Abstract
Bone marrow-derived mesenchymal stem cells (MSCs) have been reported to migrate to brain lesions of neurodegenerative diseases; however, the precise mechanisms by which MSCs migrate remain to be elucidated. In this study, we carried out an in vitro migration assay to investigate the chemoattractive factors for MSCs in the brains of prion-infected mice. The migration of immortalized human MSCs (hMSCs) was reduced by their pretreatment with antibodies against the chemokine receptors, CCR3, CCR5, CXCR3, and CXCR4 and by pretreatment of brain extracts of prion-infected mice with antibodies against the corresponding ligands, suggesting the involvement of these receptors, and their ligands in the migration of hMSCs. In agreement with the results of an in vitro migration assay, hMSCs in the corpus callosum, which are considered to be migrating from the transplanted area toward brain lesions of prion-infected mice, expressed CCR3, CCR5, CXCR3, and CXCR4. The combined in vitro and in vivo analyses suggest that CCR3, CCR5, CXCR3, and CXCR4, and their corresponding ligands are involved in the migration of hMSCs to the brain lesions caused by prion propagation. In addition, hMSCs that had migrated to the right hippocampus of prion-infected mice expressed CCR1, CX3CR1, and CXCR4, implying the involvement of these chemokine receptors in hMSC functions after chemotactic migration. Further elucidation of the mechanisms that underlie the migration of MSCs may provide useful information regarding application of MSCs to the treatment of prion diseases.
INTRODUCTION
Prion diseases are fatal neurodegenerative disorders in humans and animals that are characterized by the accumulation of a disease-specific isoform of the prion protein (PrPSc), astrocytosis, microglial activation, spongiosis, and neuronal cell death in the central nervous system (CNS). Although the etiology of the diseases is not clear, conversion of the normal prion protein to PrPSc plays a key role in the neuropathological changes (44). Therefore, compounds that inhibit PrPSc formation are considered as therapeutic candidates of the diseases, and many compounds have been reported to inhibit PrPSc formation in cell cultures and cell-free systems (reviewed in reference 56). However, only a few of these inhibitors, such as amphotericin B and its derivative (13), pentosan polysulfate (14), porphyrin derivatives (27), certain amyloidophilic compounds (25), and FK506 (37) have been reported to prolong the survival of prion-infected mice even when administered in the middle-late stage of infection but still before clinical onset. We recently reported that intraventricular infusion of anti-PrP antibodies (50) slowed down the progression of the disease even when initiated just after clinical onset. However, in addition to inhibition of PrPSc formation, the protection of neurons or restoration of degenerated neurons is thought to be important for functional recovery.
Bone marrow-derived mesenchymal stem cells (MSCs) differentiate into cells of mesodermal origin such as adipocytes, osteoblasts, and endothelial and muscle cells (41, 43). In addition, MSCs are known to transdifferentiate into neuronal and glial cells. MSCs have been shown to migrate to damaged neuronal tissues and to alleviate the deficits in experimental animal models of cerebral ischemia (10), spinal cord injury (20), Parkinson's disease (19, 33), and amyotrophic lateral sclerosis (59). MSCs also secrete various neurotrophic factors that may protect neuronal tissues from degradations, as well as stimulate the activity of endogenous neural stem cells (38). Therefore, despite their mesodermal origin, MSCs are considered to be a candidate for cell-mediated therapy for neurodegenerative diseases. One of the characteristics of MSCs is their migration to brain lesions caused by neurodegenerative diseases, including prion diseases (10, 19, 39, 51). This feature may be of further use for cell-mediated therapy of neurodegenerative diseases, particularly for prion diseases, Multiple sclerosis and Alzheimer's disease, which have diffuse pathological lesions.
Since many cytokines, chemokines, and adhesion molecules are involved in the homing of immune cells (9, 36, 53), evidence that a variety of chemokines and growth factors, as well as their cognate receptors, have a pivotal role in the migration of MSCs has been accumulated. These factors include CXCL12 and its receptor CXCR4 (30, 40; reviewed in reference 52), CCL2 (15, 62, 66), CCL3 (62), interleukin-8 (48, 62), hepatocyte growth factor (16), platelet-derived growth factor AB (PDGF-AB), insulin-like growth factor 1 (IGF-1), CCL5 and CCL22 (42), and integrin β1 (23). Regarding the migration of MSCs to injury in the CNS, the involvement of CCL2 (61), CXCL12/CXCR4, and CX3CL1/CX3CR1 (24) has been reported. However, knowledge of the mechanism by which MSCs migrate to pathological lesions of neurodegenerative diseases is insufficient, and further efforts are required to elucidate this mechanism.
We recently reported that human MSCs (hMSCs) migrate to CNS lesions and prolong the survival of mice infected with prions (51). In the present study, we investigated factors that are involved in the migration of hMSCs to brain lesions of prion diseases.
MATERIALS AND METHODS
Cell culture.
Human bone marrow-derived MSCs that were immortalized with the human telomerase catalytic subunit gene (26) and that stably expressed the LacZ gene (hMSCs [51]) were used. The hMSCs were cultured in Dulbecco modified Eagle medium (DMEM; Sigma Chemical Co., St. Louis, MO) containing 10% fetal bovine serum (FBS) in a humidified atmosphere under normoxic (21% O2 and 5% CO2) or hypoxic (2% O2 and 5% CO2) conditions at 37°C.
Mice and prion inoculation.
All animal experiments were carried out according to protocols approved by the Institutional Committee for Animal Experiments. Four-week-old female ICR mice were purchased from CLEA Japan, Inc. (Japan), and the mice were acclimatized for a week prior to use. Brain homogenates (10% [wt/wt] in phosphate-buffered saline [PBS]) that were used for inoculation were prepared from brains of mice infected with the Chandler prion strain at the terminal stage of the disease and from age-matched uninfected mice. Mice were intracerebrally inoculated with 20 μl of 10% brain homogenates.
In vitro migration assay.
To examine the migration of hMSCs in vitro, the brains of infected mice 120 days postinoculation (dpi) with the Chandler strain or of age-matched mock-infected mice were homogenized to 20% (wt/wt) in DMEM. The homogenates were centrifuged at 10,000 × g for 10 min at 4°C, and the resulting supernatants were passed through a 0.22-μm-pore-size filter. Aliquots of the brain extracts were stored at −80°C until use. Migration of hMSCs to the brain extracts was assessed using a QCM chemotaxis cell migration assay kit (Chemicon, Temecula, CA). The hMSCs that were starved for a day in serum-free medium were harvested, and 300 μl of cell suspension (5 × 104 cells) was added to the insert well. The lower chambers were supplied with serum-free DMEM containing 1% brain extract. At 24 h after incubation, hMSCs on the polycarbonate membrane (pore size, 8.0 μm) were stained with the cell stain solution provided in the kit. Nonmigratory cells that stayed on the upper side of the polycarbonate membrane were removed using a cotton swab. The migrated hMSCs, which had passed through the pores and clung to the underside of the membrane, were counted using the NIH Image J program. Three random high-magnification (×100) light microscopic images were captured using the Olympus BX-51 microscope and were used for cell counting.
To examine the involvement of receptors expressed on hMSCs in their migration, hMSCs were preincubated with antibodies against specific receptors for 30 min before adding the insert wells. To examine the involvement of chemokines and growth factors in migration, 1% brain homogenates were incubated with antibodies against specific chemokines and growth factors for 30 min at 4°C prior to adding them to the insert wells. Antibodies against various proteins were purchased as follows: PDGF-αβ receptor (PDGF-αβR, ab34074), CCR2 (ab1668), CCR4 (ab1669), CX3CR1 (ab7201), CXCR3 (clone 2Ar1), and CCL3 (ab10381) were from Abcam (Cambridge, MA); IGF-1 receptor (IGF-1R; clone 33255), CXCR4 (clone 12G5), IGF-1 (AF791), CCL2 (clone 123602), CCL4 (AF-451-NA), CCL5 (AF-478), CCL7 (AF-456-NA), CCL17 (AF-529), CCL24 (clone 106521), CX3CL1 (clone 126315), CXCL10 (AF-466-NA), CXCL12 (clone 79014), and CXCL13 (AF-470) were from R&D Systems (Minneapolis, MN); CCR1 (clone 141-2) and CCR3 (clone 444-11) were both from MRL; CCR5 (clone 45523.111) was from (Sigma Chemical Co.); and PDGF-AB (06-127) was from Millipore (Billerica, MA). All antibodies were used at a concentration of 10 μg/ml. Migration assays were performed as three independent experiments (each experiment was carried out in triplicate).
Transplantation of hMSCs.
The hMSCs were transplanted into the thalamus as described previously (51). Briefly, after anesthesia, mouse scalps were incised. The mice were then placed onto a stereotaxic apparatus (Narishige, Japan), and burr holes were drilled to accommodate stereotaxic placement into the thalamus (caudal, 2.0 mm; lateral, 2.1 mm; depth, 3.2 mm [Bregma]). The hMSCs (105 cells in 2 μl of DMEM) were transplanted over a period of 15 min using a Hamilton syringe with a 31-gauge needle.
Flow cytometric analysis.
The hMSCs were treated with 1 mM EDTA and dispersed in 0.5% FBS in PBS (FBS-PBS) by pipetting. The cells were then incubated with primary mouse antibodies against IGF-1R, CCR1, CCR3, CCR5, CXCR3, and CXCR4, primary goat antibodies against PDGF-αβR, CCR2, and CCR4, and a primary rabbit antibody against CX3CR1 (all at a 1:200 dilution) in 0.5% FBS-PBS for 30 min on ice. The primary antibodies were omitted for negative controls. The cells were washed three times with 0.5% FBS-PBS and incubated with anti-mouse Alexa Fluor 546, anti-goat Alexa Fluor 555, or anti-rabbit Alexa Fluor 555 (Molecular Probes, Eugene, OR) at a 1:1,000 dilution for 30 min on ice. After washing, the cells were stained with 5 μg/ml of propidium iodide (Molecular Probes) in PBS for 5 min and analyzed using an EPICS XL-ADC flow cytometer (Beckman Coulter, Miami, FL).
Quantitative reverse transcription-PCR (qRT-PCR).
Total RNA was obtained from hMSCs or from mouse brains using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA). First-strand cDNA was synthesized from 2.5 μg of the total RNA using a First-Strand synthesis kit (Amersham Biosciences, United Kingdom) according to the manufacturer's instructions. Quantitative PCR was carried out using a TaqMan assay. The amplification reaction mixtures contained template cDNA, 1× predesigned TaqMan gene expression assays, and 1× TaqMan universal PCR master mix (Applied Biosystems, Foster City, CA) in a final reaction volume of 20 μl. The following TaqMan gene expression assays were purchased from Applied Biosystems: human genes for PDGF-αβR (assay identification Hs00182163), CCR3 (Hs00266213), CCR4 (Hs99999919), CCR5 (Hs99999149), CX3CR1 (Hs00365842), CXCR3 (Hs01847760), and CXCR4 (Hs00607978) and mouse genes for CCL3 (Mm00441259), CCL4 (Mm00443111), CCL5 (Mm01302427), CCL7 (Mm00443113), CCL17 (Mm01244826), CX3CL1 (Mm00436454), CXCL10 (Mm00445235), and CXCL12 (Mm00445553). The human RNase P gene or the mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was amplified as an internal marker using human RNase P control reagents (catalog no. 4316844; Applied Biosystems) or TaqMan rodent GAPDH control reagents (catalog no. 4308313; Applied Biosystems). TaqMan assays were carried out using an ABI Prism 7900HT sequence detection system (Applied Biosystems). The amplification profiles were analyzed using a threshold cycle (CT) relative quantification method and were normalized to the expression of the human RNase P control gene or the mouse GAPDH gene, which were used as human and mouse reference genes as described previously (58).
IFA.
Immunofluorescence assay (IFA) was performed on hMSCs cultured in an 8-well chamber slide (Nalge Nunc, Naperville, IL) or on cryosections (5 μm thick) of mouse brains after transplantation of hMSCs. The cells or sections were fixed with cold methanol for 20 min at −20°C and treated with PBS containing 0.1% polyoxyethylene (20) and sorbitan monolaurate (Tween 20) (PBST) for 10 min. After blocking with 5% FBS in PBST for 30 min, the cells or sections were incubated for 1 h with primary antibodies for the receptors described above using a 1:500 dilution. After being washed with PBST, they were then incubated with a 1:2,000 dilution of anti-mouse Alexa Fluor 546, anti-goat Alexa Fluor 555, or anti-rabbit Alexa Fluor 555 for 1 h at room temperature. To investigate the colocalization of hMSCs with their receptors, the sections were incubated for 90 min with a mouse anti-β-Gal antibody (catalog no. Z3783; Promega, Madison, WI) conjugated with Alexa Fluor 488 (51). After being washed with PBST, samples were then mounted with Vectashield containing DAPI (4′,6′-diamidino-2-phenylindole; Vector Laboratories, Burlingame, CA). Samples were observed under a Nikon C1 laser confocal microscope.
RESULTS
Migration of hMSCs to the brain extracts.
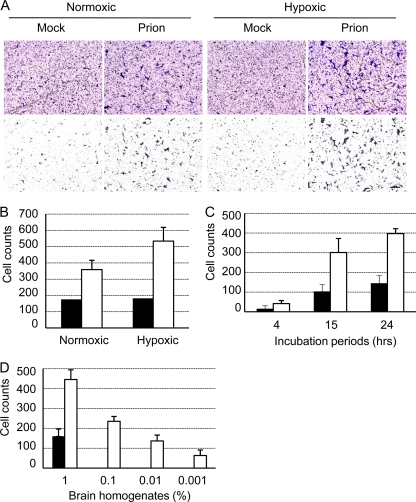
Hypoxic preconditioning of MSCs is reported to enhance their migratory activity (2, 22). To determine the effects of oxygen conditions on the migration of hMSCs to brain homogenates of mock- or prion-infected mice, hMSCs that were cultured under normoxic or hypoxic conditions were analyzed using an in vitro migration assay. The hMSCs that were precultured under hypoxic conditions migrated to brain extracts of prion-infected mice more efficiently than those precultured under normoxic conditions (Fig. 1A). Quantitative analysis revealed that twice as many hMSCs migrated to the brain extracts of prion-infected mice than to those of mock-infected mice following normoxic preconditioning, while 2.7 times more hMSCs migrated to the brain extracts of prion-infected mice following hypoxic preconditioning (Fig. 1B). No differences were observed in the migration of hMSCs to the brain extracts of mock-infected mice between normoxic and hypoxic preconditioning. We therefore used hMSCs that were precultured under hypoxic conditions for all subsequent experiments to facilitate discrimination between subtle differences in cell migration. These hMSCs showed a time-dependent increase in migration for up to 24 h (Fig. 1C), and their migration increased in a brain extract concentration-dependent manner (Fig. 1D). Migration of hMSCs to 0.01 and 0.001% brain homogenates of prion-infected mice was observed, whereas only migration to 1% brain homogenates, but not to lower percentages of brain extracts of mock-infected mice was observed.
Fig. 1.
Chemotactic migration of hMSCs to brain extracts of mock- or prion-infected mice. The migration of hMSCs to brain extracts of mock- or prion-infected mice that were prepared at 120 dpi was analyzed using a QCM 96-well cell migration assay kit. (A) hMSCs migrated to the underside of the membrane of the insert well. The hMSCs that were precultured under normoxic or hypoxic conditions (Normoxic or Hypoxic) were added to the insert well, and the lower chambers were supplied with serum-free DMEM containing 1% brain extracts of mock- or prion-infected mice (Mock or Prion). At 24 h after incubation, hMSCs that had passed through the pores of the membrane and clung to its underside were stained (upper panel). Stained cells with a size of >200 μm2 were selected (lower panel) and counted using the NIH ImageJ program. Bar, 100 μm. (B) Quantification of migrated hMSCs. The migration of hMSCs, assayed as described in panel A, was quantified. Three random areas (3.84 × 105 μm2/area) of the underside of the membrane were photographed, and the number of hMSCs was counted. The graphs show cell counts per 3.84 × 105 μm2 (means and standard deviations [SDs] are shown; n = 3). Black and white bars indicate the numbers of hMSCs that migrated to 1% brain extracts of mock- and prion-infected mice, respectively. (C) Time-dependent increase in hMSC migration. The migration assay was performed for 4, 15, and 24 h. (D) Dose dependency of hMSC migration. The migration of hMSCs to various doses (0.001, 0.01, 0.1, and 1%) of the brain extracts was analyzed. The graph shows cell counts per 3.84 × 105 μm2 at the underside of the membrane (means and SDs, n = 3).
Chemotactic factors involved in the migration of hMSCs.
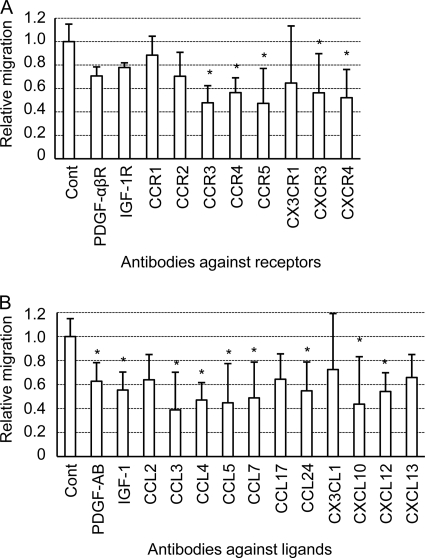
The chemokine CXCL12 and its receptor, CXCR4, are known to be involved in the chemotactic migration of MSCs to brain lesions of neurodegenerative diseases (24, 63). However, since MSCs express a variety of receptors for growth factors, chemokines and cytokines that are associated with cell migration (8, 52), it was anticipated that other cytokines, chemokines and growth factors also play a role in the migration of MSCs. We therefore selected 10 receptors for chemokines and growth factors, and 13 of their ligands, as indicated in Table 1, and analyzed their involvement in hMSC migration using an in vitro migration assay (Fig. 2). These factors were chosen with reference to cell surface expression of those receptors on MSCs in previous studies (50) and to the pathway and networks of receptors and their ligands obtained using Ingenuity Pathway Analysis 5.0 (Ingenuity System, Inc., Redwood City, CA). The migration of hMSCs to the brain extracts of prion-infected mice was significantly decreased when hMSCs were pretreated with antibodies against CCR3, CCR4, CCR5, CXCR3, and CXCR4 compared to the migration of untreated hMSCs (P < 0.05, Dunnett's test) (Fig. 2A). When antibodies against the ligands of these receptors were tested (Table 1), the migration of hMSCs was significantly decreased by treatment of the brain extracts with antibodies against the ligands for CCR3 (CCL5, CCL7, and CCL24), CCR5 (CCL3, CCL4, and CCL5), CXCR3 (CXCL10), and CXCR4 (CXCL12) (P < 0.05) (Fig. 2B). Neither the antibody against CX3CR1, nor that against its corresponding ligand, CX3CL1, decreased the migration of hMSCs. In addition, the antibody against CCR4 decreased hMSC migration, whereas the antibody against its ligand, CCL17, did not. When the involvement of growth factors and their receptors in migration was analyzed, antibodies against the PDGF-αβR or the IGF-1R did not decrease migration, whereas antibodies against their respective ligands, PDGF-AB and IGF-1, did decrease migration. In addition, an antibody against CCR2 or its ligand CCL2 did not decrease migration, but an antibody against CCL7, another ligand for CCR2, did decrease migration. Taking into account the biological relationship between the receptors and ligands that were analyzed here, the migration assays suggested that at least CCR3, CCR5, CXCR3, and CXCR4, and their ligands were involved in the migration of hMSCs to the brain extracts of prion-infected mice.
Table 1.
Growth factor and chemokine receptors and their corresponding ligandsa
| Receptorb | Corresponding ligand(s) |
|---|---|
| HGFR | HGF |
| IGF-1R | IGF-1 |
| PDGF-αβR | PDGF-AB |
| CCR1 | CCL3, CCL5, CCL7, CCL13 |
| CCR2 | CCL2, CCL7, CCL8, CCL13 |
| CCR3 | CCL5, CCL7, CCL8, CCL11, CCL24 |
| CCR4 | CCL17, CCL22 |
| CCR5 | CCL3, CCL4, CCL5, CCL8, CCL11 |
| CX3CR1 | CX3CL1 |
| CXCR3 | CXCL9, CXCL10, CXCL11 |
| CXCR4 | CXCL12 |
| CXCR5 | CXCL13 |
The table was adapted from that of Spaeth et al. (52).
HGFR, hepatocyte growth factor receptor; IGFR, insulin-like growth factor receptor; PDGF-αβR, platelet-derived growth factor-αβ receptor.
Fig. 2.
Screening of chemotactic factors involved in the migration of hMSCs. The migration of hMSCs to 1% brain extracts of prion-infected mice was assessed after pretreatment of hMSCs with antibodies against receptors for chemokines or growth factors (A) or after pretreatment of brain extracts with antibodies against growth factors or chemokines (B). Migration assays, in which antibody pretreatments of hMSCs and brain extracts were omitted, were assigned as a control (Cont). The graphs show relative numbers of hMSCs that migrated to the brain extracts of prion-infected mice compared to numbers of hMSCs that migrated to brain extracts in control experiments. Means and SDs from three independent assays (each assay was carried out in triplicate) are shown. *, P < 0.05 (Dunnett's post hoc test).
Expression of chemokine and chemokine receptor genes.
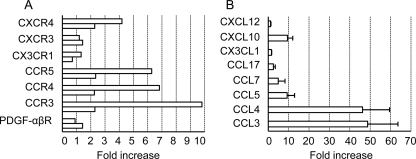
We next examined the expression of chemokine genes in the brains of prion-infected mice and that of chemokine receptor genes in hMSCs treated with the brain extracts of prion-infected mice. For the latter analysis, hMSCs were incubated with DMEM containing 1% brain extracts of prion- or mock-infected mice. At 24 h after incubation, total RNA was recovered, and gene expression was analyzed using qRT-PCR. The expression of CCR3, CCR4, CCR5, and CXCR4 genes was elevated 2- to 9-fold in hMSCs treated with the brain extracts of prion-infected mice compared to their expressions in hMSCs that were treated with the brain extracts of mock-infected mice (Fig. 3A). In contrast, the expression of PDGF-αβR, CX3CR1, and CXCR3 genes was not upregulated by stimulation of the hMSCs with brain extracts from prion-infected mice.
Fig. 3.
Expression of chemokine receptor genes in hMSCs and chemokine genes in the brains of prion-infected mice. (A) Expression of chemokine and growth factor receptor genes in hMSCs stimulated with brain extracts of prion-infected mice. The hMSCs were incubated for a day in DMEM containing 1% brain extracts of prion-infected mice prepared at 120 dpi or of age-matched mock-infected mice. Expression of the mRNA of the indicated genes was then analyzed using qRT-PCR. The graph shows the fold increase in gene expression in hMSCs incubated with brain extracts of prion-infected mice compared to hMSCs incubated with brain extracts of mock-infected mice. The results of two independent experiments are shown. (B) Expression of chemokine genes in the brains of prion-infected mice. The graph shows the fold increases in gene expression in the brains of prion-infected mice at 120 dpi compared to the brains of age-matched mock-infected mice. Means and SDs (n = 3) of the fold increase are shown.
The expression of chemokine genes in the brains of prion-infected mice or of age-matched control mice was also analyzed at 120 dpi. The expression of CCL3, CCL4, CCL5, and CXCL10 genes was upregulated by 10-fold (CXCL10 and CCL5) to nearly 50-fold (CCL3 and CCL4) in the brains of prion-infected mice (Fig. 3B). In addition, the mRNA levels of CCL7 and CCL17 (a ligand for CCR4) were moderately increased (ca. 3- to 6-fold) in the brains of prion-infected mice. In contrast, the expression of the CX3CL1 and CXCL12 genes was not upregulated.
Expression of chemokine receptors on hMSCs.
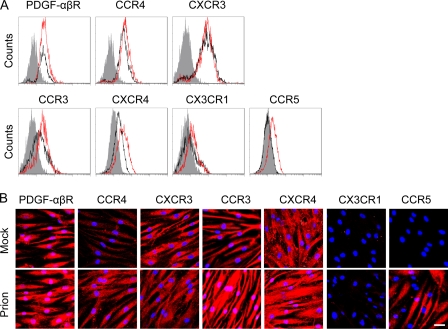
Flow cytometric analysis and IFA were carried out to confirm the expression of chemokine and growth factor receptors on hMSCs. The hMSCs treated with brain extracts of mock-infected mice expressed PDGF-αβR, CCR3, CCR4, CXCR4, and CXCR3 on the cell surface. Of these receptors, expression of CCR3, CCR4, and CXCR4 was increased by stimulation with brain extracts of prion-infected mice, whereas no increase in expression of PDGF-αβR and CXCR3 was observed. Although it is difficult to distinguish signals on the plasma membrane from those in the cytoplasm using IFA, a similar tendency was observed in IFA; CCR3, CCR4, and CXCR4 fluorescent signals appeared to be more intense in cells treated with brain extracts of prion-infected mice than in cells treated with brain extracts of mock-infected mice. In contrast to the expression of these receptors, hMSCs expressed a trace level of CX3CR1, and CCR5 was not detectable on the cell surface. However, the expression of CCR5 was specifically induced in response to brain extracts of prion-infected mice (Fig. 4A). The prion-specific induction of CCR5 expression was also confirmed by IFA (Fig. 4B).
Fig. 4.
Expression of chemokine receptors on hMSCs. (A) Flow cytometric analysis. The expression of chemokine receptors on the cell surface was examined after incubation of the cells with brain extracts of mock (black)- or prion (red)-infected mice prepared at 120 dpi. Gray histograms indicate the negative control (omitted primary antibodies). (B) IFA. The hMSCs that were incubated with brain extracts of mock- or prion-infected mice (Mock or Prion) for 24 h in 8-chamber slides were stained with antibodies against chemokine receptors (red) and were counterstained with DAPI (blue). Bar, 20 μm.
Differential expression of chemokine receptors on hMSCs after transplantation into the brains of prion-infected mice.
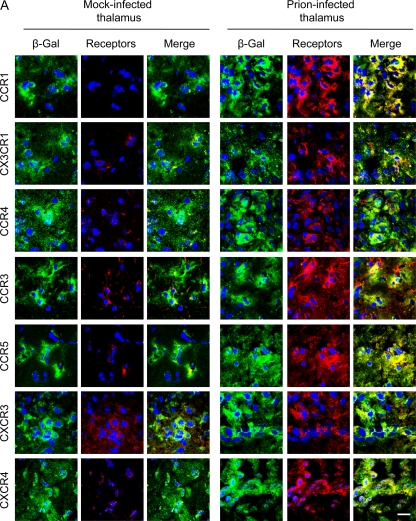
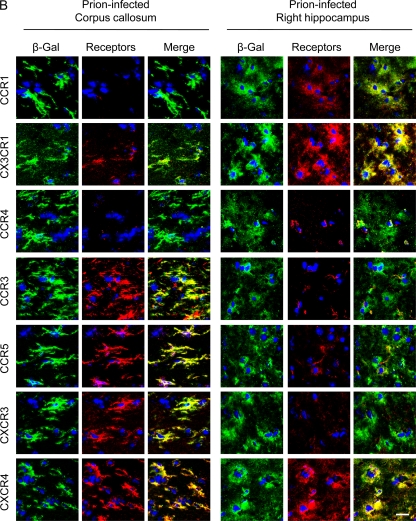
It is known that MSCs transplanted into the left hippocampus or thalamus migrate to the contralateral (right) hippocampus through the corpus callosum (3, 19, 51). Therefore, we hypothesized that receptors expressed on hMSCs in the corpus callosum are possibly involved in the migration of hMSCs to neuropathological lesions. We therefore transplanted hMSCs into the left thalamus of prion- or mock-infected mice at 120 dpi and analyzed the expression of growth factor/chemokine receptors on hMSCs. Two days after transplantation of hMSCs into the left thalami of prion-infected mice, hMSCs in the transplanted region expressed CCR1, CCR3, CCR4, CCR5, CX3CR1, CXCR3, and CXCR4 (Fig. 5A), as well as PDGF-αβR, IGF-1R, and CCR2 (data not shown). However, the hMSCs that were transplanted into the mock-infected mice showed weak expression of these receptors except for CXCR3, suggesting that the expression of these chemokine receptors was increased by stimulation with factors produced in the brains of prion-infected mice. At 2 days posttransplantation, hMSCs in the corpus callosum of prion-infected mice still strongly expressed CCR3, CCR5, CXCR3, and CXCR4, but the expression of CCR1, CX3CR1, and CCR4 appeared to be lower than that on hMSCs in the transplanted area (Fig. 5B). The expression of chemokine receptors on hMSCs in the contralateral hippocampus also differed from that on hMSCs in the transplanted region and in the corpus callosum; hMSCs preferentially expressed only CCR1, CX3CR1, and CXCR4 in the contralateral hippocampus at 1 week posttransplantation (Fig. 5B). These data suggest that CCR1, CX3CR1, and CXCR4 may be associated with specific activities of hMSCs after their migration to the target lesions.
Fig. 5.
Expression of chemokine receptors in hMSCs transplanted into the brains of mock- or prion-infected mice. The hMSCs (105 cells) were transplanted into the left thalamus of mock- or prion-infected mice at 120 dpi, and cryosections of mouse brains were prepared at 2 days or a week posttransplantation. Sections were double stained with anti-β-Gal antibodies conjugated with Alexa Fluor 488 (green) for staining of hMSCs and with antibodies against chemokine receptors (red). Nuclei were counterstained with DAPI (blue). (A) Expression of chemokine receptors in hMSCs at the transplanted area. Images of the left thalamus (transplanted side) of mock- and prion-infected mice were taken at 2 days posttransplantation. (B) Expression of chemokine receptors in hMSCs in the corpus callosum and the right hippocampus. Images of the corpus callosum and the right hippocampus (contralateral side) of prion-infected mice were taken at 2 days and 1 week posttransplantation, respectively. Bar, 20 μm.
DISCUSSION
MSCs are known to migrate to neuropathological lesions of neurodegenerative diseases (8, 54). The migrated MSCs can contribute to the functional recovery of damaged nervous tissues by secretion of various trophic factors (12), neuronal differentiation or cell fusion (1, 29), and stimulation of the proliferation and differentiation of endogenous neural stem cells (38). To date, transplantation of MSCs has been reported to ameliorate the symptoms not only of the experimental animal models of neurodegenerative diseases (10, 19, 20, 33, 59) but also of human patients with multiple system atrophy (32), amyotrophic lateral sclerosis (35), or stroke (4). The involvement of CXCL12 and its cognate receptor CXCR4 in MSC migration to injured tissues is well established (17) in spite of some exceptions (23). In vitro migration assays reported to date have identified growth factors and chemokines that are possibly involved in MSC migration (42, 49, 60, 61). However, the mechanisms of MSC migration are expected to differ with tissue microenvironments induced by diseases. Targeting of MSCs to neuropathological lesions is essential for functional recovery; therefore, an understanding of the mechanisms that underlie the migration of MSCs to lesions in the CNS could contribute to the development of MSC-mediated cell therapy by facilitating site-specific migration of MSCs. The involvement of CCL2, CXCL12/CXCR4, and CXCL1/CX3CR1 in the migration of MSCs to brain lesions has been reported (24, 62, 63). However, the mechanisms that underlie the migration of MSCs to neuropathological lesions are largely unknown.
We recently showed that hMSCs can migrate to neuropathological lesions induced by prion propagation (51). Since brain extracts of prion-infected mice were considered to contain chemoattractive factors (5, 46, 57, 65), we analyzed factors that induce hMSC migration by blocking experiments using antibodies against receptors for growth factors and chemokines and their ligands. To increase the accuracy of the interpretation of in vitro migration assays, we defined a ligand-receptor interaction as chemoattractive if antibodies against both the ligand and its cognate receptor reduced the migration of hMSCs. Based on this criterion, we expected that CCR3, CCR5, CXCR3, and CXCR4, and their ligands are possibly involved in the migration of hMSCs to the brain extracts of prion-infected mice (Fig. 2). The involvement of CXCL12/CXCR4 signaling in hMSCs migration is consistent with findings in hypoglossal nerve injury (24), ischemic (63), and glioma (11) models. Although an effect of CCR3, CCR5, or CXCR3 on MSC migration in brain injury has not been reported, CXCR3 and CCR5 are known to modulate resident microglial migration to brain lesions (6, 45). In prion diseases, impairment of microglial migration, associated with the increased accumulation of PrPSc but prolongation of survival, has been reported in CXCR3 gene deficient mice infected with prions (47). Microglial recruitment in retina after intraocular injection of homogenates from prion-infected neuroblastoma cells was inhibited by CCR5 antagonist, suggesting the involvement of CCR5 in microglial response to prion infection (34), although ablation of CCR5 gene did not influence the incubation period after prion infection (55). Since MSCs are able to migrate to brain lesions, the mechanisms by which they do so are expected to show some similarity with the mechanisms that underlie microglial migration. CCL2 has been reported to mediate MSC migration to ischemic brain lesions (62). However, neither an anti-CCL2 antibody, nor an antibody against its receptor, CCR2, reduced hMSC migration to brain extracts of prion-infected mice, implying that CCL2/CCR2 interaction may not mediate the migration of MSCs to prion-specific brain lesions. PDGF-AB and IGF-1 have been reported to be strong chemoattractants for MSC migration in vitro (42). In the present study, antibodies against these two growth factors reduced hMSC migration, but antibodies against their receptors did not inhibit hMSC migration despite the surface expression of these receptors on hMSCs (Fig. 4). Because of the complexity of the brain extracts and the limitations of blocking experiments, the lack of inhibition by these antibodies against those receptors does not necessarily mean that these ligand-receptor interactions have no functional relevance. Further detailed analysis will define additional factors that possibly mediate the migration of MSCs in response to damage of nervous tissue caused by prion propagation.
Regarding the expression of chemokine genes, the gene expression of CCR5 ligands (CCL3, CCL4, and CCL5), CCR3 ligands (CCL5 and CCL7), and the ligand for CXCR3 (CXCL10) was upregulated. The upregulation of CXCL10 gene in the CNS following viral infection has been reported to attract T lymphocytes bearing CXCR3 into the CNS (64), suggesting that MSCs share some mechanism of trafficking with lymphocyte trafficking. The hMSCs transplanted into the brains of mock-infected mice do not migrate, and the degree of hMSC migration in the brains of prion-infected mice correlates with the progression of neuropathological lesions (51). These results indicate that constitutive expression levels of chemokines and/or growth factors in the brain do not, but increased levels of those factors do, induce hMSC migration. Therefore, although CXCR4 and its ligand CXCL12 have been reported to play an important role in migration of MSCs in the CNS (24, 63), the lack of upregulation of CXCL12 gene expression in prion-infected mice suggests that CXCL12-CXCR4 interaction does not have an important role in the initial event on the attraction of hMSCs. Indeed, cell migration is dependent on a multitude of signals. Therefore, cytokines, chemokines, and growth factors, whose expression is upregulated in the brains of prion-infected mice, e.g., the CXCL10, CCL3 to CCL5, and CCL7 factors that we analyzed in the present study, as well as other factors reported previously (5, 57, 65), will stimulate hMSCs to initiate migration.
The corpus callosum is known to be one of the sites through which MSCs transplanted into one hemisphere migrate to the contralateral hemisphere (3, 19). We recently reported that hMSCs transplanted into the left thalamus of prion-infected mice were detected in the corpus callosum, the contralateral hippocampus and thalamus 2 days after transplantation (51). This result suggests that the hMSCs that were detected in the corpus callosum were migrating to the brain lesions in the contralateral hemisphere. Based on this idea, we therefore analyzed the expression of chemokine receptors on hMSCs that were transplanted into prion-infected mice. Interestingly, in agreement with the interpretation of the in vitro migration assays, hMSCs in the corpus callosum clearly expressed CCR3, CCR5, CXCR3, and CXCR4 2 days after transplantation (Fig. 5B), suggesting the involvement of these chemokine receptors in the migration of hMSCs in vivo. In contrast to hMSCs in the corpus callosum, hMSCs that had migrated to the contralateral hippocampus showed reduced expression of CCR3, CCR5, and CXCR3, whereas strong expression of CXCR4 was still observed. These results suggest that CXCR4 plays a role not only in migration but also in regulating hMSC activity following chemotactic migration. A role in the regulation of hMSC activity following chemotactic migration may also apply to the expression of CCR1 and CX3CR1; the weak expression of CCR1 and CX3CR1 on hMSCs in the corpus callosum (Fig. 5B) appears to be consistent with the results of the in vitro migration assay (Fig. 2). However, in contrast, these receptors were clearly expressed on hMSCs in the transplanted thalamus at 2 days posttransplantation and in the contralateral hippocampus of prion-infected mice a week after transplantation, implying that CCR1 and CX3CR1 may play a role in regulating MSC activity after migration to the targeted site.
Although neuroprotective functions of MSCs that have migrated to brain lesions are not fully understood, the temporal and spatial differences in the expression of chemokine receptors on hMSCs transplanted into the brains of prion-infected mice that we observed in the present study is intriguing, i.e., hMSCs that are considered to be in the process of migration in the corpus callosum expressed CCR3, CCR5, and CXCR3, whereas hMSCs that had migrated to the target site showed reduced expression of these receptors but elevated expression of CCR1 and CX3CR1. The stimulation of CX3CR1 by its ligand CX3CL1 plays a role in modulating the release of proinflammatory cytokines from microglia (7, 21) and in producing neuroprotective substances (31). Therefore, the expression of CX3CR1 on MSCs that had migrated to the contralateral hippocampus might be an indicator of functional alteration of hMSCs, i.e., alteration from active migration toward the target site to exhibition of neuroprotective potential. The hMSCs that had migrated to the contralateral hippocampus still expressed CCR1, CX3CR1, and CXCR4 at 3 weeks posttransplantation (data not shown). We have previously shown that hMSCs transplanted into prion-infected mice efficiently produced various neurotrophic factors at 1 to 3 weeks posttransplantation (51). It is thus conceivable that signaling via these receptors may facilitate alteration of the phenotype of hMSCs to that of a neuroprotective phenotype following chemotactic migration. The interaction between endogenous nucleotide and purinergic receptors on microglia is known to regulate the activation state of microglia. The interaction of ATP with the P2Y12 receptor induces microglial chemotaxis to local CNS injury. However, a decrease in the expression of the P2Y12 receptor accompanies morphological change of microglia from a ramified to an amoeboid state (18). In contrast, upregulation of the expression of P2Y6 receptors by neuronal damage and interaction of UDP released from damaged neurons with the P2Y6 receptor triggers phagocytic activity of microglia (28). It is therefore of interest to investigate whether a temporal and spatial change in the expression profile of chemokine receptors, similar to the control of microglial activity by endogenous nucleotides and purinergic receptors, accompanies alteration in the activation states of MSCs.
In the present study, we showed that, in addition to CXCR4, the chemokine receptors CCR3, CCR5, and CXCR3 are involved in the migration of hMSCs to brain lesions caused by prion infection. The fact that the results of the in vitro migration assay are consistent with the expression of chemokine receptors on hMSCs transplanted into the brains of prion-infected mice provides strong evidence for the involvement of these chemokine receptors in the migration of hMSCs. Although factors that regulate the migration of hMSCs in the CNS may vary with diseases and with the microenvironment of the lesions, our results provide an insight into the mechanism of MSC migration toward neuropathological lesions. In addition, our results also show that comparison of MSC receptor expression in migrating MSCs with that of MSCs that had homed to the targeted site provides a clue to identification of factors that facilitate the homing of MSCs. Further investigation of the host cells that produce relevant ligands for those receptors in response to the progression of neuropathological lesions, the temporal order of receptor expression on MSCs, and of the mechanisms that regulate the activity of MSCs, may facilitate the application of MSCs to prion diseases.
ACKNOWLEDGMENTS
This study was supported by a Grant-in-Aid for Science Research (A) (grant 23248050), a grant from the global COE Program (F-001), and the Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. This study was also supported by grants for TSE research (H23-Shokuhin-Ippan-005) and Research on Measures for Intractable Diseases from the Ministry of Health, Labor, and Welfare of Japan. This study was also partly supported by a grant-in-aid from the BSE Control Project of the Ministry of Agriculture, Forestry, and Fisheries of Japan.
We thank Zensho Co., Ltd., for the BSL3 facility.
Footnotes
Published ahead of print on 3 August 2011.
REFERENCES
- 1. Alvarez-Dolado M., et al. 2003. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes, and hepatocytes. Nature 425:968–973 [DOI] [PubMed] [Google Scholar]
- 2. Annabi B., et al. 2003. Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells 21:337–347 [DOI] [PubMed] [Google Scholar]
- 3. Azizi S. A., Stokes D., Augelli B. J., DiGirolamo C., Prockop D. J. 1998. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats: similarities to astrocyte grafts. Proc. Natl. Acad. Sci. U. S. A. 95:3908–3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bang O. Y., Lee J. S., Lee P. H., Lee G. 2005. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 57:874–882 [DOI] [PubMed] [Google Scholar]
- 5. Campbell I. L., Eddleston M., Kemper P., Oldstone M. B., Hobbs M. V. 1994. Activation of cerebral cytokine gene expression and its correlation with onset of reactive astrocyte and acute-phase response gene expression in scrapie. J. Virol. 68:2383–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carbonell W. S., Murase S., Horwitz A. F., Mandell J. W. 2005. Migration of perilesional microglia after focal brain injury and modulation by CC chemokine receptor 5: an in situ time-lapse confocal imaging study. J. Neurosci. 25:7040–7047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cardona A. E., et al. 2006. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 9:917–924 [DOI] [PubMed] [Google Scholar]
- 8. Chamberlain G., Fox J., Ashton B., Middleton J. 2007. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 25:2739–2749 [DOI] [PubMed] [Google Scholar]
- 9. Charo I. F., Ransohoff R. M. 2006. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354:610–621 [DOI] [PubMed] [Google Scholar]
- 10. Chen J., et al. 2001. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J. Neurol. Sci. 189:49–57 [DOI] [PubMed] [Google Scholar]
- 11. Chien L. Y., et al. 2011. In vivo magnetic resonance imaging of cell tropism, trafficking mechanism, and therapeutic impact of human mesenchymal stem cells in a murine glioma model. Biomaterials 32:3275–3284 [DOI] [PubMed] [Google Scholar]
- 12. Chopp M., Li Y. 2002. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 1:92–100 [DOI] [PubMed] [Google Scholar]
- 13. Demaimay R., et al. 1997. Late treatment with polyene antibiotics can prolong the survival time of scrapie-infected animals. J. Virol. 71:9685–9689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doh-ura K., et al. 2004. Treatment of transmissible spongiform encephalopathy by intraventricular drug infusion in animal models. J. Virol. 78:4999–5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dwyer R. M., et al. 2007. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin. Cancer Res. 13:5020–5027 [DOI] [PubMed] [Google Scholar]
- 16. Forte G., et al. 2006. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells 24:23–33 [DOI] [PubMed] [Google Scholar]
- 17. Fox J. M., Chamberlain G., Ashton B. A., Middleton J. 2007. Recent advances into the understanding of mesenchymal stem cell trafficking. Br. J. Haematol. 137:491–502 [DOI] [PubMed] [Google Scholar]
- 18. Haynes S. E., et al. 2006. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 9:1512–1519 [DOI] [PubMed] [Google Scholar]
- 19. Hellmann M. A., Panet H., Barhum Y., Melamed E., Offen D. 2006. Increased survival and migration of engrafted mesenchymal bone marrow stem cells in 6-hydroxydopamine-lesioned rodents. Neurosci. Lett. 395:124–128 [DOI] [PubMed] [Google Scholar]
- 20. Hofstetter C. P., et al. 2002. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc. Natl. Acad. Sci. U. S. A. 99:2199–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang D., et al. 2006. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J. 20:896–905 [DOI] [PubMed] [Google Scholar]
- 22. Hung S. C., et al. 2007. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS One 2:e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ip J. E., et al. 2007. Mesenchymal stem cells use integrin beta1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol. Biol. Cell 18:2873–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ji J. F., He B. P., Dheen S. T., Tay S. S. 2004. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells 22:415–427 [DOI] [PubMed] [Google Scholar]
- 25. Kawasaki Y., et al. 2007. Orally administered amyloidophilic compound is effective in prolonging the incubation periods of animals cerebrally infected with prion diseases in a prion strain-dependent manner. J. Virol. 81:12889–12898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kobune M., et al. 2003. Telomerized human multipotent mesenchymal cells can differentiate into hematopoietic and cobblestone area-supporting cells. Exp. Hematol. 31:715–722 [DOI] [PubMed] [Google Scholar]
- 27. Kocisko D. A., et al. 2006. A porphyrin increases survival time of mice after intracerebral prion infection. Antimicrob. Agents Chemother. 50:759–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koizumi S., et al. 2007. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature 446:1091–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kopen G. C., Prockop D. J., Phinney D. G. 1999. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc. Natl. Acad. Sci. U. S. A. 96:10711–10716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lapidot T. 2001. Mechanism of human stem cell migration and repopulation of NOD/SCID and B2mnull NOD/SCID mice: the role of SDF-1/CXCR4 interactions. Ann. N. Y. Acad. Sci. 938:83–95 [DOI] [PubMed] [Google Scholar]
- 31. Lauro C., et al. 2008. Activity of adenosine receptors type 1 Is required for CX3CL1-mediated neuroprotection and neuromodulation in hippocampal neurons. J. Immunol. 180:7590–7596 [DOI] [PubMed] [Google Scholar]
- 32. Lee P. H., et al. 2008. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin. Pharmacol. Ther. 83:723–730 [DOI] [PubMed] [Google Scholar]
- 33. Li Y., et al. 2001. Intracerebral transplantation of bone marrow stromal cells in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Neurosci. Lett. 316:67–70 [DOI] [PubMed] [Google Scholar]
- 34. Marella M., Chabry J. 2004. Neurons and astrocytes respond to prion infection by inducing microglia recruitment. J. Neurosci. 24:620–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mazzini L., et al. 2008. Stem cell treatment in amyotrophic lateral sclerosis. J. Neurol. Sci. 265:78–83 [DOI] [PubMed] [Google Scholar]
- 36. Miyasaka M., Tanaka T. 2004. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat. Rev. Immunol. 4:360–370 [DOI] [PubMed] [Google Scholar]
- 37. Mukherjee A., et al. 2010. Calcineurin inhibition at the clinical phase of prion disease reduces neurodegeneration, improves behavioral alterations and increases animal survival. PLoS Pathog. 6:e1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Munoz J. R., Stoutenger B. R., Robinson A. P., Spees J. L., Prockop D. J. 2005. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc. Natl. Acad. Sci. U. S. A. 102:18171–18176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nakamura K., et al. 2004. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 11:1155–1164 [DOI] [PubMed] [Google Scholar]
- 40. Peled A., et al. 1999. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 283:845–848 [DOI] [PubMed] [Google Scholar]
- 41. Pittenger M. F., et al. 1999. Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147 [DOI] [PubMed] [Google Scholar]
- 42. Ponte A. L., et al. 2007. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells 25:1737–1745 [DOI] [PubMed] [Google Scholar]
- 43. Prockop D. J. 1997. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276:71–74 [DOI] [PubMed] [Google Scholar]
- 44. Prusiner S. B., Scott M. R., DeArmond S. J., Cohen F. E. 1998. Prion protein biology. Cell 93:337–348 [DOI] [PubMed] [Google Scholar]
- 45. Rappert A., et al. 2004. CXCR3-dependent microglial recruitment is essential for dendrite loss after brain lesion. J. Neurosci. 24:8500–8509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Riemer C., et al. 2004. Gene expression profiling of scrapie-infected brain tissue. Biochem. Biophys. Res. Commun. 323:556–564 [DOI] [PubMed] [Google Scholar]
- 47. Riemer C., et al. 2008. Accelerated prion replication in, but prolonged survival times of, prion-infected CXCR3−/− mice. J. Virol. 82:12464–12471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ringe J., et al. 2007. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2, and CCR2 and migrate upon stimulation with CXCL8 but not CCL2. J. Cell Biochem. 101:135–146 [DOI] [PubMed] [Google Scholar]
- 49. Son B. R., et al. 2006. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells 24:1254–1264 [DOI] [PubMed] [Google Scholar]
- 50. Song C. H., et al. 2008. Effect of intraventricular infusion of anti-prion protein monoclonal antibodies on disease progression in prion-infected mice. J. Gen. Virol. 89:1533–1544 [DOI] [PubMed] [Google Scholar]
- 51. Song C. H., et al. 2009. Effect of transplantation of bone marrow-derived mesenchymal stem cells on mice infected with prions. J. Virol. 83:5918–5927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Spaeth E., Klopp A., Dembinski J., Andreeff M., Marini F. 2008. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 15:730–738 [DOI] [PubMed] [Google Scholar]
- 53. Springer T. A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76:301–314 [DOI] [PubMed] [Google Scholar]
- 54. Sykova E., Jendelova P. 2007. Migration, fate and in vivo imaging of adult stem cells in the CNS. Cell Death Differ. 14:1336–1342 [DOI] [PubMed] [Google Scholar]
- 55. Tamguney G., et al. 2008. Genes contributing to prion pathogenesis. J. Gen. Virol. 89:1777–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Trevitt C. R., Collinge J. 2006. A systematic review of prion therapeutics in experimental models. Brain 129:2241–2265 [DOI] [PubMed] [Google Scholar]
- 57. Tribouillard-Tanvier D., Striebel J. F., Peterson K. E., Chesebro B. 2009. Analysis of protein levels of 24 cytokines in scrapie agent-infected brain and glial cell cultures from mice differing in prion protein expression levels. J. Virol. 83:11244–11253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Uryu M., Karino A., Kamihara Y., Horiuchi M. 2007. Characterization of prion susceptibility in Neuro2a mouse neuroblastoma cell subclones. Microbiol. Immunol. 51:661–669 [DOI] [PubMed] [Google Scholar]
- 59. Vercelli A., et al. 2008. Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 31:395–405 [DOI] [PubMed] [Google Scholar]
- 60. Von Luttichau I., et al. 2005. Human adult CD34− progenitor cells functionally express the chemokine receptors CCR1, CCR4, CCR7, CXCR5, and CCR10 but not CXCR4. Stem Cells Dev. 14:329–336 [DOI] [PubMed] [Google Scholar]
- 61. Wang L., et al. 2002. Ischemic cerebral tissue and MCP-1 enhance rat bone marrow stromal cell migration in interface culture. Exp. Hematol. 30:831–836 [DOI] [PubMed] [Google Scholar]
- 62. Wang L., et al. 2002. MCP-1, MIP-1, IL-8 and ischemic cerebral tissue enhance human bone marrow stromal cell migration in interface culture. Hematology 7:113–117 [DOI] [PubMed] [Google Scholar]
- 63. Wang Y., Deng Y., Zhou G. Q. 2008. SDF-1alpha/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells toward ischemic brain lesion in a rat model. Brain Res. 1195:104–112 [DOI] [PubMed] [Google Scholar]
- 64. Wilson E. H., Weninger W., Hunter C. A. 2010. Trafficking of immune cells in the central nervous system. J. Clin. Invest. 120:1368–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xiang W., et al. 2004. Identification of differentially expressed genes in scrapie-infected mouse brains by using global gene expression technology. J. Virol. 78:11051–11060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang F., et al. 2009. Transforming growth factor-beta promotes recruitment of bone marrow cells and bone marrow-derived mesenchymal stem cells through stimulation of MCP-1 production in vascular smooth muscle cells. J. Biol. Chem. 284:17564–17574 [DOI] [PMC free article] [PubMed] [Google Scholar]