Abstract
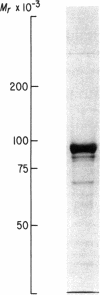
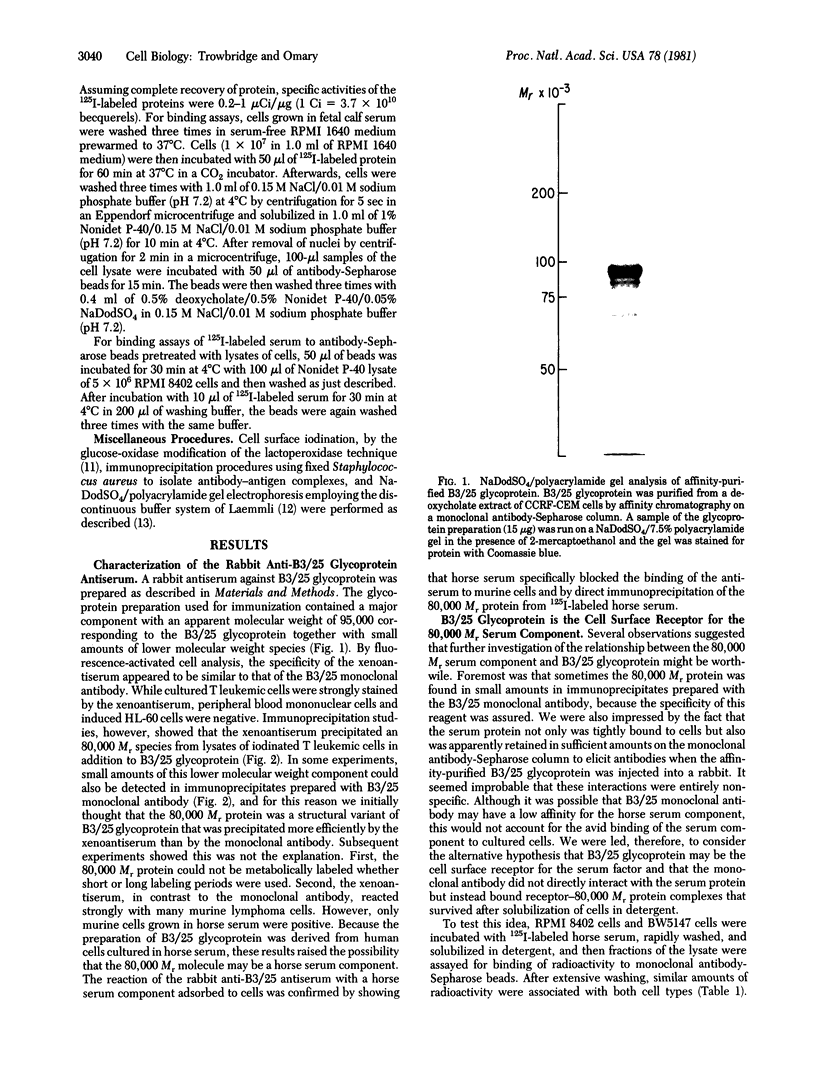
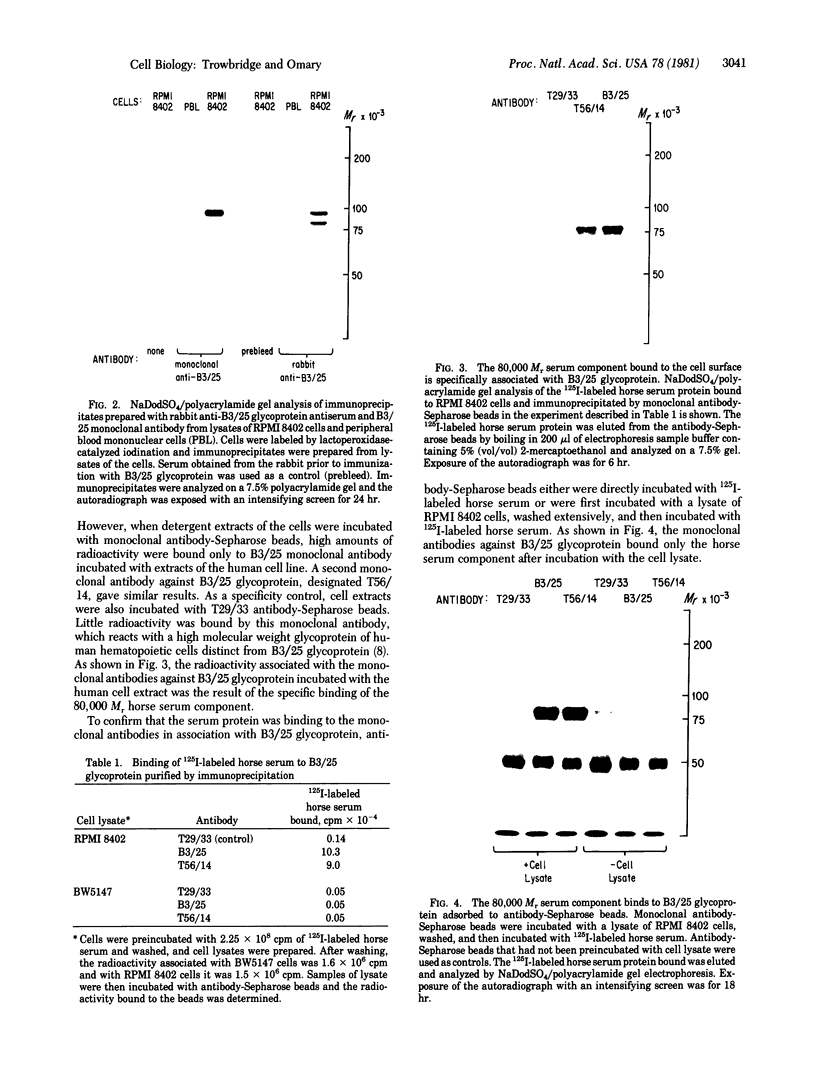
A cell surface glycoprotein antigen with an apparent molecular weight of about 100,000 that is selectively expressed on proliferating cells was purified from deoxycholate-solubilized membranes of a cultured human leukemic thymus-derived (T) cell line by affinity chromatography on a monoclonal antibody-Sepharose column. A conventional xenoantiserum prepared by immunization with the affinity-purified glycoprotein was found to contain antibodies against a serum component that bound tightly to cultured cells. This molecule was shown to be specifically associated with the cell surface glycoprotein purified by immunoprecipitation from lysates of cells. We have identified the serum component as transferrin and conclude that the membrane glycoprotein is the cell surface transferrin receptor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D., Sato G. Methods for growth of cultured cells in serum-free medium. Anal Biochem. 1980 Mar 1;102(2):255–270. doi: 10.1016/0003-2697(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Breitman T. R., Collins S. J., Keene B. R. Replacement of serum by insulin and transferrin supports growth and differentiation of the human promyelocytic cell line, HL-60. Exp Cell Res. 1980 Apr;126(2):494–498. doi: 10.1016/0014-4827(80)90296-7. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Faulk W. P., Galbraith G. M. Trophoblast transferrin and transferrin receptors in the host--parasite relationship of human pregnancy. Proc R Soc Lond B Biol Sci. 1979 Mar 26;204(1154):83–97. doi: 10.1098/rspb.1979.0014. [DOI] [PubMed] [Google Scholar]
- Galbraith G. M., Galbraith R. M., Temple A., Faulk W. P. Demonstration of transferrin receptors on human placental trophoblast. Blood. 1980 Feb;55(2):240–242. [PubMed] [Google Scholar]
- Guilbert L. J., Iscove N. N. Partial replacement of serum by selenite, transferrin, albumin and lecithin in haemopoietic cell cultures. Nature. 1976 Oct 14;263(5578):594–595. doi: 10.1038/263594a0. [DOI] [PubMed] [Google Scholar]
- Hamilton T. A., Wada H. G., Sussman H. H. Identification of transferrin receptors on the surface of human cultured cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6406–6410. doi: 10.1073/pnas.76.12.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi I., Larner J., Sato G. Hormonal growth control of cells in culture. In Vitro. 1978 Jan;14(1):23–30. doi: 10.1007/BF02618171. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman R., Stallings V. Complementation patterns of Thy-1 variants and evidence that antigen loss variants "pre-exist" in the parental population. J Natl Cancer Inst. 1974 Feb;52(2):429–436. doi: 10.1093/jnci/52.2.429. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Melchers F. Complete replacement of serum by albumin, transferrin, and soybean lipid in cultures of lipopolysaccharide-reactive B lymphocytes. J Exp Med. 1978 Mar 1;147(3):923–933. doi: 10.1084/jem.147.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd W., Poodry C. A., Strominger J. L. Novel surface antigen expressed on dividing cells but absent from nondividing cells. J Exp Med. 1980 Nov 1;152(5):1430–1435. doi: 10.1084/jem.152.5.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURELL C. B., MORGAN E. IRON EXCHANGE BETWEEN TRANSFERRIN AND THE PLACENTA IN THE RAT. Acta Physiol Scand. 1964 Nov;62:271–279. doi: 10.1111/j.1748-1716.1964.tb03974.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larrick J. W., Cresswell P. Modulation of cell surface iron transferrin receptors by cellular density and state of activation. J Supramol Struct. 1979;11(4):579–586. doi: 10.1002/jss.400110415. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Messmer T. O. Nature of the iron requirement for Chinese hamster V79 cells in tissue culture medium. Exp Cell Res. 1973 Mar 15;77(1):404–408. doi: 10.1016/0014-4827(73)90594-6. [DOI] [PubMed] [Google Scholar]
- Minowada J., Janossy G., Greaves M. F., Tsubota T., Srivastava B. I., Morikawa S., Tatsumi E. Expression of an antigen associated with acute lymphoblastic leukemia in human leukemia-lymphoma cell lines. J Natl Cancer Inst. 1978 Jun;60(6):1269–1277. doi: 10.1093/jnci/60.6.1269. [DOI] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S., Battifora H. A. Human homologue of murine T200 glycoprotein. J Exp Med. 1980 Oct 1;152(4):842–852. doi: 10.1084/jem.152.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Disposition of T200 glycoprotein in the plasma membrane of a murine lymphoma cell line. J Biol Chem. 1980 Feb 25;255(4):1662–1669. [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S., Minowada J. Human cell-surface glycoprotein with unusual properties. Nature. 1980 Aug 28;286(5776):888–891. doi: 10.1038/286888a0. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman P. A., Schleicher R. B., Allen R. H. Isolation and characterization of the transferrin receptor from human placenta. J Biol Chem. 1979 Oct 25;254(20):9943–9946. [PubMed] [Google Scholar]
- Wada H. G., Hass P. E., Sussman H. H. Transferrin receptor in human placental brush border membranes. Studies on the binding of transferrin to placental membrane vesicles and the identification of a placental brush border glycoprotein with high affinity for transferrin. J Biol Chem. 1979 Dec 25;254(24):12629–12635. [PubMed] [Google Scholar]