Abstract
Gut-associated lymphoid tissue (GALT) is a major site of HIV replication and CD4+ T cell depletion. Furthermore, microbial translocation facilitated by mucosal damage likely contributes to the generalized immune activation observed in HIV infection. Regulatory T cells (Treg) help maintain homeostasis and suppress harmful immune activation during infection; however, in the case of persistent viral infections such as HIV, their role is less clear. Although a number of studies have examined Treg in blood during chronic infection, few have explored Treg in the gastrointestinal mucosa. For this study, paired blood and rectal biopsy samples were obtained from 12 HIV noncontrollers (viral load of >10,000 copies/ml plasma), 10 HIV controllers (viral load of <500 copies/ml plasma for more than 5 years), and 12 HIV seronegative control subjects. Noncontrollers had significantly higher percentages of Treg in rectal mononuclear cells (RMNC), but not in blood, compared to seronegative subjects (P = 0.001) or HIV controllers (P = 0.002). Mucosal Treg positively correlated with viral load (P = 0.01) and expression of immune activation markers by CD4+ (P = 0.01) and CD8+ (P = 0.07) T cells. Suppression assays indicated that mucosal and peripheral Treg of noncontrollers and controllers maintained their capacity to suppress non-Treg proliferation to a similar extent as Treg from seronegative subjects. Together, these findings reveal that rather than experiencing depletion, mucosal Treg frequency is enhanced during chronic HIV infection and is positively correlated with viral load and immune activation. Moreover, mucosal Treg maintain their suppressive ability during chronic HIV infection, potentially contributing to diminished HIV-specific T cell responses and viral persistence.
INTRODUCTION
Regulatory T cells (Treg) are important mediators of immune homeostasis that act by suppressing the activation and effector functions of innate and adaptive immune cells (7, 58, 61, 65, 86, 90, 93, 96). Although first described in the 1970s as “suppressor T cells” (38, 39), little was known of these cells until the mid-1990s, when Sakaguchi et al. identified a minor subset of CD4+ T cells responsible for protection from autoimmunity in mice. These cells were characterized by expression of the high-affinity interleukin-2 (IL-2) receptor alpha chain, CD25 (74). CD25 proved to be a reliable Treg marker in mice; however, human effector memory T cells can also express intermediate levels of CD25, blurring the distinction between regulatory and nonregulatory cells (81). In 2003, several groups linked defects in CD4+ CD25+ T cells to mutations in the Foxp3 gene of “scurfy” mice, which suffer from aggressive X-linked autoimmune disease (15, 35, 46, 52). In humans, similar mutations in FOXP3 cause IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome), a multiorgan autoimmune disease often accompanied by severe intestinal inflammation (9, 18, 36). With the addition of FOXP3, the identification of human Treg was significantly improved. This transcription factor is necessary for Treg generation and function and remains the most definitive marker of Treg to date (35, 48, 99). Nonetheless, transient, low-level expression of FOXP3 has been observed in human T cells after T cell receptor (TCR) stimulation; thus, FOXP3 expression alone is not always sufficient to differentiate Treg from non-Treg (75).
Importantly, Treg not only act to suppress autoreactive cells, but also play a central role in protecting host tissues from immune-mediated damage by limiting immune activation and proliferation during infection (8). In the case of persistent viral infections such as HIV, however, the benefits of a vigorous Treg response are debatable (7, 32, 60). HIV presents several unique challenges to fully understanding how Treg impact disease progression. The central question involves determining whether Treg are predominantly beneficial or harmful to the infected individual. Studies with the macaque model suggested an early Treg response may be responsible for premature and persistent suppression of effector responses, contributing to the establishment of a chronic disease state (29, 30). In agreement with this hypothesis, Treg have been shown to inhibit HIV-specific T cell responses ex vivo and thus may act in vivo by suppressing HIV-specific immunity, allowing HIV to persist (1, 53, 55, 98). Transient Treg depletion during chronic Friend virus infection in mice resulted in the reactivation of virus-specific CD8 T cells and a reduction in virus load, again indicating a potentially harmful role for Treg in retroviral infection (22). Furthermore, Favre et al. recently postulated that in gut-associated lymphoid tissue (GALT), expansion of Treg in response to inflammation may support disease progression by preventing the recovery of Th17 cells, resulting in persistent mucosal barrier dysfunction and failure to clear translocated microbial products (31). However, other reports have suggested that Treg play a largely beneficial role, either by suppressing HIV-replication in conventional CD4+ cells (66) or by limiting nonspecific immune activation (17, 51). Indeed, because immune activation closely correlates with disease progression (19, 40, 63, 88), it is possible that in the context of chronic infection the beneficial aspects of the Treg response outweigh the disadvantages.
To date, the majority of studies on Treg in HIV infection have focused on peripheral blood; however, HIV also affects lymphoid tissues, notably the gastrointestinal mucosa (12, 14, 41, 43, 44, 83, 84, 95). CD4+ T cells in the gut are depleted early and rapidly after infection with HIV or simian immunodeficiency virus (SIV) (14, 43, 59, 64, 87, 94). The depletion of gut CD4+ T cells, including Th17 cells, is associated with decreased integrity of the mucosal barrier, resulting in gut “leakiness” and microbial translocation, a likely contributor to increased immune activation (11, 13, 28, 31, 37, 44, 77). Although studies of peripheral Treg levels have had varied results (3, 24, 54, 56, 92, 101), a general consensus appears to be emerging regarding Treg in tissues. Several studies have found evidence of increased Treg frequencies in lymph nodes and tonsils of untreated SIV-positive (SIV+) macaques and HIV+ patients with high viral loads (2, 3, 10, 27, 29, 31, 69, 78); however, very few have examined mucosal Treg from HIV-infected humans (27, 31), and to our knowledge, none have directly tested their functional capacity. Moreover, while Treg may accumulate in SIV- and HIV-infected tissue sites, the causes and effects of this increase remain unclear. By comparing levels of peripheral and mucosal Treg present in HIV controllers, who are able to maintain low-to-undetectable levels of viremia in the absence of antiretroviral therapy, to those in noncontrollers with progressive infection, we may begin to answer some of these challenging questions.
This study examined Treg phenotype, frequency, and function in both blood and rectal tissue of HIV controllers and noncontrollers. We observed increased frequencies of Treg in rectal mucosae of noncontrollers compared to controllers or seronegative individuals regardless of the method used to identify Treg. In contrast, no significant differences between groups were detected in blood. Moreover, although the number of CD4+ T cells was decreased in rectal mucosae of noncontrollers, Treg numbers did not differ significantly between patient groups. The percentage of rectal Treg correlated positively with plasma viral load and with mucosal immune activation, suggesting that these factors may drive mucosal accumulation and/or conversion of Treg. We also demonstrated, for the first time, the suppressive capacity of mucosal Treg isolated from HIV+ patients. Although mucosal Treg of noncontrollers appeared more highly activated, they suppressed proliferation of autologous CD4+ CD25− T cells to a similar extent as Treg from controllers or seronegatives. Thus, noncontrollers maintain a functional Treg response to viral infection that is nonetheless unable to effectively control mucosal immune activation. Collectively, these results point to the gastrointestinal mucosa as a central player in HIV pathogenesis throughout the course of disease. Increases in Treg frequency in rectal mucosa may contribute to T effector cell dysfunction and viral persistence in HIV noncontrollers by perpetuating a destructive cycle of effector cell suppression, virus replication, and immune activation.
MATERIALS AND METHODS
Study subjects and clinical samples.
HIV-positive (n = 23) and -negative (n = 12) subjects were enrolled and samples collected at the University of California—Davis Medical Center (UCDMC) in Sacramento, CA, or at San Francisco General Hospital (SFGH), University of California—San Francisco (UCSF). Informed consent was obtained from all subjects under protocols approved by the Committee on Human Subjects Research, UCSF, and the University of California—Davis Institutional Review Board. Each patient provided approximately 20 ml of blood, collected in Vacutainer tubes coated with EDTA (BD Pharmingen, San Jose, CA), and 20 to 25 rectal biopsy specimens, collected in R15 medium (RMPI 1640 containing 15% fetal bovine serum, 100 IU/ml penicillin, 100 IU/ml streptomycin, and 2 mM glutamine). The HIV+ participants, none of whom were on antiretroviral therapy, were considered “noncontrollers” if they maintained plasma HIV RNA levels of >10,000 copies/ml and “controllers” if they maintained plasma HIV RNA levels of <2,000 copies/ml, as previously defined by Emu et al. (20, 26). All controllers in our study had plasma HIV RNA levels <500 copies/ml, well below the established criteria for controllers.
Cell isolation.
Patient blood and rectal biopsy samples were processed the same day they were collected. Peripheral blood mononuclear cells (PBMC) were isolated from whole blood using Ficoll-Hypaque (GE Healthcare, United Kingdom) gradient centrifugation. Rectal biopsies were obtained by flexible sigmoidoscopy as described previously (34) and subjected to three rounds of tissue digestion in medium containing 0.5 mg/ml collagenase II (Sigma-Aldrich, St. Louis, MO) followed by mechanical disruption with a 12-ml syringe equipped with an 18-gauge blunt-end needle, and passage through a 70-μm-pore cell strainer to yield a single-cell suspension of rectal mononuclear cells (RMNC). RMNC were pooled and washed in R15. Red blood cell lysis was performed as needed on PBMC and RMNC using ammonium chloride-potassium carbonate-EDTA (ACK). Cells destined for immunophenotyping were rested overnight at 37°C with 5% CO2, while all remaining cells were used to establish the suppression assays. Zosyn (piperacillin-tazobactam; Wyeth, Madison, NJ) was added at 0.5 mg/ml to all rectal cell cultures to prevent bacterial growth.
Treg immunophenotyping.
Following isolation, 1 × 106 PBMC and 1 × 106 RMNC were immunophenotyped using multiparameter flow cytometry. Surface staining for CD3, CD4, CD8, CD25, CD127, CD38, and programmed death 1 receptor (PD-1), followed by intracellular staining for FOXP3 was performed using the eBioscience method and FOXP3 staining buffer set (eBioscience, San Diego, CA). LIVE/DEAD fixable Aqua dead cell stain (Invitrogen, Carlsbad, CA), was included in the surface stain in order to identify viable cells. Fluorescence minus one (FMO) controls (72) were included as needed. BD CompBeads (BD Pharmingen) were used to assess compensation settings prior to acquisition and to establish a compensation matrix for data analysis. All samples were fixed in 1% paraformaldehyde before acquisition on an LSRII flow cytometer (BD Pharmingen). Flow cytometry data were analyzed using FlowJo software (Tree-Star, Inc., Ashland, OR). The number of Treg per 10,000 CD3+ T cells was calculated from flow data using the following formula: (total CD4+ FOXP3+ CD25+ cell count/total CD3+ cell count) × 10,000, as previously described (24, 68).
Purification of T cell subsets and suppression assays.
PBMC and RMNC were separated into CD4+ CD25− and CD4+ CD25+ fractions using EasySep particles and magnets according to the manufacturer's suggested protocol (StemCell Technologies, Inc., Vancouver, British Columbia, Canada). Briefly, after incubation with the EasySep human CD4 enrichment cocktail, magnetic beads (“D particles”) were used to negatively select for CD4+ T cells. The CD4-enriched cells were then incubated with EasySep human CD25 selection cocktail and magnetic nanoparticles to positively select for the CD4+ CD25-bright population. The CD4+ CD25− responder cells remaining in the supernatant fraction were resuspended in prewarmed phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA) and incubated with 2 μM carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen) for 10 min at 37°C with 5% CO2. Staining was arrested by the addition of 5 times the volume of ice-cold R15 followed by a 5-min incubation on ice. Responder cells were washed twice more in R15 before counting.
For the suppression assays, wells of a 96-well round-bottom plate were either left uncoated or coated with 0.2 μg/ml anti-CD3, clone OKT3 (eBioscience), for 4 h at 37°C. Autologous PBMC were irradiated at 4,000 rads; 1 × 105 irradiated feeder cells were added to the culture wells along with 1 × 104 to 11 × 104 CFSE-labeled responder cells and the indicated number of CD4+ CD25+ Treg in R15 supplemented with 10 mM HEPES, 1 mM sodium pyruvate, and 0.1 mM nonessential amino acids. Plates were centrifuged at 700 rpm for 5 min to initiate cell contact and incubated at 37°C with 5% CO2 for 60 to 84 h. After the indicated culture period, cells were stained with anti-CD3, anti-CD4, anti-CD8, anti-CD25, and anti-FOXP3 using the FOXP3 staining buffer set according to the manufacturer's instructions. Anti-OX40/CD134 antibodies were included in the stain for the majority of samples. Cell phenotype and proliferation of responder cells (via CFSE dye dilution) were determined by flow cytometry. In order to control for Treg remaining in the responder cell population, the ratio of CFSE-negative (CFSE−) CD4+ CD25+ FOXP3+ to CFSE-positive (CFSE+) CD4+ CD25+ FOXP3+ cells was used in the correlation analyses where the percentage of proliferation was determined directly from histogram plots of CFSE+ cells.
Antibodies.
The fluorochrome-labeled antibodies used for this study include anti-CD3 (clone UCHT1, Pacific Blue labeled; BD Pharmingen), anti-CD4 (SFCI12T4D11, electron-coupled dye (ECD) labeled; Beckman Coulter, Brea, CA; or L200, peridinin chlorophyll protein [PerCP]-Cy5.5 labeled; BD Pharmingen), anti-CD8 (SK1 or 3B5, Qdot605 labeled; Invitrogen), anti-CD25 (M-A251, phycoerythrin [PE]-Cy7 labeled; BD Pharmingen), anti-CD127 (hIL-7R-M21, Alexa Fluor 647 labeled; BD Pharmingen), anti-PD-1 (eBioJ105, PE labeled; eBioscience), anti-CD38 (HIT2, PerCP-Cy5.5 or PE-Cy5 labeled; BD Pharmingen), anti-FOXP3 (PCH101, Alexa Fluor 700 labeled; eBioscience), and anti-OX40/CD134 (ACT35, PE labeled; BD Pharmingen).
Statistical analysis.
GraphPad Prism (GraphPad Software, La Jolla, CA) was used to graph and analyze data for statistical significance. Intergroup comparisons were analyzed using a two-tailed Mann-Whitney test. The Wilcoxon matched-pairs test was used for intragroup comparisons between blood and rectal samples. The Spearman rank test was used for bivariate correlations, while linear regression analysis was used to produce an accompanying best-fit line. P values between 0.1 and 0.05 were considered trends; P values of ≤0.05 were considered significant.
RESULTS
Patient groups.
This study examined paired blood and rectal biopsy specimens from three groups of individuals: 12 HIV-positive noncontrollers with a viral load over 10,000 copies/ml plasma, 10 HIV controllers able to maintain a viral load of less than 500 copies/ml plasma for more than 5 years in the absence of antiretroviral therapy, and 12 HIV-seronegative control subjects. No subjects were on therapy at the time of sample collection, although three had documented prior antiretroviral treatment (Table 1).
Table 1.
Characteristics of the patients in this study
| Patient identification no. and group | Gender | Ethnicitya | Age (yr) | No. of yr infected | Viral load (copies/ml) | CD4 count (cells/μl) |
|---|---|---|---|---|---|---|
| Seropositive | ||||||
| Noncontrollers | ||||||
| 106b | Male | AA | 36 | 6.3 | 35,800 | 502 |
| G126 | Male | White | 36 | 8.6 | 23,000 | 157 |
| 131 | Male | White | 47 | 3.6 | 14,600 | 512 |
| 143 | Male | AA | 48 | 11.4 | 26,300 | 238 |
| G135 | Female | AA | 38 | 17.4 | 20,606 | 396 |
| G138c | Male | White | 53 | 23.2 | 10,073 | 350 |
| G137 | Female | AA | 37 | 5.7 | 13,200 | 523 |
| S1335 | Male | AA | 53 | 24.2 | 48,105 | 482 |
| S1597 | Male | H/L, Iberian | 49 | 12.8 | 12,593 | 510 |
| S1580 | Male | White | 43 | 1.8 | 31,966 | 467 |
| S1613 | Male | AA, NAm | 40 | 0.3 | 41,812 | 561 |
| S1359 | Male | H/L | 34 | 2.7 | 52,459 | 714 |
| Median | 42 | 7 | 24,650 | 492 | ||
| Controllers | ||||||
| 48 | Female | White | 54 | 16.6 | 50 | 1,710 |
| 97 | Male | H/L | 49 | 18.8 | 114 | 927 |
| 105 | Male | White | 52 | 5.6 | 738 | 500 |
| G15d | Female | AA | 55 | 21.6 | 50 | 679 |
| S1116 | Male | White | 53 | 25.8 | 69 | 535 |
| S1198 | Male | White | 54 | 23.3 | 40 | 728 |
| S1581 | Male | White | 56 | 23.8 | 40 | 1,227 |
| S1548 | Female | AA | 47 | 18.3 | 73 | 718 |
| S1572 | Male | AA | 56 | 19.3 | 40 | 909 |
| S1495 | Male | White | 55 | 18.3 | 323 | 542 |
| S1541 | Male | White | 47 | 14.3 | 47 | 1,895 |
| Median | 54 | 19 | 50 | 728 | ||
| Seronegative | ||||||
| 129 | Female | White | 41 | NAe | NA | 695 |
| 130 | Male | White | 42 | NA | NA | 642 |
| 135 | Female | White | 46 | NA | NA | 1,499 |
| 137 | Male | AA | 46 | NA | NA | 651 |
| 138 | Male | AA | 38 | NA | NA | 772 |
| 142 | Male | White | 24 | NA | NA | 1,183 |
| G53 | Female | AA | 44 | NA | NA | 866 |
| G64 | Male | AA | 56 | NA | NA | 892 |
| G54 | Female | White | 47 | NA | NA | 813 |
| S1939 | Male | White | 30 | NA | NA | 769 |
| S1812 | Male | White | 51 | NA | NA | 699 |
| S1913 | Male | AA | 48 | NA | NA | 873 |
| Median | 45 | 793 |
AA, African American; H/L, Hispanic or Latino; NAm, Native American.
Was on antiretroviral therapy (ART) previously.
Was off ART for 4 months previous to the time of sample collection.
Was on ART during pregnancy only, 18 years ago.
NA, not applicable.
Regulatory T cell frequency is increased in the mucosae of HIV+ patients with high viral loads.
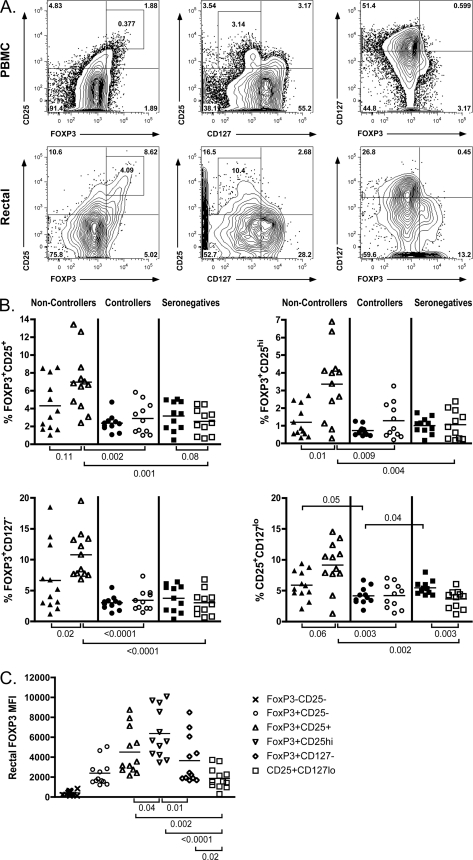
Human Treg are typically identified as either CD4+ CD25+ CD127lo (5, 62, 80) or CD4+ FOXP3+ CD25+ (35, 73, 99). The designation FOXP3+ CD25hi has also been used in an attempt to avoid inclusion of recently activated non-Treg that express an intermediate level of CD25, although this approach typically underestimates the total number of Treg (32, 80). In order to investigate the relationship between Treg frequency and progressive infection, we first identified Treg using multiple approaches, as shown in Fig. 1A. Regardless of the gating strategy employed, the frequency of Treg within the CD3+ CD4+ gate was consistently higher in the rectal mucosae of noncontrollers than controllers (P < 0.009 for all gating strategies) or seronegative subjects (P < 0.004 for all gating strategies) (Fig. 1B). In the peripheral blood, however, significant differences in Treg percentages were only observed when CD25+ CD127lo was used to define the subset, where CD25+ CD127lo Treg were significantly decreased in the blood of controller samples compared to uninfected seronegative subjects (P = 0.04). Additionally, within the controller and seronegative groups, Treg frequencies in the periphery and rectal mucosa were similar, whereas the noncontroller group consistently exhibited an increased frequency of Treg in rectal mucosa compared to blood (P = 0.11 for FOXP3+ CD25+, P = 0.01 for FOXP3+ CD25hi, P = 0.02 for FOXP3+ CD127−, and P = 0.06 for CD25+ CD127lo). Notably, all four Treg gating strategies indicated that subjects with progressive HIV infection experienced an increase in Treg frequency in mucosal tissue to a degree that was not seen in the periphery. In contrast, controllers maintained levels of Treg that were similar to those of uninfected controls in both compartments.
Fig. 1.
Treg frequency is increased in the rectal mucosae of noncontrollers regardless of the gating strategy used to identify Treg. (A) Representative flow cytometry plots depicting four different approaches for identifying CD4+ Treg in human PBMC (top panels) and rectal cells (bottom panels) in the FOXP3+ CD25+ (left), FOXP3+ CD25hi (left, inset), CD25+ CD127lo (middle), and FOXP3+ CD127− (right) groups. Numbers within the gates represent the frequencies of the gated CD4+ subpopulations. (B) Treg frequencies in PBMC (solid symbols) and RMNC (open symbols) of noncontrollers (triangles), controllers (circles), and seronegative subjects (squares) were analyzed using the gating strategies described above. (C) FOXP3 expression in the rectal mucosae was determined by MFI and compared between each of the four Treg gating approaches. FOXP3− CD25− non-Treg and the FOXP3+ CD25− subsets were included in the analysis as reference groups. FOXP3 MFI was highest within the CD4+ FOXP3+ CD25hi and CD4+ FOXP3+ CD25+ gates. This graph contains data from the noncontroller patients; a similar pattern of expression was seen in controllers and seronegative subjects. Horizontal lines within the groups correspond to the mean. The numbers above and below the brackets are P values. P values of ≤0.05 were considered significant; P values between 1 and 0.05 were considered trends.
FOXP3 expression level varies depending on the method used to identify Treg but is not significantly different between patient groups.
Although a similar distribution of FOXP3 expression was found in all patient groups, there was considerable variation in the FOXP3 median fluorescence intensity (MFI), depending on the Treg gating strategy examined. Within the various Treg populations, expression of FOXP3 was brightest in the FOXP3+ CD25hi population, followed by the FOXP3+ CD25+ and FOXP3+ CD127− groups, and lowest in the FOXP3+ CD25− and CD25+ CD127lo populations for all patient groups (Fig. 1C). CD127 is known to be dysregulated in HIV infection (16, 23), and loss of CD127 expression has been used as a measure of immune activation. Additionally, a previous study of Treg found that the CD25hi CD127lo phenotype appeared to be reflecting the high levels of immune activation found in viremic progressors and did not correlate with levels of FOXP3+ CD25hi T cells (21). Thus, while the CD25hi CD127lo Treg phenotype may have discriminatory value in patient populations with lower levels of immune activation (i.e., HIV controllers and HIV-uninfected individuals) (47), it is a less consistent Treg marker in untreated viremic participants. For these reasons, the FOXP3+ CD25+ population was chosen for all subsequent analyses of Treg phenotype in order to avoid a falsely high estimation by using CD25+ CD127lo or falsely low estimation by using FOXP3+ CD25hi. Although no significant differences between patient groups were observed, FOXP3 MFI was significantly higher in rectal FOXP3+ CD25+, FOXP3+ CD25hi, and FOXP3+ CD127− Treg in all patient groups compared to their peripheral blood counterparts (P ≤ 0.05 for all; data not shown), similar to what has previously been described in the lymph nodes of HIV+ individuals (53).
The number of mucosal Treg does not differ significantly between noncontrollers, controllers, and seronegative subjects.
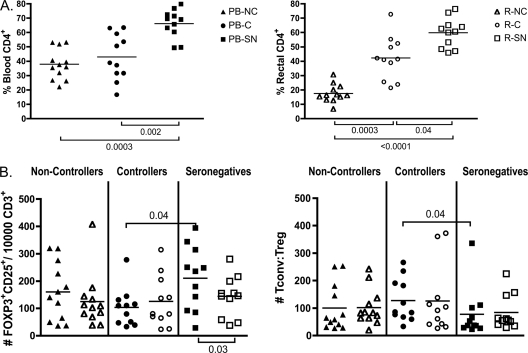
The gastrointestinal mucosa experiences a significant loss in the percentage and total number of CD4+ T cells after HIV or SIV infection coupled with an increase in CD8+ T cells as the body attempts to control the virus (85, 94). As expected, in our cohort HIV noncontrollers revealed the greatest loss of blood and rectal CD4+ T cells, but controllers also had significant CD4 loss compared to seronegative subjects (Fig. 2A). Because such perturbations in the major lymphocyte populations occur, it is important to assess not only the frequency of CD4+ Treg present within the CD4+ T cell population, but also their frequency relative to total CD3+ cells. Although the mean value was highest for seronegative subjects in both blood and rectal mucosa, no significant difference in the number of Treg normalized per 10,000 CD3+ T cells was observed between groups in the mucosa. These data suggest that multiple, competing factors influence Treg abundance in mucosal tissues. Treg can be infected by HIV (67, 70), yet they may not be as readily depleted as conventional CD4+ T cells, resulting in a stable number but increased frequency of Treg within the CD4+ subset. Treg accumulation in lymphoid tissues is associated with disease progression, and their survival may be promoted by interactions between CD4 and gp120 (50, 69).
Fig. 2.
Numbers of CD4+ FOXP3+ CD25+ Treg are not significantly different between patient groups despite a decline in total CD4+ T cells of noncontrollers and controllers. (A and B) Treg frequencies in PBMC (PB-; solid symbols) and RMNC (R-; open symbols) of noncontrollers (NC; triangles), controllers (C; circles), and seronegative subjects (SN; squares) were determined by staining as described in Materials and Methods, and data were acquired on an LSRII flow cytometer. (A) Percentage of CD4+ T cells in the blood (left) and rectal mucosae (right). (B) The number of Treg per 10,000 CD3+ T cells was determined by dividing the number of CD4+ FOXP3+ CD25+ T cells acquired during flow cytometry sample acquisition by the total number of CD3+ T cells acquired and multiplying by 10,000 (right). The ratio of conventional T cells to Treg was determined by the following formula where the number of cells was determined by flow cytometry data: [CD8+ T cells + (CD4+ T cells − CD4+ CD25+ FOXP3+ Treg)]/CD4+ CD25+ FOXP3+ Treg (left). Horizontal lines within the groups correspond to the mean. The numbers above and below the brackets are P values. P values of ≤0.05 were considered significant; P values between 1 and 0.05 were considered trends.
Interestingly, HIV controllers had a significantly lower number of Treg in the blood compared to seronegative subjects (P = 0.04), which was likewise reflected in a higher ratio of conventional CD4+ and CD8+ T cells to Treg (P = 0.04) (Fig. 2B). These data are consistent with prior findings from our group, which involved different subjects and different phenotypic panels to define the Treg population (47). As previously argued, these lower numbers of Treg may allow for the more robust, polyfunctional HIV-specific responses often seen in HIV controllers, while also contributing to higher generalized immune activation (47). Despite these consistent differences in blood, we saw no differences in Treg frequencies or numbers (defined multiple ways) in the gut mucosa when comparing HIV-seronegative subjects and controllers. A larger study focused on HIV controllers may be needed to define the complex impact that Treg might have on HIV progression in these individuals.
Markers of immune activation are elevated in conventional T cells and Treg of noncontrollers.
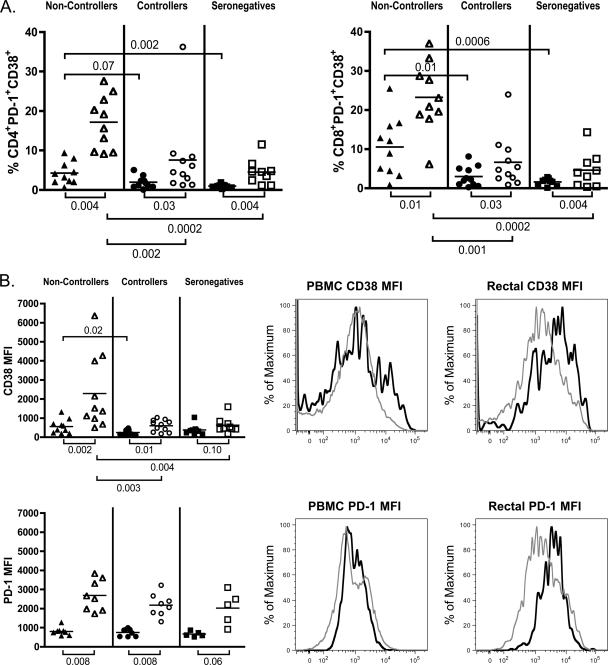
High-level expression of markers of immune activation has been shown to correlate with clinical measures of progressive infection (40, 63, 88). Recently, it was demonstrated that the coexpression of activation marker CD38 and the inhibitory molecule PD-1 on T cells is highly enriched in HIV-specific CD8 T cells of progressors compared to controllers and closely correlates with viral load and CD4+ T cell loss (25, 97). PD-1 expression in HIV-infected subjects has also been linked to persistent viral replication as well as CD4+ T cell activation (25, 76). In our patient cohort, the frequency of activated CD4+ and CD8+ PD-1+ CD38+ cells was very significantly increased in rectal mucosae of noncontrollers compared to controllers (P = 0.002 and P = 0.001) or seronegative subjects (P = 0.0002 and P = 0.0002) (Fig. 3A). In the periphery, activation measured by coexpression of CD38 and PD-1 was always significantly lower than in the mucosa and showed the same trends that were seen in the mucosa with respect to intergroup comparisons: noncontrollers maintained the highest levels of activated CD4+ and CD8+ T cells in blood compared to controllers (P = 0.07 and P = 0.01) or seronegative subjects (P = 0.002 and P = 0.0006) (Fig. 3A). In agreement with the study referenced above (97), CD38–PD-1 coexpression on total CD8+ cells in PBMC and mucosae negatively correlated with CD4 count (P = 0.003 and P = 0.001 for PBMC and mucosa, respectively) and positively correlated with plasma viral load (P = 0.003 and P = 0.004, respectively). Similar relationships were observed for activated CD38+ PD-1+ CD4+ T cells, where P = 0.003 in the blood and P = 0.02 in the mucosae are with respect to CD4 count, and P = 0.06 in the blood and P = 0.004 in the mucosae are with respect to viral load. Notably, utilizing coexpression of CD38 and PD-1 in the correlation analyses consistently resulted in greater significance than use of CD38 alone (data not shown). In summary, compared to controllers and seronegative subjects, T cells of noncontrollers displayed increased expression of CD38 and PD-1, which was particularly striking in rectal mucosa. Controllers, in contrast, did not display significant activation compared to seronegative subjects.
Fig. 3.
Markers of T cell activation are elevated in total CD8+, CD4+, and Treg populations in the mucosae of noncontrollers. (A and B) Treg frequencies in PBMC (solid symbols) and RMNC (open symbols) of noncontrollers (triangles), controllers (circles), and seronegative subjects (squares) were stained as described in Materials and Methods, and data were acquired on an LSRII flow cytometer. Horizontal lines within the groups correspond to the mean. The numbers above and below the brackets are P values. P values of ≤0.05 were considered significant; P values between 1 and 0.05 were considered trends. (A) Coexpression of CD38 and PD-1 was used to assess T cell activation in total CD4+ (left) and CD8+ (right) T cells in the blood and rectal mucosae of the three patient groups. (B) MFI of CD38 (top panel) and PD-1 (bottom panel) in Treg populations of noncontrollers, controllers, and seronegative subjects (left). Shown are representative histograms from a noncontroller comparing MFI in CD4+ FOXP3− CD25− non-Treg (gray lines) to CD4+ FOXP3+ CD25+ Treg (black lines) for CD38 (top panels) and PD-1 (bottom panels) in the peripheral blood and rectal mucosae (right).
Further analysis of the CD4+ CD25+ FOXP3+ Treg population also revealed increased immune activation. Although both CD38 MFI and PD-1 MFI were consistently higher on rectal Treg versus peripheral Treg for all patient groups, only CD38 expression was specifically increased in mucosal Treg of noncontrollers compared to controllers or uninfected controls (P = 0.003 and P = 0.004) (Fig. 3B). In comparison to CD25− FOXP3− conventional CD4+ cells (non-Treg) or CD4+ CD25+ FOXP3− cells, Treg consistently expressed higher levels of PD-1, with an overall expression pattern that differed little between groups, whereas CD38 expression exhibited greater variation (data not shown).
Correlation analyses between Treg, clinical parameters of infection, and immune activation.
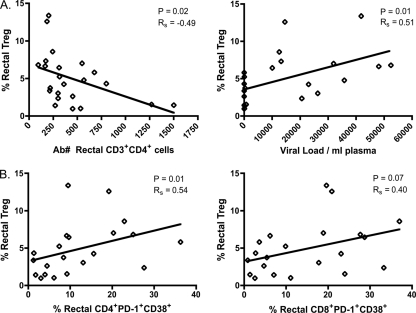
Although Treg frequency was increased in the mucosae of noncontrollers, it is difficult to determine what effect this increase may have on disease progression. By examining the relationship between Treg frequency, viral load, CD4 count, and immune activation in HIV+ patient samples, we can begin to assess the impact of Treg on chronic HIV infection and vice versa. Rectal Treg frequency was negatively correlated with the absolute number of mucosal CD4+ T cells (P = 0.02) and positively correlated with plasma viral load (P = 0.01) (Fig. 4A). In contrast, no significant relationships existed for blood Treg and plasma viral load or peripheral CD4 count (data not shown).
Fig. 4.
Mucosal Treg frequency negatively correlates with mucosal CD4 numbers and positively correlates with plasma viral load and mucosal T cell activation. (A) Relationship between rectal Treg frequencies in HIV+ subjects and mucosal CD3+ CD4+ cell number based on events acquired by flow cytometry, calculated by dividing the number of CD4+ cells by the number of live cells and multiplying by 10,000 (left) or the viral load determined by quantitative PCR (qPCR) (right). Ab#, absolute number. (B) Relationship between rectal Treg frequencies in HIV+ subjects and percentage of mucosal CD38+ PD-1+ CD4 T cells (left) or CD38+ PD-1+ CD8 T cells (right). Regression analysis was used to produce best-fit lines on all graphs, while the P values and rho values were determined using the Spearman rank test for bivariate correlations.
Increasing immune activation is also known to correlate with high viral load and low CD4 counts, but the relationship between immune activation and Treg levels is less clear (17, 31, 51). Although regression analysis revealed a positive relationship for both rectal and peripheral Treg frequencies with respect to CD38+ PD-1+ T cells, once again a significant correlation was present only in the mucosa. The relationship was strongest for rectal Treg and mucosal CD4+ activation (P = 0.01); however, a strong trend also existed between rectal Treg and mucosal CD8+ activation (P = 0.07) (Fig. 4B).
Rectal Treg of noncontrollers are suppressive and are able to suppress polyclonally stimulated non-Treg to a similar extent as Treg of controllers or seronegatives.
Given that both mucosal Treg frequency and immune activation are elevated in chronic infection of noncontrollers, we hypothesized that mucosal Treg of noncontrollers might be functionally impaired in their ability to limit immune activation compared to Treg from controllers or HIV-negative individuals. Alternatively, Treg in noncontrollers might more effectively (yet inappropriately) suppress HIV-specific adaptive responses, providing a competitive advantage to the virus. To compare the functionality of Treg from controllers, noncontrollers, and seronegative subjects, we performed a series of suppression assay experiments by coculturing CD4+ CD25+ Treg and CD4+ CD25− non-Treg isolated from peripheral blood and rectal cell suspensions. Non-Treg were incubated alone or with increasing numbers of Treg and left unstimulated or stimulated with anti-CD3 for 3 days; proliferation was assessed using CFSE dye dilution (Fig. 5A). Although peripheral Treg suppressed proliferation to a greater extent than did mucosal Treg (Fig. 5B), no significant differences in the suppression of proliferation were observed between noncontrollers, controllers, and seronegative subjects in either blood or rectal cell cultures.
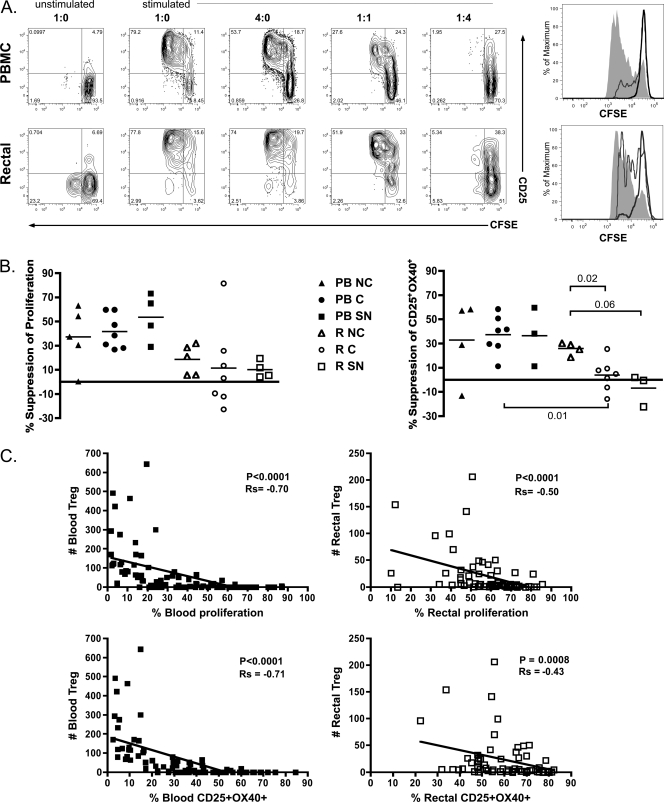
Fig. 5.
Peripheral and mucosal Treg of noncontrollers, controllers, and seronegative subjects are suppressive ex vivo. CD4+ CD25− non-Treg and CD4+ CD25+ Treg were separated from PBMC and RMNC using magnetic beads as described in Materials and Methods. A total of 1 × 104 CFSE-labeled non-Treg were added to 96-well round-bottom plates containing 1 × 105 irradiated, autologous PBMC and cultured alone or with increasing numbers of Treg. Cells were unstimulated or stimulated with 0.2 μg/ml immobilized anti-CD3 for 60 to 84 h. (A) Representative flow cytometry gating on CFSE+ cells from one experiment. Proliferation in cultures containing PBMC (top panels) or rectal cells (bottom panels) was assessed using CFSE. Contour plots show the results of cultures containing the indicated ratio of non-Treg to Treg. Histograms depict CFSE expression in non-Treg for cultures at non-Treg/Treg ratios of 1:0 (shaded areas), 1:1 (gray lines), and 1:4 (black lines). (B) Treg-mediated suppression was measured in PBMC (PB-; solid symbols) and RMNC (R-; open symbols) of noncontrollers (NC; triangles), controllers (C; circles), and seronegative subjects (SN; squares). Percent suppression was determined by dividing the level of proliferation (left) or CD25-OX40 coexpression (right) in the 1:1 cocultures by the same parameter in the 1:0 cultures, multiplying the result by 100, and subtracting this percentage from 100. Horizontal lines within boxes correspond to the mean. The numbers above and below the brackets are P values. P values of ≤0.05 were considered significant; P values between 1 and 0.05 were considered trends. (C) Graphs illustrate the relationship between FOXP3+ CD25+ Treg numbers present in PBMC (solid symbols) and RMNC (open symbols) cultures and the percent proliferation (top panels) or percent CD25-OX40 coexpression (bottom panels). Regression analysis was used to produce a best-fit line for both graphs; P values and rho values were determined using the Spearman rank test for bivariate correlations.
A previous study found that simultaneous expression of CD25 and CD134 (OX40) was induced after TCR engagement and that CD25-OX40 coexpression strongly correlated with antigen-specific gamma interferon secretion and proliferation (100). We also observed a highly significant correlation between the proliferation and CD25+ OX40+ expression in stimulated T cell cultures (P < 0.0001 and Spearman's rank order coefficient [Rs] = 0.89 for PBMC and P < 0.0001 and Rs = 0.53 for RMNC; data not shown). Using coexpression of CD25 and OX40 as a measure of the T cell response to stimulation, we again observed no significant differences in the suppressive abilities of peripheral Treg isolated from noncontrollers, controllers, or seronegative subjects. In mucosal cultures, however, Treg from noncontrollers were significantly more suppressive than those from controllers (P = 0.02) and exhibited a strong trend toward increased suppression compared to Treg from seronegative subjects (P = 0.06) (Fig. 5B). It is interesting to note that this effect was seen for non-Treg CD25-OX40 coexpression but not proliferation inhibition. This may indicate Treg can modulate effector functions to different degrees depending on the tissue microenvironment and specific pathways engaged.
Although the degree of Treg-mediated suppression was greater in cells isolated from peripheral blood compared to rectal mucosa, regardless of patient group (Fig. 5B), our data suggest that this was most likely due to reduced purity of non-Treg and Treg fractions from rectal cell suspensions compared to PBMC (data not shown). Factors potentially affecting purity include clumping of mucosal mononuclear cells and reduced CD4 staining intensity following collagenase digestion (our unpublished observation). However, in preliminary experiments, cell viability and yield were greater following magnetic bead isolation than flow cytometry-based cell sorting of rectal Treg. To account for the reduced purity of Treg and non-Treg fractions in rectal samples, correlation analyses were performed comparing the percentage of non-Treg proliferation to the number of FOXP3+ CD25+ Treg present in the CD4+ CFSE− population divided by the number of FOXP3+ CD25+ Treg present in the CD4+ CFSE+ (non-Treg) population (Fig. 5C). These analyses clearly showed a very close correlation between total number of Treg present in a culture and suppression for both the peripheral blood and rectal mucosa (P < 0.0001 for PBMC and P < 0.0001 for RMNC), confirming the suppressive nature of the isolated Treg. Similar results were obtained for the CD25+ OX40+ coexpression and Treg number (P < 0.0001 for PBMC and P < 0.0008 for RMNC) (Fig. 5C).
DISCUSSION
The impact of Treg on HIV progression has been the subject of some debate (24, 27, 32, 45, 69, 79). Adding to the confusion is the variety of approaches used to identify Treg. The combination of markers most commonly used includes CD4+ CD25+, CD4+ CD25+ CD127lo, CD4+ FOXP3+, CD4+ CD25+ FOXP3+, and CD4+ CD25+ FOXP3+ CD127lo. Additionally, the majority of studies have focused on peripheral Treg, while comparatively few have sampled mucosal tissues despite the severe impact HIV infection is known to have on the gut (12, 84). For these reasons, we used a multiparameter flow cytometry approach to determine the phenotype of Treg present in the blood and rectal mucosae and established a suppression assay to evaluate Treg function in subjects from across the clinical spectrum.
Regardless of the method we used to identify Treg, a significantly increased frequency was evident in the rectal mucosae of noncontrollers compared to controllers or seronegative subjects. This is in agreement with previous studies examining Treg in lymphoid and mucosal tissues of HIV+ progressors (3, 27, 31, 53, 56, 78). We did not observe a corresponding increase in peripheral blood of noncontrollers, in contrast to what has been reported previously (71, 78, 89). This discrepancy may be due to differences in patient characteristics, specifically a lower median CD4 count in the noncontroller cohort, and/or due to differences in Treg gating strategies.
This increase in frequency was observed as a percentage of CD4+ T cells; however, the number of Treg normalized per 10,000 CD3+ T cells was not increased in noncontrollers relative to the other groups. This finding suggests that HIV may preferentially deplete conventional CD4+ T cells while sparing the regulatory population; alternatively, both populations may be infected and depleted to a similar extent, but Treg may be more quickly replaced. Indeed, multiple mechanisms may influence Treg numbers in the mucosae, including recruitment and trafficking (3, 50), increased survival (50, 69), active proliferation (3, 27), and peripheral conversion of non-Treg into Treg (56). A recent study of SIV-infected rhesus macaques found high levels of HIV DNA present in Treg but low levels of HIV RNA, indicating Treg were permissive to viral entry and integration, but not productively infected. Additionally, Treg proliferated along with the total CD4+ T cell pool but were less susceptible to SIV-related cell death, resulting in an increased percentage and absolute number of Treg (2). A similar situation may be at play in chronic HIV infection. Treg themselves are a target of viral infection (67, 70), and the balance between proliferation, immune activation, and death may be what keeps Treg numbers stable as non-Treg decline.
Importantly, controllers maintain low mucosal Treg frequencies similar to seronegative subjects. Although this might simply be a reflection of the low level of plasma viremia and relative CD4+ T cell preservation, controllers do experience a significant loss of gut CD4+ T cells compared to uninfected persons without a corresponding increase in Treg frequency. Our group has previously reported that HIV-specific CD4+ and CD8+ T cell responses in the rectal mucosae of controllers were of greater magnitude and more polyfunctional than responses in noncontrollers (33, 34). Additionally, controllers had a significantly lower number of Treg and higher ratio of conventional T cells to Treg in the periphery compared to HIV-negative controls, a situation that could potentially allow for more robust anti-HIV responses.
The level of T cell activation was significantly higher in noncontrollers compared to controllers or seronegative patients in both the blood and mucosal compartments, as expected. CD38–PD-1 coexpression was higher in the rectal compartment compared to the blood for all patient groups, and the difference in activation was comparable for CD4+ T cells and CD8+ T cells. Mucosal Treg likewise expressed increased levels of CD38 and PD-1, measured by MFI, compared to blood Treg. Noncontroller Treg had a significantly higher CD38 MFI in the mucosae compared to controllers or seronegative subjects as well as increased CD38 MFI in the periphery compared to controllers only. Thus, it appears that mucosal Treg of noncontrollers are highly activated and may be responding to increased levels of immune activation in the gut. In support of this, the frequency of rectal Treg exhibited a positive correlation with both plasma viral load and CD4 and CD8 mucosal T cell activation. Importantly, significant correlations between Treg frequency, viral load, and immune activation were seen only in the gut, suggesting that in addition to the early assault endured during primary infection, this compartment continues to be a dynamic site of HIV replication throughout the course of disease.
The gastrointestinal mucosa must maintain a delicate balance between tolerance to dietary and commensal organisms and responsiveness to pathogenic intruders. A number of studies have underscored the importance of Treg in maintaining a healthy gut environment (48). Treg suppressive function in the blood and lymph node of HIV+ subjects has been examined in numerous studies (1, 4, 6, 49, 53–55, 57, 82, 91, 92, 98), but to our knowledge, this is the first to perform functional experiments on mucosal Treg in HIV infection. Studies of peripheral Treg function found a decreased suppressive capacity in Treg from patients with high viral loads and low CD4 counts (54, 92). In contrast, Kinter et al. described an increased capacity for suppression in Treg isolated from lymph nodes of HIV+ progressors (53). Despite difficulties purifying mucosal Treg, we did observe Treg-mediated suppression of non-Treg proliferation as well as a decrease in coexpression of CD25 and OX40. The degrees of suppression of proliferation were similar in all patient groups for both blood and mucosal cultures.
Interestingly, noncontrollers did have significantly greater suppression of CD25-OX40 coexpression in mucosa compared to controllers. Griseri et al. recently described an important role for OX40 expression on Treg in a mouse model of colitis (42). OX40 provided survival signals necessary for the accumulation of Treg in the colon, and under inflammatory conditions, OX40+ Treg were able to suppress colitogenic T cell responses, presumably by competing with effector T cells for access to OX40L, thereby limiting their ability to sustain an effective response. Further studies will be necessary to determine whether expression of OX40 on mucosal Treg of humans has a role in the suppression of effector responses in HIV.
As the body of work examining Treg in HIV infection continues to grow, a model of how Treg are impacted by infection and how they in turn impact disease progression is beginning to emerge. Although Treg can be infected and depleted by HIV, their frequency relative to conventional CD4+ T cells increases in progressive infection. This increase is most evident in the lymphoid tissues and gastrointestinal tract, where the majority of early HIV replication occurs. The relationship between mucosal Treg frequency, viral load, and immune activation in our study suggests that the frequency of Treg increases in the gastrointestinal mucosa in response to high levels of immune activation in HIV noncontrollers. They may then suppress antiviral immune responses, contributing to an environment permissive to ongoing viral replication, which in turn further exacerbates immune activation. Additional studies will be required to address the mechanisms driving the accumulation of Treg in mucosal tissues and to test whether strategies designed to modulate mucosal Treg function could boost adaptive responses and/or limit immune activation in HIV noncontrollers.
ACKNOWLEDGMENTS
We thank the study volunteers for their willingness to participate in this research. We also thank Becky Hoh, Lee Gilman, the clinical staff at San Francisco General Hospital, and the SCOPE study for their assistance with patient recruitment and clinical procedures. We also thank Lishoma Ndhlovu, University of Hawaii, for assistance during the early phase of the project.
This research was supported by NIH/NIAID R01-AI057020 to B.L.S. The SCOPE cohort was supported in part by NIH/NIAID (R01-AI087145, K24-AI069994), the UCSF CFAR (P01-AI027763), the UCSF CTSI (UL1-RR024131), the Cleveland Immunopathogenesis Consortium (R01-AI076174), and the CFAR Network of Integrated Systems (R24-AI067039). This investigation was conducted in a facility constructed with support from the Research Facilities Improvement Program (grant C06-RR012088) to UC Davis from NIH/NCRR. The LSR-II violet laser was upgraded with funding from the James B. Pendleton Charitable Trust.
Footnotes
Published ahead of print on 31 August 2011.
REFERENCES
- 1. Aandahl E. M., Michaelsson J., Moretto W. J., Hecht F. M., Nixon D. F. 2004. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J. Virol. 78:2454–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allers K., et al. 2010. Gut mucosal FOXP3+ regulatory CD4+ T cells and nonregulatory CD4+ T cells are differentially affected by simian immunodeficiency virus infection in rhesus macaques. J. Virol. 84:3259–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andersson J., et al. 2005. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J. Immunol. 174:3143–3147 [DOI] [PubMed] [Google Scholar]
- 4. Antons A. K., Wang R., Kalams S. A., Unutmaz D. 2008. Suppression of HIV-specific and allogeneic T cell activation by human regulatory T cells is dependent on the strength of signals. PLoS One 3:e2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baecher-Allan C., Brown J. A., Freeman G. J., Hafler D. A. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167:1245–1253 [DOI] [PubMed] [Google Scholar]
- 6. Baker C. A., et al. 2007. Peripheral CD4 loss of regulatory T cells is associated with persistent viraemia in chronic HIV infection. Clin. Exp. Immunol. 147:533–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Belkaid Y. 2007. Regulatory T cells and infection: a dangerous necessity. Nat. Rev. Immunol. 7:875–888 [DOI] [PubMed] [Google Scholar]
- 8. Belkaid Y., Rouse B. T. 2005. Natural regulatory T cells in infectious disease. Nat. Immunol. 6:353–360 [DOI] [PubMed] [Google Scholar]
- 9. Bennett C. L., et al. 2001. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 27:20–21 [DOI] [PubMed] [Google Scholar]
- 10. Boasso A., et al. 2007. Regulatory T-cell markers, indoleamine 2,3-dioxygenase, and virus levels in spleen and gut during progressive simian immunodeficiency virus infection. J. Virol. 81:11593–11603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brenchley J. M., et al. 2008. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 112:2826–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brenchley J. M., Price D. A., Douek D. C. 2006. HIV disease: fallout from a mucosal catastrophe? Nat. Immunol. 7:235–239 [DOI] [PubMed] [Google Scholar]
- 13. Brenchley J. M., et al. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12:1365–1371 [DOI] [PubMed] [Google Scholar]
- 14. Brenchley J. M., et al. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brunkow M. E., et al. 2001. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 27:68–73 [DOI] [PubMed] [Google Scholar]
- 16. Carini C., McLane M. F., Mayer K. H., Essex M. 1994. Dysregulation of interleukin-7 receptor may generate loss of cytotoxic T cell response in human immunodeficiency virus type 1 infection. Eur. J. Immunol. 24:2927–2934 [DOI] [PubMed] [Google Scholar]
- 17. Chase A. J., Yang H. C., Zhang H., Blankson J. N., Siliciano R. F. 2008. Preservation of FoxP3+ regulatory T cells in the peripheral blood of human immunodeficiency virus type 1-infected elite suppressors correlates with low CD4+ T-cell activation. J. Virol. 82:8307–8315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chatila T. A., et al. 2000. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J. Clin. Invest. 106:R75–R81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deeks S. G., et al. 2004. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 104:942–947 [DOI] [PubMed] [Google Scholar]
- 20. Deeks S. G., Walker B. D. 2007. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 27:406–416 [DOI] [PubMed] [Google Scholar]
- 21. del Pozo-Balado M. M., Leal M., Mendez-Lagares G., Pacheco Y. M. 2010. CD4+CD25+/hiCD127lo phenotype does not accurately identify regulatory T cells in all populations of HIV-infected persons. J. Infect. Dis. 201:331–335 [DOI] [PubMed] [Google Scholar]
- 22. Dietze K. K., et al. 2011. Transient depletion of regulatory T cells in transgenic mice reactivates virus-specific CD8+ T cells and reduces chronic retroviral set points. Proc. Natl. Acad. Sci. U. S. A. 108:2420–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dunham R. M., et al. 2008. CD127 and CD25 expression defines CD4+ T cell subsets that are differentially depleted during HIV infection. J. Immunol. 180:5582–5592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eggena M. P., et al. 2005. Depletion of regulatory T cells in HIV infection is associated with immune activation. J. Immunol. 174:4407–4414 [DOI] [PubMed] [Google Scholar]
- 25. Eller M. A., et al. 2011. Innate and adaptive immune responses both contribute to pathological CD4 T cell activation in HIV-1 infected Ugandans. PLoS One 6:e18779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Emu B., et al. 2008. HLA class I-restricted T-cell responses may contribute to the control of human immunodeficiency virus infection, but such responses are not always necessary for long-term virus control. J. Virol. 82:5398–5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Epple H.-J., et al. 2006. Mucosal but not peripheral FOXP3+ regulatory T cells are highly increased in untreated HIV infection and normalize after suppressive HAART. Blood 108:3072–3078 [DOI] [PubMed] [Google Scholar]
- 28. Estes J. D., et al. 2010. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 6:e1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Estes J. D., et al. 2006. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J. Infect. Dis. 193:703–712 [DOI] [PubMed] [Google Scholar]
- 30. Estes J. D., et al. 2007. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J. Infect. Dis. 195:551–561 [DOI] [PubMed] [Google Scholar]
- 31. Favre D., et al. 2010. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV Dis. Sci. Transl. Med. 2:32ra36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fazekas de St Groth B., Landay A. L. 2008. Regulatory T cells in HIV infection: pathogenic or protective participants in the immune response? AIDS 22:671–683 [DOI] [PubMed] [Google Scholar]
- 33. Ferre A. L., et al. 2009. Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood 113:3978–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferre A. L., et al. 2010. HIV controllers with HLA-DRB1*13 and HLA-DQB1*06 alleles have strong, polyfunctional mucosal CD4+ T-cell responses. J. Virol. 84:11020–11029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fontenot J. D., Gavin M. A., Rudensky A. Y. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330–336 [DOI] [PubMed] [Google Scholar]
- 36. Gambineri E., Torgerson T. R., Ochs H. D. 2003. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr. Opin. Rheumatol. 15:430–435 [DOI] [PubMed] [Google Scholar]
- 37. George M. D., Reay E., Sankaran S., Dandekar S. 2005. Early antiretroviral therapy for simian immunodeficiency virus infection leads to mucosal CD4+ T-cell restoration and enhanced gene expression regulating mucosal repair and regeneration. J. Virol. 79:2709–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gershon R. K., Kondo K. 1970. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology 18:723–737 [PMC free article] [PubMed] [Google Scholar]
- 39. Gershon R. K., Kondo K. 1971. Infectious immunological tolerance. Immunology 21:903–914 [PMC free article] [PubMed] [Google Scholar]
- 40. Giorgi J. V., et al. 1999. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 179:859–870 [DOI] [PubMed] [Google Scholar]
- 41. Gordon S. N., et al. 2010. Disruption of intestinal CD4+ T cell homeostasis is a key marker of systemic CD4+ T cell activation in HIV-infected individuals. J. Immunol. 185:5169–5179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Griseri T., Asquith M., Thompson C., Powrie F. 2010. OX40 is required for regulatory T cell-mediated control of colitis. J. Exp. Med. 207:699–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guadalupe M., et al. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77:11708–11717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Haase A. T. 2005. Perils at mucosal front lines for HIV and SIV and their hosts. Nat. Rev. Immunol. 5:783–792 [DOI] [PubMed] [Google Scholar]
- 45. Holmes D., Jiang Q., Zhang L., Su L. 2008. Foxp3 and Treg cells in HIV-1 infection and immuno-pathogenesis. Immunol. Res. 41:248–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hori S., Nomura T., Sakaguchi S. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057–1061 [DOI] [PubMed] [Google Scholar]
- 47. Hunt P. W., et al. 2011. A low T regulatory cell response may contribute to both viral control and generalized immune activation in HIV controllers. PLoS One 6:e15924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Izcue A., Coombes J. L., Powrie F. 2006. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol. Rev. 212:256–271 [DOI] [PubMed] [Google Scholar]
- 49. Jesser R. D., Li S., Weinberg A. 2006. Regulatory T cells generated during cytomegalovirus in vitro stimulation of mononuclear cells from HIV-infected individuals on HAART correlate with decreased lymphocyte proliferation. Virology 352:408–417 [DOI] [PubMed] [Google Scholar]
- 50. Ji J., Cloyd M. W. 2009. HIV-1 binding to CD4 on CD4+CD25+ regulatory T cells enhances their suppressive function and induces them to home to, and accumulate in, peripheral and mucosal lymphoid tissues: an additional mechanism of immunosuppression. Int. Immunol. 21:283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jiao Y., et al. 2009. The decrease of regulatory T cells correlates with excessive activation and apoptosis of CD8(+) T cells in HIV-1-infected typical progressors, but not in long-term non-progressors. Immunology 128(Suppl. 1):e366–e375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Khattri R., Cox T., Yasayko S. A., Ramsdell F. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4:337–342 [DOI] [PubMed] [Google Scholar]
- 53. Kinter A., et al. 2007. Suppression of HIV-specific T cell activity by lymph node CD25+ regulatory T cells from HIV-infected individuals. Proc. Natl. Acad. Sci. U. S. A. 104:3390–3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kinter A. L., et al. 2004. CD25(+)CD4(+) regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4(+) and CD8(+) HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J. Exp. Med. 200:331–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kinter A. L., et al. 2007. CD25+ regulatory T cells isolated from HIV-infected individuals suppress the cytolytic and nonlytic antiviral activity of HIV-specific CD8+ T cells in vitro. AIDS Res. Hum. Retroviruses 23:438–450 [DOI] [PubMed] [Google Scholar]
- 56. Krathwohl M. D., Schacker T. W., Anderson J. L. 2006. Abnormal presence of semimature dendritic cells that induce regulatory T cells in HIV-infected subjects. J. Infect. Dis. 193:494–504 [DOI] [PubMed] [Google Scholar]
- 57. Legrand F. A., et al. 2006. Strong HIV-1-specific T cell responses in HIV-1-exposed uninfected infants and neonates revealed after regulatory T cell removal. PLoS One 1:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Levings M. K., Sangregorio R., Roncarolo M. G. 2001. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 193:1295–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li Q., et al. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148–1152 [DOI] [PubMed] [Google Scholar]
- 60. Li S., Gowans E. J., Chougnet C., Plebanski M., Dittmer U. 2008. Natural regulatory T cells and persistent viral infection. J. Virol. 82:21–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liang B., et al. 2008. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J. Immunol. 180:5916–5926 [DOI] [PubMed] [Google Scholar]
- 62. Liu W., et al. 2006. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 203:1701–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu Z., et al. 1997. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the multicenter AIDS cohort study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J. Acquir. Immune Defic. Syndr. 16:83–92 [DOI] [PubMed] [Google Scholar]
- 64. Mattapallil J. J., et al. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093–1097 [DOI] [PubMed] [Google Scholar]
- 65. Misra N., Bayry J., Lacroix-Desmazes S., Kazatchkine M. D., Kaveri S. V. 2004. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J. Immunol. 172:4676–4680 [DOI] [PubMed] [Google Scholar]
- 66. Moreno-Fernandez M. E., Rueda C. M., Rusie L. K., Chougnet C. A. 2011. Regulatory T cells control HIV replication in activated T cells through a cAMP-dependent mechanism. Blood 117:5372–5380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Moreno-Fernandez M. E., Zapata W., Blackard J. T., Franchini G., Chougnet C. A. 2009. Human regulatory T cells are targets for human immunodeficiency virus (HIV) infection, and their susceptibility differs depending on the HIV type 1 strain. J. Virol. 83:12925–12933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nigam P., et al. 2010. Expansion of FOXP3+ CD8 T cells with suppressive potential in colorectal mucosa following a pathogenic simian immunodeficiency virus infection correlates with diminished antiviral T cell response and viral control. J. Immunol. 184:1690–1701 [DOI] [PubMed] [Google Scholar]
- 69. Nilsson J., et al. 2006. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood 108:3808–3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Oswald-Richter K., et al. 2004. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2:E198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rallon N. I., et al. 2009. Level, phenotype and activation status of CD4+FoxP3+ regulatory T cells in patients chronically infected with human immunodeficiency virus and/or hepatitis C virus. Clin. Exp. Immunol. 155:35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Roederer M. 2001. Spectral compensation for flow cytometry: visualization artifacts, limitations, and caveats. Cytometry 45:194–205 [DOI] [PubMed] [Google Scholar]
- 73. Roncador G., et al. 2005. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur. J. Immunol. 35:1681–1691 [DOI] [PubMed] [Google Scholar]
- 74. Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151–1164 [PubMed] [Google Scholar]
- 75. Sakaguchi S., Yamaguchi T., Nomura T., Ono M. 2008. Regulatory T cells and immune tolerance. Cell 133:775–787 [DOI] [PubMed] [Google Scholar]
- 76. Salisch N. C., et al. 2010. Inhibitory TCR coreceptor PD-1 is a sensitive indicator of low-level replication of SIV and HIV-1. J. Immunol. 184:476–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sankaran S., et al. 2008. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J. Virol. 82:538–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Schulze zur Wiesch J., et al. 2011. Comprehensive analysis of frequency and phenotype of T regulatory cells in HIV infection: CD39 expression of FoxP3+ T regulatory cells correlates with progressive disease. J. Virol. 85:1287–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Seddiki N., Kelleher A. D. 2008. Regulatory T cells in HIV infection: who's suppressing what? Curr. HIV/AIDS Rep. 5:20–26 [DOI] [PubMed] [Google Scholar]
- 80. Seddiki N., et al. 2006. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 203:1693–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Seddiki N., et al. 2006. Persistence of naive CD45RA+ regulatory T cells in adult life. Blood 107:2830–2838 [DOI] [PubMed] [Google Scholar]
- 82. Seddiki N., et al. 2009. Proliferation of weakly suppressive regulatory CD4+ T cells is associated with over-active CD4+ T-cell responses in HIV-positive patients with mycobacterial immune restoration disease. Eur. J. Immunol. 39:391–403 [DOI] [PubMed] [Google Scholar]
- 83. Shacklett B. L. 2008. Mucosal immunity to HIV: a review of recent literature. Curr. Opin. HIV AIDS 3:541–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shacklett B. L., Anton P. A. 2010. HIV infection and gut mucosal immune function: updates on pathogenesis with implications for management and intervention. Curr. Infect. Dis. Rep. 12:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Shacklett B. L., et al. 2003. Trafficking of human immunodeficiency virus type 1-specific CD8+ T cells to gut-associated lymphoid tissue during chronic infection. J. Virol. 77:5621–5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shevach E. M. 2009. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 30:636–645 [DOI] [PubMed] [Google Scholar]
- 87. Smit-McBride Z., Mattapallil J. J., McChesney M., Ferrick D., Dandekar S. 1998. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4(+) T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J. Virol. 72:6646–6656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sousa A. E., Carneiro J., Meier-Schellersheim M., Grossman Z., Victorino R. M. M. 2002. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J. Immunol. 169:3400–3406 [DOI] [PubMed] [Google Scholar]
- 89. Suchard M. S., et al. 2010. FOXP3 expression is upregulated in CD4+ T cells in progressive HIV-1 infection and is a marker of disease severity. PLoS One 5:e11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Taams L. S., et al. 2002. Antigen-specific T cell suppression by human CD4+CD25+ regulatory T cells. Eur. J. Immunol. 32:1621–1630 [DOI] [PubMed] [Google Scholar]
- 91. Thorborn G., et al. 2010. Increased sensitivity of CD4+ T-effector cells to CD4+CD25+ Treg suppression compensates for reduced Treg number in asymptomatic HIV-1 infection. PLoS One 5:e9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tsunemi S., et al. 2005. Relationship of CD4+CD25+ regulatory T cells to immune status in HIV-infected patients. AIDS 19:879–886 [DOI] [PubMed] [Google Scholar]
- 93. van Mierlo G. J., et al. 2008. Cutting edge: TNFR-shedding by CD4+CD25+ regulatory T cells inhibits the induction of inflammatory mediators. J. Immunol. 180:2747–2751 [DOI] [PubMed] [Google Scholar]
- 94. Veazey R. S., et al. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427–431 [DOI] [PubMed] [Google Scholar]
- 95. Veazey R. S., Lackner A. A. 2004. Getting to the guts of HIV pathogenesis. J. Exp. Med. 200:697–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Vignali D. A., Collison L. W., Workman C. J. 2008. How regulatory T cells work. Nat. Rev. Immunol. 8:523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Vollbrecht T., et al. 2010. Impact of changes in antigen level on CD38/PD-1 co-expression on HIV-specific CD8 T cells in chronic, untreated HIV-1 infection. J. Med. Virol. 82:358–370 [DOI] [PubMed] [Google Scholar]
- 98. Weiss L., et al. 2004. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood 104:3249–3256 [DOI] [PubMed] [Google Scholar]
- 99. Yagi H., et al. 2004. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int. Immunol. 16:1643–1656 [DOI] [PubMed] [Google Scholar]
- 100. Zaunders J. J., et al. 2009. High levels of human antigen-specific CD4+ T cells in peripheral blood revealed by stimulated coexpression of CD25 and CD134 (OX40). J. Immunol. 183:2827–2836 [DOI] [PubMed] [Google Scholar]
- 101. Zhang Z., et al. 2008. Relationship of frequency of CD4+CD25+Foxp3+ regulatory T cells with disease progression in antiretroviral-naive HIV-1 infected Chinese. Jpn. J. Infect. Dis. 61:391–392 [PubMed] [Google Scholar]