding retrovirus and selection in the presence of puromycin (0.5 μg/ml). E5-expressing cell lines were generated from immortalized HECs by infection with retroviruses encoding E5 or the empty pLXSN expression vector and selection in the presence of Geneticin G418 (100 μg/ml). Nonimmortalized HECs expressing HPV-16 E6 were generated by infection with E6-encoding retrovirus and selection. HEC lines were grown at 37°C and 5% CO2 in keratinocyte growth medium (KGM; Invitrogen, Carlsbad, CA), supplemented with gentamicin sulfate (10 μg/ml). To maintain constant levels of E5 expression, G418 was administered to cell cultures at alternating passages.
Human foreskin keratinocytes (HFKs) were isolated from neonatal foreskins (46) and cultured in KGM. Immortalized HFK cell lines were generated by infection with retroviruses encoding E6/E7 and either E5 or the empty pLXSN expression vector (with selection).
COS cells were grown in Dulbecco's modified Eagle medium containing 10% fetal bovine serum (FBS), 100 U of penicillin G/ml, and 100 μg of streptomycin sulfate/ml (Invitrogen).
Retroviral infection.
HECs and HFKs were grown as described in 25- or 75-cm2 tissue culture flasks. Prior to infection, the growth medium was removed, and cell culture supernatants containing retroviruses and Polybrene (6 μg/ml) were added to the cells for 1.5 h at 37°C while shaking.
Transfection.
COS cells were grown to 90% confluence in antibiotic-free Opti-MEM (Invitrogen) containing 4% FBS. For each 10- or 15-cm tissue culture dish, 32 or 72 μg of DNA (in pJS55) was mixed with 4 or 9 ml of serum-free Opti-MEM, and 40 or 90 μl of Lipofectamine 2000 transfection reagent (Invitrogen) and added to the cells for 4 h.
HFKs and HECs were grown to 80% confluence in antibiotic-free KGM in four-chamber slides (Nunc, Rochester, NY), on 22-by-22-mm sterile glass coverslips in six-well tissue culture plates or in10-cm tissue culture dishes. For each slide chamber, coverslip, or dish, 0.8, 4, or 32 μg of small interfering RNA (siRNA; Ambion, Austin, TX) was mixed with 0.1, 0.5, or 4 ml of KSFM (Invitrogen) and 1, 5, or 40 μl of Lipofectamine 2000 and added to the cells for 4 h.
Immunoprecipitation and immunoblotting.
Cells were lysed in radioimmunoprecipitation assay (48) or CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} (26) buffer as indicated for the isolation and microsequencing of E5-associated proteins (27) and for immunoprecipitation and immunoblot detection of E5, ANXA2, and p11 (48). The following antibodies were used for immunoblotting: a 1:5,000 dilution of rabbit anti-AU1 (Covance, Princeton, NJ), a 1:1,000 dilution of mouse anti-p11 (BD Biosciences, San Jose, CA), a 1:200 dilution of mouse anti-ANXA2 (Santa Cruz Biotechnology, Santa Cruz, CA), and a 1:10,000 dilution of mouse anti-β-actin (Sigma, St. Louis, MO). Portions (4 μg) of the same antibodies were used for immunoprecipitations.
Immunofluorescence microscopy.
Immunofluorescence detection of E5, p11, and ANXA2 was performed on HFKs grown on 22-by-22-mm glass coverslips, fixed in 4% (wt/vol) paraformaldehyde (20 min), permeabilized in phosphate-buffered saline (PBS) containing 0.1% (wt/vol) saponin (10 min), and blocked in Pgel-S (PBS plus 0.2% [wt/vol] gelatin plus 0.1% saponin) containing 10% normal donkey serum (20 min), as described previously (29). The following primary antibodies were used: rabbit anti-AU1 (1:1,500 dilution), mouse anti-ANXA2 (1:300), and mouse anti-p11 (1:1,000).
To assay perinuclear membrane fusion, HECs that stably express HPV-16 E6 were infected with retroviruses encoding E5 or the empty pLXSN expression vector (28). Three days later, the cells were pulse-labeled with Alexa Fluor 594-conjugated cholera toxin B subunit (CTB; Molecular Probes, Carlsbad, CA) at 1 μg/ml for 30 min at 37°C. Six hours later, the same cells were pulse-labeled (30 min at 37°C) with an Alexa Fluor 488 conjugate of CTB (1 μg/ml; Molecular Probes). At 24 h after the second pulse, the HECs were fixed and imaged using a fluorescence microscope, and the data were exported to CorelDRAW X3 (Corel Corp., Ottawa, Ontario, Canada). To confirm that fusion events were occurring in the perinuclear area, the “local equalization” feature of Corel Photo-Paint X3 (Corel Corp.) was used to outline the periphery of cells.
Histological staining.
HECs were grown on four-chamber microscope slides, fixed in 4% (wt/vol) paraformaldehyde, and stained with hematoxylin and eosin (H&E) according to standard histological procedures. An Olympus BH-2 microscope was used for microphotography, and a Zeiss Axioskop microscope was used for counting cells.
RESULTS
E5 associates with calpactin I.
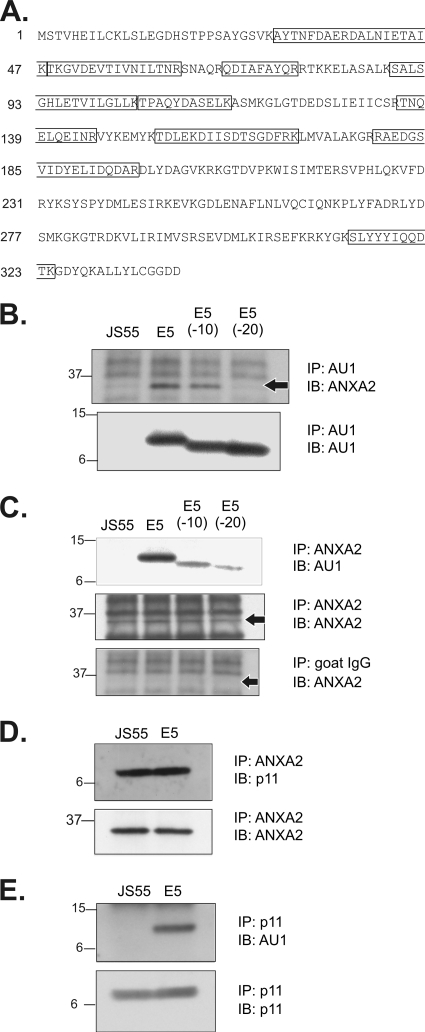
To identify new cellular targets for the E5 oncoprotein, COS cells were transfected with DNA encoding AU1 epitope-tagged E5 and immunoprecipitated with an anti-AU1 monoclonal antibody. An E5-associated, 36-kDa polypeptide was isolated and microsequenced. As shown in Fig. 1A, nine proteolytic fragments of the 36-kDa polypeptide exactly matched the amino acid sequence of human ANXA2. To verify the binding interaction between E5 and ANXA2, COS cells were transfected with DNA encoding AU1 epitope-tagged full-length E5, E5 deletion mutants lacking 10 or 20 C-terminal amino acids [E5(−10) and E5(−20)], or the empty pJS55 expression vector (27). Immunoprecipitations were performed with antibodies recognizing ANXA2 or AU1, and the immunoprecipitates were analyzed on Western blots labeled with anti-AU1 or anti-ANXA2 antibodies (27). The results (Fig. 1B and C, upper panels) demonstrated an association between ANXA2 and full-length E5. In contrast, we observed reduced binding of E5(−10) to ANXA2 and almost no binding of E5(−20) to ANXA2 (Fig. 1B and C, upper panels), demonstrating a critical role for the E5 C terminus in this interaction. Since the E5 constructs were expressed at equivalent levels (Fig. 1B, lower panel), these results could not be attributed to variations in E5 protein expression. Due to the known ability of ANXA2 to form a heterotetrameric calpactin I complex with p11 subunits, we used immunoprecipitation and immunoblotting techniques to demonstrate interactions between ANXA2 and p11 (Fig. 1D, upper panel) and between p11 and E5 (Fig. 1E, upper panel) in COS cells. Equivalent amounts of ANXA 2 and p11 were present in all transfected cells (Fig. 1D and E, lower panels).
Fig. 1.
Association of E5 and calpactin I in COS cells. (A) A 36-kDa polypeptide that coimmunoprecipitates with E5 was excised from a Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gel, digested with trypsin, and microsequenced by ProtTech, Inc. (Norristown, PA). Tryptic peptides showing complete sequence homology with human ANXA2 (NCBI protein database) are framed. (B) Association of ANXA2 with E5. Immunoprecipitates from COS cells transfected with AU1 epitope-tagged full-length E5 and E5 C-terminal deletion mutants were immunoblotted to detect associated ANXA2. The immunoblot was relabeled (lower panel) to demonstrate equivalent levels of the E5 proteins. An arrow indicates the location of ANXA2. (C) Association of E5 with ANXA2. ANXA2 immunoprecipitates from COS cells transfected with the indicated E5 constructs were immunoblotted to detect coprecipitation of E5. The immunoblot was relabeled (middle panel) to demonstrate equivalent levels of ANXA2. An ANXA2 immunoblot of proteins immunoprecipitated with a nonspecific anti-goat IgG antibody (lower panel) demonstrates the specificity of ANXA2 labeling. Arrows indicate the location of ANXA2. (D) Association of ANXA2 and p11 by immunoprecipitation and immunoblotting in COS cells. (E) Association of p11 and E5 in COS cells. Cells were transfected with E5 constructs in the pJS55 expression vector and lysed 24 h later. Molecular mass markers (in kDa) are shown on the left. IP, immunoprecipitation; IB, immunoblotting.
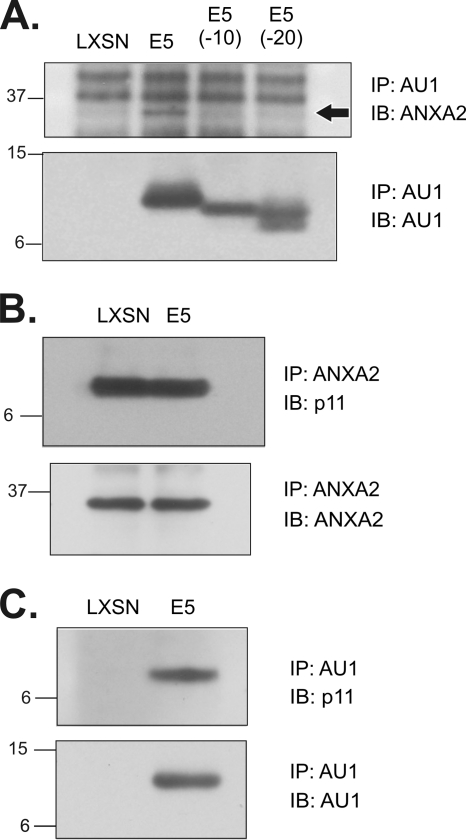
In the preceding experiments, the pJS55 vector induced high levels of E5 expression in COS cells (29). To verify that the association of ANXA2, p11, and E5 occurred in stable lines derived from a natural HPV host cell in the presence of other HPV-16 early genes, we analyzed HPV-16 E6/E7-immortalized HEC lines that expressed full-length and mutant E5 proteins (27). Strong associations between E5 (but not E5 C-terminal deletion mutants) and ANXA2 (Fig. 2A, upper panel), ANXA2 and p11 (Fig. 2B, upper panel), and E5 and p11 (Fig. 2C, upper panel) were observed. Relabeling the immunoblots with the antibodies used for immunoprecipitation showed that E5 proteins and ANXA2 were expressed at similar levels in the HEC lines (Fig. 2A and B, lower panels). The observation that the C-terminal E5(−20) deletion mutant is largely defective for binding in COS cells and is completely defective in HECs indicates that the E5-calpactin I interaction requires the 20 amino acids at the C terminus of E5.
Fig. 2.
Association of E5 and calpactin I in HPV-16 E6/E7-immortalized HEC lines. (A) Association of ANXA2 with E5. AU1 immunoprecipitates from stable cell lines expressing AU1 epitope-tagged full-length E5 and E5 C-terminal deletion mutants were immunoblotted to detect associated ANXA2 (upper panel; arrow). The immunoblot was relabeled (lower panel) to show levels of E5 proteins in the immunoprecipitates. (B) Association of p11 with ANXA2 by immunoprecipitation and immunoblotting. (C) Association of p11 and E5 by immunoprecipitation and immunoblotting in lysates prepared using modified CHAPS buffer (23). Molecular mass markers (in kilodaltons) are indicated on the left. IP, immunoprecipitation; IB, immunoblotting.
E5 alters the intracellular localization of ANXA2 and p11.
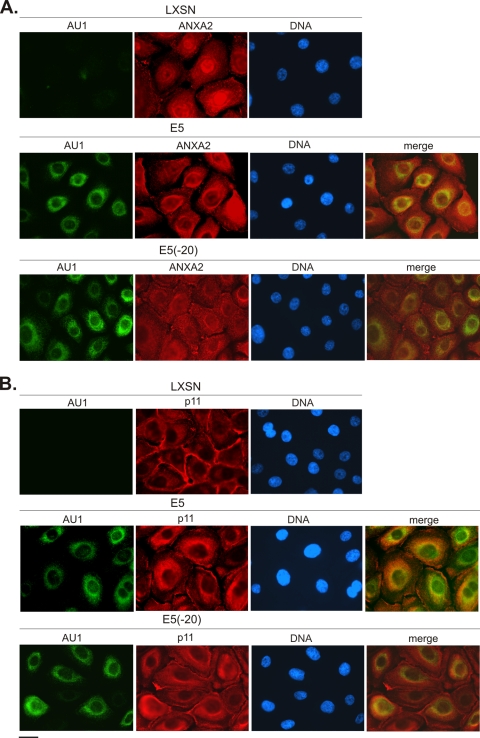
Since E5 resides in membranes of the ER and nuclear envelope (7, 49) with its C terminus exposed to the cytoplasm (29), we used immunofluorescence microscopy to investigate whether the association of calpactin I with E5 alters the localization of ANXA2 and p11 subunits in stable cell lines expressing epitope-tagged E5, E5(−20), or harboring the empty pLXSN expression vector. In control (LXSN) cells, ANXA2 was localized to the cytoplasmic face of the plasma membrane and exhibited diffuse cytoplasmic fluorescence, as has been reported previously (51, 56) (Fig. 3A, upper panel). A similar pattern of ANXA2 localization was observed in cells expressing the E5(−20) mutant that does not bind ANXA2 (Fig. 3A, lower panel). In contrast, ANXA2 was predominantly perinuclear in E5-expressing cells, largely mirroring the distribution of E5 (Fig. 3A, middle panel). p11 was found primarily near the plasma membrane in LXSN cells (Fig. 3B, upper panel), which is consistent with previous reports (51, 56). In cells expressing E5(−20), p11 localized near the plasma membrane and also exhibited some diffuse cytoplasmic staining (Fig. 3B, lower panel). However, as with ANXA2, p11 was predominantly perinuclear in all cells expressing full-length E5 (Fig. 3B, middle panel). Therefore, E5 induces perinuclear redistribution of both calpactin I subunits, presumably as a result of its capacity to associate with calpactin I.
Fig. 3.
Intracellular localization of calpactin I subunits in HPV-16 E6/E7-immortalized HFK cell lines. Immunofluorescence localization of E5 and an E5 deletion mutant lacking 20 C-terminal amino acids are indicated in the left panels (AU1; green). Immunofluorescence localization of ANXA 2 (A) and p11 (B) are shown in red. ANXA2 and p11 are predominantly perinuclear in E5-expressing HFKs, largely mirroring the distribution of E5 (merge). Scale bar, 10 μm.
E5 promotes perinuclear membrane fusion.
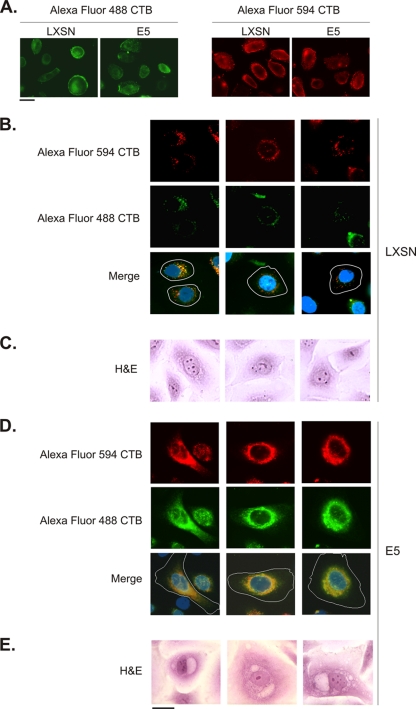
We have shown previously that E5 (in cooperation with HPV-16 E6) induces the formation of perinuclear vacuoles in HECs and HFKs grown in two-dimensional cultures (28). This vacuolization mimics the process of koilocytosis, a characteristic morphological change observed in HPV-infected keratinocytes in vivo (55). Because calpactin I promotes membrane fusion and is targeted to the perinuclear area by E5, we hypothesized that calpactin I might be involved in the observed vacuolization. To test this hypothesis, HECs that stably express HPV-16 E6 were infected with a retrovirus encoding E5 or the empty pLXSN expression vector and 3 days later were pulse-labeled for 30 min with Alexa Fluor 594-conjugated CTB. Six hours later, the same cells were pulse-labeled with an Alexa Fluor 488 conjugate of CTB. In ca. 95% of LXSN-infected cells, small nonmerged vesicles labeled with Alexa Fluor 594 (red) and Alexa Fluor 488 (green) CTB were present in the perinuclear area (Fig. 4B). A small number (5%) of these cells exhibited perinuclear vacuoles that showed evidence of fusion (fluorescence colocalization). The control cells appeared to be morphologically similar to nonvacuolated, nonkoilocytotic LXSN/E6-HECs stained with H&E (Fig. 4C). In contrast, ca. 15% of E5-infected cells exhibited large perinuclear areas of complete Alexa Fluor 488 CTB and Alexa Fluor 594 CTB colocalization (Fig. 4D), indicating that two distinct populations of membrane vesicles had undergone fusion. These cells appeared morphologically similar to E5/E6-HECs stained with H&E (Fig. 4E). Consistent with these data, H&E staining of LXSN/E6-HECs verified that 5 to 9% of the cells developed perinuclear vacuoles, whereas 2.5-fold more E5/E6-HECs evidenced perinuclear vacuolization (Fig. 5B). Furthermore, the observed increase in perinuclear membrane fusion in E5/E6-HECs could not be attributed to increased Alexa Fluor-CTB labeling of these cells relative to LXSN/E6-HECs (Fig. 4A).
Fig. 4.
E5 promotes perinuclear membrane fusion. (A) HECs that stably express HPV-16 E6 were infected with a retrovirus encoding E5 (or harboring the empty pLXSN expression vector). Three days later, the cells were labeled for 30 min at 4°C with Alexa Fluor 488 (green) or Alexa Fluor 594 (red) conjugates of CTB, washed, and fixed. Similar levels of CTB bind to the plasma membrane of E5- and LXSN-infected cells. (B) E6-HECs were infected with a retrovirus containing the empty pLXSN expression vector and, 3 days later, were pulse-labeled for 30 min (at 37°C) with Alexa Fluor 594 CTB and Alexa Fluor 488 CTB 6 h apart. Small, nonmerged vesicles labeled with Alexa Fluor 594 CTB (red) and Alexa Fluor 488 CTB (green) were present in the perinuclear area in ca. 95% of the cells (merge). Nuclei (blue) and the boundaries of cells (white lines) are indicated. (C) H&E staining of LXSN/E6-HECs reveals morphologically similar nonvacuolated cells. (D) E6-HECs were infected with a retrovirus encoding E5 and pulse-labeled as in panel B. Large perinuclear areas of complete Alexa Fluor 488 CTB and Alexa Fluor 594 CTB colocalization were present in ca. 15% of the cells (merge). (E) H&E staining of E5/E6-HECs shows morphologically similar vacuolated cells. Scale bar, 10 μm.
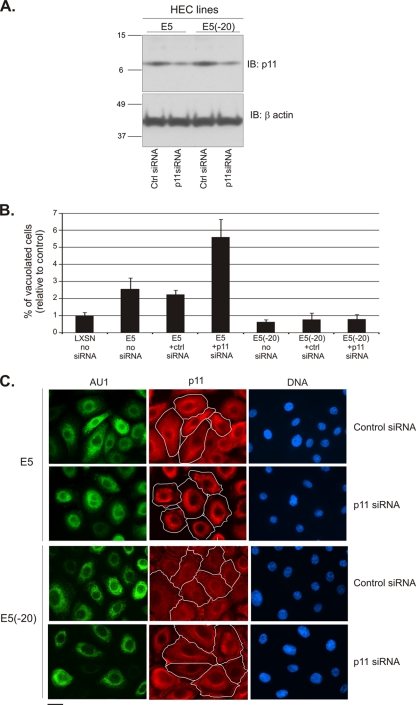
Fig. 5.
siRNA mediated p11 knockdown synergizes with E5 to promote perinuclear vacuole formation. (A) siRNA targeting p11 similarly decreases the level of p11 in HPV-16 E6/E7-immortalized HECs that express full-length E5 or the E5(−20) mutant. Molecular mass markers (in kilodaltons) are indicated on the left. IB, immunoblotting. (B) Measurement of perinuclear vacuolization in H&E-stained E5/E6- and E5(−20)/E6-HECs transfected with p11 or control siRNA. (C) Exclusively perinuclear localization of p11 in E5-HFKs transfected with siRNA targeting p11, but not with control siRNA (p11, red, upper panels). Predominantly diffuse cytoplasmic localization of p11 in E5(−20)-HFKs transfected with control or p11 siRNA (p11, red, lower panels). The boundaries of cells (white lines) are indicated. Scale bar, 10 μm.
To provide additional evidence that perinuclear membrane fusion and vacuolization are dependent upon the ability of E5 to relocalize the calpactin I complex, we studied the effect of siRNA-mediated p11knockdown on E5-dependent perinuclear vacuole formation. As shown in Fig. 5A, p11 knockdown was equally effective in HECs that express E5 or E5(−20). Although we anticipated that p11 knockdown would inhibit E5-dependent vacuolization, 29% of E5/E6-HECs transfected with p11 siRNA formed perinuclear vacuoles compared to 11% of cells transfected with control siRNA (Fig. 5B). In contrast, p11 knockdown had no effect on vacuole formation in E6-HECs expressing E5(−20) (Fig. 5B), which does not induce a marked perinuclear redistribution of calpactin I subunits (Fig. 3). To better understand this unexpected result, p11 was localized by immunofluorescence microscopy in E5/E6-HECs and E5(−20)/E6-HECs transfected with p11 or control siRNA. As shown (Fig. 5C), the p11 remaining after knockdown was exclusively perinuclear in cells expressing E5, but not in cells expressing E5(−20). In fact, while overall levels of p11 were reduced in the siRNA experiment, the level of perinuclear p11 appeared to increase in cells expressing full-length E5 (but not the C-terminal deletion), which correlated with enhanced perinuclear vacuole formation.
DISCUSSION
In the present study we identify calpactin I as a new cellular target for the HPV-16 E5 oncoprotein. Calpactin I is a heterotetrameric protein complex that consists of two subunits, ANXA2 and p11, and promotes membrane fusion (9, 25, 32, 42). We show that E5 is associated with both calpactin I subunits in COS cells and, more importantly, in stable HEC lines that do not overexpress E5 and are the natural host cell for HPV infection. We further demonstrate that E5 induces a perinuclear redistribution of calpactin I and that both the binding interaction with E5 and perinuclear localization are mediated by the C terminus of E5, which is known to be exposed to the cytoplasm (29).
We previously have reported that E5 cooperates with the HPV E6 protein to induce koilocytosis, a pathognomonic, long-recognized feature of papillomavirus infection (28). Koilocytes are epithelial cells that contain an acentric, hyperchromatic, moderately enlarged nucleus displaced by a large perinuclear vacuole that is clearly visible in samples stained with H&E (21). Importantly, E5 is present in these vacuole membranes, and its C terminus is required for vacuole formation (28). Given the capacity of calpactin I to promote membrane fusion, it is reasonable to hypothesize that its perinuclear targeting by E5 contributes to the formation of koilocytotic vacuoles. To test this hypothesis, we developed an in vivo immunofluorescence assay for perinuclear membrane fusion based on the selective, high-affinity binding of CTB to the ganglioside GM1 component of lipid rafts (39). Since the association of CTB with lipid rafts limits its diffusion in biological membranes, it is a useful tool for following membrane trafficking (5, 52). Pulse-labeling cells with red and green fluorescent CTB conjugates 6 h apart results in the tagging of distinct populations of endocytotic vesicles that undergo retrograde transport to the perinuclear region. These vesicles remain predominantly distinct in control cells, but exhibit extensive colocalization secondary to fusion in a significant percentage of E5-expressing cells.
Experiments in which siRNA was used to knock down the intracellular levels of p11 further support for a role for E5-dependent perinuclear targeting of calpactin I in the formation of koilocytotic vacuoles. Immunofluorescence localization indicates that the p11 remaining after knockdown is exclusively perinuclear in E5-expressing cells but not in cells expressing E5(−20), which does not bind calpactin I. This result suggests that E5 may have a high affinity for calpactin I. More importantly, the level of perinuclear p11 appears to be elevated following knockdown in E5 cells, which correlates with an almost 3-fold increase in the number of H&E-stained cells exhibiting perinuclear vacuoles. In contrast, no increase in vacuolization occurs after p11 knockdown in E5(−20) cells. The fact that SNAP-23, another protein involved in membrane fusion, associates with calpactin I in vitro and in vivo (54) may also contribute to increased perinuclear vacuolization in these experiments, since it may exclusively be recruited to the perinuclear region when p11 is exclusively perinuclear.
Although the presence of koilocytes in cervical smears and biopsy specimens is characteristic of papillomavirus infection, the biological role of koilocytosis is unclear. Vacuolization of the perinuclear cytoplasm may lead to increased cell fragility, thereby augmenting viral egress and infection. It has been suggested previously that the HPV E4 protein may promote viral release from the upper layer of the epithelium by altering assembly of the cornified envelope and keratin cytoskeleton (8), and it is possible that the E4 and E5 proteins might cooperate in potentiating viral spread.
ACKNOWLEDGMENTS
This study was supported by grant R01-CA053371 from the National Cancer Institute (to R.S.). J.D.H. was supported by a grant to Georgetown University from the Howard Hughes Medical Institute through the Precollege and Undergraduate Science Education Program.
Footnotes
Published ahead of print on 17 August 2011.
REFERENCES
- 1. Ashrafi G. H., Haghshenas M., Marchetti B., Campo M. S. 2006. E5 protein of human papillomavirus 16 downregulates HLA class I and interacts with the heavy chain via its first hydrophobic domain. Int. J. Cancer 119:2105–2112 [DOI] [PubMed] [Google Scholar]
- 2. Baege A. C., Berger A., Schlegel R., Veldman T., Schlegel R. 2002. Cervical epithelial cells transduced with the papillomavirus E6/E7 oncogenes maintain stable levels of oncoprotein expression but exhibit progressive, major increases in hTERT gene expression and telomerase activity. Am. J. Pathol. 160:1251–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bodily J., Laimins L. A. 2011. Persistence of human papillomavirus infection: keys to malignant progression. Trends Microbiol. 19:33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen S. L., Lin S. T., Tsai T. C., Hsiao W. C., Tsao Y. P. 2006. ErbB4 (JM-b/CYT-1)-induced expression and phosphorylation of c-Jun is abrogated by human papillomavirus type 16 E5 protein. Oncogene 26:42–53 [DOI] [PubMed] [Google Scholar]
- 5. Chinnapen D. J., Chinnapen H., Saslowsky D., Lencer W. I. 2007. Rafting with cholera toxin: endocytosis and trafficking from plasma membrane to ER. FEMS Microbiol. Lett. 266:129–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conrad M., Bubb V. J., Schlegel R. 1993. The human papillomavirus type 6 and 16 E5 proteins are membrane-associated proteins which associate with the 16-kilodalton pore-forming protein. J. Virol. 67:6170–6178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Disbrow G. L., Sunitha I., Baker C. C., Hanover J., Schlegel R. 2003. Codon optimization of the HPV-16 E5 gene enhances protein expression. Virology 311:105–114 [DOI] [PubMed] [Google Scholar]
- 8. Doorbar J. 2005. The papillomavirus life cycle. J. Clin. Virol. 32S:7–15 [DOI] [PubMed] [Google Scholar]
- 9. Emans N., et al. 1993. Annexin II is a major component of fusogenic endosomal vesicles. J. Cell Biol. 120:1357–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Erikson E., Tomasiewicz H. G., Erikson R. L. 1984. Biochemical characterization of a 34-kilodalton normal cellular substrate of pp60v-src and an associated 6-kilodalton protein. Mol. Cell. Biol. 4:77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fehrmann F., Laimins L. A. 2003. Human papillomaviruses: targeting differentiating epithelial cells for malignant transformation. Oncogene 22:5201–5207 [DOI] [PubMed] [Google Scholar]
- 12. Filipenko N. R., Waisman D. M. 2001. The C terminus of annexin II mediates binding to F-actin. J. Biol. Chem. 276:5310–5315 [DOI] [PubMed] [Google Scholar]
- 13. Futter C. E., White I. J. 2007. Annexins and endocytosis. Traffic 8:951–958 [DOI] [PubMed] [Google Scholar]
- 14. Genther Williams S. M., et al. 2005. Requirement of epidermal growth factor receptor for hyperplasia induced by E5, a high-risk human papillomavirus oncogene. Cancer Res. 65:6534–6542 [DOI] [PubMed] [Google Scholar]
- 15. Gerke V., Creutz C. E., Moss S. E. 2005. Annexins: linking Ca2+ signaling to membrane dynamics. Nat. Rev. Mol. Cell. Biol. 6:449–461 [DOI] [PubMed] [Google Scholar]
- 16. Gerke V., Moss S. E. 2002. Annexins: from structure to function. Physiol. Rev. 82:331–371 [DOI] [PubMed] [Google Scholar]
- 17. Gerke V., Weber K. 1984. Identity of p36K phosphorylated upon Rous sarcoma virus transformation with a protein purified from brush borders; calcium-dependent binding to non-erythroid spectrin and F-actin. EMBO J. 3:227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gerke V., Weber K. 1985. Calcium-dependent conformational changes in the 36-kDa subunit of intestinal protein I related to the cellular 36-kDa target of Rous sarcoma virus tyrosine kinase. J. Biol. Chem. 260:1688–1695 [PubMed] [Google Scholar]
- 19. Glenney J. R., Jr., Tack B. F. 1985. Amino-terminal sequence of p36 and associated p10: identification of the site of tyrosine phosphorylation and homology with S-100. Proc. Natl. Acad. Sci. U. S. A. 82:7884–7888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gruener M., Bravo I. G., Momburg F., Alonso A., Tomakidi P. 2007. The E5 protein of the human papillomavirus type 16 downregulates HLA-I surface expression in calnexin-expressing but not in calnexin-deficient cells. Virol. J. 4:116–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hajdu S. I. 2006. A note from history: the link between koilocytes and human papillomaviruses. Ann. Clin. Lab. Sci. 36:485–487 [PubMed] [Google Scholar]
- 22. Hayes M. J., Shao D., Bailly M., Moss S. E. 2006. Regulation of actin dynamics by annexin 2. EMBO J. 25:1816–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heyraud S., et al. 2008. Contribution of annexin 2 to the architecture of mature endothelial adherens junctions. Mol. Cell. Biol. 28:1657–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnsson N., Marriott G., Weber K. 1988. p36, the major cytoplasmic substrate of src tyrosine protein kinase, binds to its p11 regulatory subunit via a short amino-terminal amphiphatic helix. EMBO J. 7:2435–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johnstone S. A., Hubaishy I., Waisman D. M. 1992. Phosphorylation of annexin II tetramer by protein kinase C inhibits aggregation of lipid vesicles by the protein. J. Biol. Chem. 267:25976–25981 [PubMed] [Google Scholar]
- 26. Klein O., et al. 1998. Role of glutamine 17 of the bovine papillomavirus E5 protein in platelet-derived growth factor beta receptor activation and cell transformation. J. Virol. 72:8921–8932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krawczyk E., Hanover J. A., Schlegel R., Suprynowicz F. A. 2008. Karyopherin β3: a new cellular target for the HPV-16 E5 oncoprotein. Biochem. Biophys. Res. Commun. 371:684–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krawczyk E., et al. 2008. Koilocytosis: a cooperative interaction between the human papillomavirus E5 and E6 oncoproteins. Am. J. Pathol. 173:682–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krawczyk E., Suprynowicz F. A., Sudarshan S. R., Schlegel R. 2010. Membrane orientation of the human papillomavirus type 16 E5 oncoprotein. J. Virol. 84:1696–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lazarczyk M., et al. 2008. Regulation of cellular zinc balance as a potential mechanism of EVER-mediated protection against pathogenesis by cutaneous oncogenic human papillomaviruses. J. Exp. Med. 205:35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lim P. S., et al. 1990. Distribution and specific identification of papillomavirus major capsid protein epitopes by immunocytochemistry and epitope scanning of synthetic peptides. J. Infect. Dis. 162:1263–1269 [DOI] [PubMed] [Google Scholar]
- 32. Mayorga L. S., et al. 1994. Calcium-dependent fusion among endosomes. J. Biol. Chem. 269:30927–30934 [PubMed] [Google Scholar]
- 33. Mayran N., Parton R. G., Gruenberg J. 2003. Annexin II regulates multivesicular endosome biogenesis in the degradation pathway of animal cells. EMBO J. 22:3242–3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moody C. A., Laimins L. A. 2010. Human papillomavirus oncoproteins: pathways to transformation. Nat. Rev. Cancer 10:550–560 [DOI] [PubMed] [Google Scholar]
- 35. Morel E., Gruenberg J. 2007. The p11/S100A10 light chain of annexin A2 is dispensable for annexin A2 association to endosomes and functions in endosomal transport. PLoS One 2:e1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morel E., Parton R. G., Gruenberg J. 2009. Annexin A2-dependent polymerization of actin mediates endosome biogenesis. Dev. Cell 16:445–457 [DOI] [PubMed] [Google Scholar]
- 37. Morgenstern J. P., Land H. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Orth G. 2008. Host defenses against human papillomaviruses: lessons from epidermodysplasia verruciformis. Curr. Top. Microbiol. Immunol. 321:59–83 [DOI] [PubMed] [Google Scholar]
- 39. Parton R. G. 1994. Ultrastructural localization of gangliosides: GM1 is concentrated in caveolae. J. Histochem. Cytochem. 42:155–166 [DOI] [PubMed] [Google Scholar]
- 40. Pear W. S., Nolan G. P., Scott M. L., Baltimore D. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. U. S. A. 90:8392–8396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Puisieux A., Ji J., Ozturk M. 1996. Annexin II upregulates cellular levels of p11 protein by a post-translational mechanism. Biochem. J. 313:51–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Raynor C. M., Wright J. F., Waisman D. M., Pryzdial E. L. 1999. Annexin II enhances cytomegalovirus binding and fusion to phospholipid membranes. Biochemistry 38:5089–5095 [DOI] [PubMed] [Google Scholar]
- 43. Regan J. A., Laimins L. A. 2008. Bap31 is a novel target of the human papillomavirus E5 protein. J. Virol. 82:10042–10051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rescher U., Gerke V. 2004. Annexins: unique membrane binding proteins with diverse functions. J. Cell Sci. 117:2631–2639 [DOI] [PubMed] [Google Scholar]
- 45. Rodríguez M. I., Finbow M. E., Alonso A. 2000. Binding of human papillomavirus 16 E5 to the 16-kDa subunit c (proteolipid) of the vacuolar H+-ATPase can be dissociated from the E5-mediated epidermal growth factor receptor overactivation. Oncogene 19:3727–3732 [DOI] [PubMed] [Google Scholar]
- 46. Schlegel R., Phelps W. C., Zhang Y. L., Barbosa M. 1988. Quantitative keratinocyte assay detects two biological activities of human papillomavirus DNA and identifies viral types associated with cervical carcinoma. EMBO J. 7:3181–3187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sparkowski J., Anders J., Schlegel R. 1994. Mutation of the bovine papillomavirus E5 oncoprotein at amino acid 17 generates both high- and low-transforming variants. J. Virol. 68:6120–6123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Suprynowicz F. A., Disbrow G. L., Simic V., Schlegel R. 2005. Are transforming properties of the bovine papillomavirus E5 protein shared by E5 from high-risk human papillomavirus type 16? Virology 332:102–113 [DOI] [PubMed] [Google Scholar]
- 49. Suprynowicz F. A., et al. 2008. HPV-16 E5 oncoprotein upregulates lipid raft components caveolin-1 and ganglioside GM1 at the plasma membrane of cervical cells. Oncogene 27:1071–1078 [DOI] [PubMed] [Google Scholar]
- 50. Suprynowicz F. A., et al. 2010. The human papillomavirus type 16 E5 oncoprotein inhibits epidermal growth factor trafficking independently of endosome acidification. J. Virol. 84:10619–10629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thiel C., Osborn M., Gerke V. 1992. The tight association of the tyrosine kinase substrate annexin II with the submembranous cytoskeleton depends on intact p11- and Ca2+-binding sites. J. Cell Sci. 103:733–742 [DOI] [PubMed] [Google Scholar]
- 52. Trojanowski J. Q., Gonatas J. O., Gonatas N. K. 1982. Horseradish peroxidase (HRP) conjugates of cholera toxin and lectins are more sensitive retrogradely transported markers than free HRP. Brain Res. 231:33–50 [DOI] [PubMed] [Google Scholar]
- 53. Waisman D. M. 1995. Annexin II tetramer: structure and function. Mol. Cell. Biochem. 149-.150:301–322 [DOI] [PubMed] [Google Scholar]
- 54. Wang P., Chintagari N. R., Gou D., Su L., Liu L. 2007. Physical and functional interactions of SNAP-23 with annexin A2. Am. J. Respir. Cell Mol. Biol. 37:467–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yamamoto L. S., et al. 2004. A morphological protocol and guide-list on uterine cervix cytology associated to papillomavirus infection. Rev. Inst. Med. Trop. Sao Paulo 46:189–193 [DOI] [PubMed] [Google Scholar]
- 56. Zobiack N., Rescher U., Ludwig C., Zeuschner D., Gerke V. 2003. The annexin 2/S100A10 complex controls the distribution of transferrin receptor-containing recycling endosomes. Mol. Biol. Cell 14:4896–4908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zokas L., Glenney J. R. 1987. The calpactin light chain is tightly linked to the cytoskeletal form of calpactin I: studies using monoclonal antibodies to calpactin subunits. J. Cell Biol. 105:2111–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. zur Hausen H. 2009. Papillomaviruses in the causation of human cancers: a brief historical account. Virology 384:260–265 [DOI] [PubMed] [Google Scholar]