Abstract
Attenuated poxvirus vectors expressing human immunodeficiency virus type 1 (HIV-1) antigens are considered promising HIV/AIDS vaccine candidates. Here, we describe the nature of T cell immune responses induced in healthy volunteers participating in a phase I clinical trial in Spain after intramuscular administration of three doses of the recombinant MVA-B-expressing monomeric gp120 and the fused Gag-Pol-Nef (GPN) polyprotein of clade B. The majority (92.3%) of the volunteers immunized had a positive specific T cell response at any time postvaccination as detected by gamma interferon (IFN-γ) intracellular cytokine staining (ICS) assay. The CD4+ T cell responses were predominantly Env directed, whereas the CD8+ T cell responses were similarly distributed against Env, Gag, and GPN. The proportion of responders after two doses of MVA-B was similar to that obtained after the third dose of MVA-B vaccination, and the responses were sustained (84.6% at week 48). Vaccine-induced CD8+ T cells to HIV-1 antigens after 1 year were polyfunctional and distributed mainly within the effector memory (TEM) and terminally differentiated effector memory (TEMRA) T cell populations. Antivector T cell responses were mostly induced by CD8+ T cells, highly polyfunctional, and of TEMRA phenotype. These findings demonstrate that the poxvirus MVA-B vaccine candidate given alone is highly immunogenic, inducing broad, polyfunctional, and long-lasting CD4 and CD8 T cell responses to HIV-1 antigens, with preference for TEM. Thus, on the basis of the immune profile of MVA-B in humans, this immunogen can be considered a promising HIV/AIDS vaccine candidate.
INTRODUCTION
Since 1981, more than 25 million people have died of AIDS, a dramatic pandemic caused by the human immunodeficiency virus (HIV). In 2009, UNAIDS estimated that 33.4 million people lived with HIV-1 infection. Although antiretroviral therapy (ART) can suppress viral replication, increasing life expectancy among those people infected, it cannot cure the infection. Moreover, affordable ART coverage in resource-poor regions where HIV-1 is endemic is a daunting global health problem. For these reasons, the development of a safe and efficacious vaccine represents the best long-term solution to ending the HIV-1 epidemic.
There have been strong proponents of either antibodies or T cells alone as the most effective strategy that should be followed to prevent HIV-1 infection. However, the consensus view now is that a highly effective HIV/AIDS vaccine will need to elicit coordinated B cell, CD4+, and CD8+ T cell responses (27).
More than 30 HIV/AIDS vaccine candidates, whose prototypes have elicited various degrees of protective responses in nonhuman primate models, have advanced to human clinical trials, alone or in combinations (25, 36). These include replication-competent or -incompetent viral vectors (poxvirus, adenovirus, alphavirus, adeno-associated virus) containing HIV-1 gene inserts, HIV-1 viruslike particles, HIV-1 DNA plasmids, and soluble HIV-1 proteins and peptides, with or without adjuvant formulations. Among the candidate regimens that have been extended to large-scale international phase IIb or III studies, only the RV144 trial, which evaluated a recombinant canarypox-HIV-1 vector prime and recombinant HIV-1 envelope gp120 subunit protein plus alum boost in Thailand, demonstrated low-level efficacy (31%) in reducing HIV-1 infection rates (35). These clinical findings provided for the first-time evidence that an HIV/AIDS vaccine can prevent HIV-1 infection and highlights that poxvirus vectors should be considered one of the future HIV/AIDS vaccine candidate vectors.
Among the poxviruses, the attenuated modified vaccinia Ankara (MVA) strain has received great attention in terms of vaccine development for prevention and therapeutic purposes (12). The main advantage of MVA is its safety record. Despite its limited replication in human and most mammalian cell types, MVA provides a high level of gene expression and triggers strong immune responses when delivering foreign antigens in animals and humans (12, 30, 39). In fact, in the last years, several clinical trials have been conducted using MVA-based vaccines in both healthy and HIV-1-infected human volunteers (10, 22, 24, 38, 40). These studies demonstrated that the recombinant vectors based on MVA are safe and well tolerated and are able to induce HIV-1-specific immune responses when administered alone or in combination with other vectors. However, the magnitude, response rates, and durability in immunization regimens using homologous vectors were modest. These observations highlight that more efficient MVA vectors with the ability to enhance the magnitude, breadth, polyfunctionality, and durability of the immune responses to HIV-1 antigens are desirable. This is particularly relevant if a single immunogen is targeted for mass vaccination purposes to simplify the immunization protocol and reduce the manufacture burden.
Here, we have characterized the immunogenicity of the recombinant MVA-B, expressing Env, Gag, Pol, and Nef HIV-1 antigens from clade B, in healthy volunteers enrolled in the RISVAC02 phase I clinical trial. The construction details and preclinical setting of this vaccine were published earlier (8, 11). We specifically addressed the breadth, phenotype, polyfunctionality, and longevity of the vaccine-elicited immune responses in order to provide insights into the immune protective potential of a homologous MVA-B vaccine regimen in humans.
MATERIALS AND METHODS
MVA-B vaccine.
The generation of MVA-B vector was previously described (11). It expresses simultaneously and under the same synthetic early/late viral promoter, monomeric gp120 as a cell-released product and Gag-Pol-Nef (GPN) as an intracellular polyprotein of 160 kDa. The gp120 Env protein comes from the HIV-1 primary isolate BX08. Gag-Pol-Nef is a fusion protein of 1,326 amino acids (aa), encoded by gag, pol, and nef open reading frames (ORFs) from HIV-1 clone IIIB, which has been modified to enhance its immunogenicity and for safety by removing undesirable domains. In both cases, the codon usage was adapted to highly express human genes. The good manufacturing production (GMP) clinical lots of MVA-B were produced by IDT (Germany) and kindly provided by EuroVacc. MVA-B was genetically stable, even when grown and purified at a large scale under GMP conditions as previously described (11).
Study design.
The RISVAC02 study was approved by the institutional ethical review board and by the Spanish Regulatory Authorities (government identifier NCT00679497). The study was explained to all patients in detail, and all signed written informed consent documents. A total of 30 HIV-1-negative, vaccinia-naïve volunteers, at two clinical sites in Madrid (HGM) and Barcelona (HC), were randomly allocated to receive three 1-ml injections of MVA-B (108 PFU/dose) (n = 24 subjects) or placebo (n = 6 subjects) by intramuscular route at weeks 0, 4, and 16. The duration of participant follow-up was 48 weeks.
Synthetic peptides.
All peptides used in this study were purified by high-pressure liquid chromatography (HPLC) (>80% purity) and provided by EuroVacc. Overlapping peptides (15-mers with 11 amino acids overlapping; n = 450) covered the entire Env, Gag, Pol, and Nef regions from clade B included in MVA-B. The BX08 gp120 protein (494 aa) was spanned by the Env-1 (aa 1 to 251; 60 peptides) and Env-2 (aa 241 to 494; 61 peptides) pools. The Gag-Pol-Nef fusion protein (1,326 aa) was spanned by the following pools: Gag-1 (aa 1 to 231; 55 peptides), Gag-2 (aa 221 to 431; 50 peptides), GPN-1 (aa 421 to 655; 56 peptides), GPN-2 (aa 645 to 879; 56 peptides), GPN-3 (aa 869 to 1,103; 56 peptides), and GPN-4 (aa 1,093 to 1,326; 56 peptides). For immunological analyses, we grouped the pools as follows: Env pool, Env-1 and Env-2; Gag pool, Gag-1 and Gag-2; and GPN pool, GPN-1, GPN-2, GPN-3, and GPN-4.
Cell preparation.
Whole-blood samples for analyses of the immune responses were collected in cell preparation tubes (CPT Vacutainer tubes; BD) and processed within 6 h, in accordance with the manufacturer's instructions. The yield and viability of peripheral blood mononuclear cells (PBMCs) were determined by trypan blue staining. Fresh PBMCs were used for the immunological analyses described in this study. The remaining cells were cryopreserved.
Flow cytometry analyses.
Fresh PBMCs (1 × 106 to 2 × 106) were stimulated during 6 h in complete RPMI 1640 medium containing 1 μl/ml GolgiPlug (BD Biosciences) and 5 μg/ml of the different HIV-1 peptide pools. When the antivaccinia response was assayed, the PBMCs were stimulated during 6 h in complete medium containing 1 μl/ml GolgiPlug (BD Biosciences) and autologous cells infected with MVA at 2 PFU/cell in a ratio of 10:1. For functional analyses, the following fluorochrome-conjugated antibodies were used: CD3-AmCyan; CD4-Alexa 700; CD8-PerCPCy5.5; gamma interferon (IFN-γ)-V450 or -PECy7; interleukin 2 (IL-2)- allophycocyanin (APC); tumor necrosis factor alpha (TNF-α)-PECy7; and MIP1β-phycoerythrin (PE). In addition, for phenotypic analyses, the following antibodies were used: CCR7-PE and CD45RA-fluorescein isothiocyanate (FITC). All antibodies were from BD Biosciences. At the end of the stimulation period, cells were stained for the surface markers, permeabilized (Cytofix/Cytoperm kit; BD Biosciences), and stained intracellularly using the appropriate fluorochromes. Cells were collected on an LSR II flow cytometer (BD Immunocytometry Systems). Analyses of the data were performed using FlowJo software version 8.5.3 (Tree Star, Ashland, OR). The number of lymphocyte-gated events ranged between 105 and 106. After gating, Boolean combinations of single functional gates were then created using FlowJo software to determine the frequency of each response based on all possible combinations of cytokine expression or all possible combinations of differentiation marker expression. Background responses detected in negative-control tubes were subtracted from those detected in stimulated samples for every specific functional combination.
Data analysis and statistics.
To correct measurements of the medium response (RPMI), we used a novel statistical approach previously described (8, 29). An intracellular cytokine stain (ICS) was considered positive if the percentages of cytokine-positive cells in the stimulated samples were 3 times more than the values obtained in the unstimulated controls and if the background-subtracted magnitudes were higher than 0.02%. The background for the different cytokines in the unstimulated controls never exceeded 0.015%. Each participant was classified as a responder if there was at least one positive IFN-γ ICS response against any of the HIV-1 peptide pools at weeks 6, 18, or 48 and as a nonresponder if responses at these weeks were all negative.
The magnitudes of the ICS responses and other continuous variables were compared between groups using the nonparametric Wilcoxon rank sum test and the Mann-Whitney U test. The differences among cumulative proportions have been tested by comparing two binomial distributions as described in reference 41 (implemented by the R function prop.test). For correlation analysis between variables, Pearson's correlation coefficient test was used.
The data analysis program Simplified Presentation of Incredibly Complex Evaluations (SPICE; version 4.1.5; M. Roederer, Vaccine Research Center, NIAID, NIH) was used to analyze and generate graphical representations of T cell responses detected by polychromatic flow cytometry. All values used for analyzing proportionate representation of responses are background subtracted.
RESULTS
Study design.
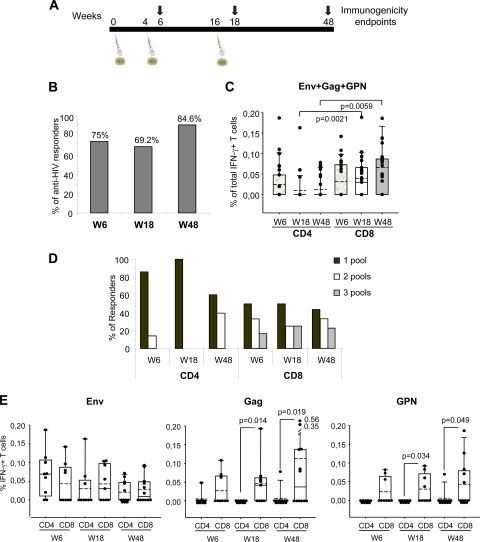
The main objective of this study was to characterize the magnitude, breadth, phenotype, function, and durability of the T cell responses induced by the single recombinant MVA-B vaccine administered in three doses in human healthy volunteers enrolled in the RISVAC02 phase I clinical trial in Spain. The MVA-B vaccine is a nonreplicating viral vector in human cells that expresses simultaneously the gp120 Env protein from the BX08 HIV-1 isolate as a cell-released product and Gag-Pol-Nef (GPN) from the IIIB HIV-1 isolate as an intracellular polyprotein (11). GPN has been engineered by the removal of immunosuppressed sequences and to prevent viruslike particle (VLP) formation. A total of 30 healthy, HIV-1-negative volunteers, naïve for the smallpox vaccine, were enrolled. The study was randomized and double-blinded with respect to active vaccine or placebo. The participants received three 1-ml injections of MVA-B (108 PFU/dose) intramuscularly in the deltoid at weeks 0, 4, and 16. The immune responses were evaluated at weeks 6, 18, and 48 by polychromatic intracellular cytokine staining (ICS) (Fig. 1A). This assay was done in 16 volunteers due to rapid availability of freshly isolated peripheral blood mononuclear cells (PBMCs) to ensure no loss of functional activity of T cells.
Fig. 1.
MVA-B-induced HIV-1-specific T cell responses across the study. (A) Chronological diagram showing the vaccination schedule followed in the RISVAC02 study and the immunogenicity endpoints. W6, week 6. (B) Percentage of responders at the different time points. The percentage of responders was calculated on the basis of volunteers with a positive IFN-γ ICS. (C) Magnitudes of vaccine-specific CD4+ and CD8+ T cells at the different time points. The mean values for the total responses (Env, Gag, and GPN) in each T cell population are shown. The box plots show the distribution of responses in positive responders only. The boxes indicate the median (solid line), mean (dashed line), and interquartile range (IQR). P values for significant differences were determined using the Mann-Whitney U test and are represented. (D) Breadth of CD4+ and CD8+ T cell responses at the different time points. Percentages of responders that recognized 1, 2, or 3 HIV-1 peptide pools in both T cell subsets are shown. (E) Percentages of CD4+ and CD8+ T cells producing IFN-γ in response to Env, Gag, or GPN peptide pools as measured by ICS at the different time points. The box plots show the distribution of responses in positive responders only. The boxes indicates the median (solid line), mean (dash line), and IQR. P values for significant differences were determined using the Wilcoxon rank sum test with continuity correction and are represented. All data are background subtracted.
Analyses of the demographics of the trial population and of the safety of the vaccine will be described elsewhere (F. García, J. C. López Bernaldo de Quirós, C. E. Gómez, B. Perdiguero, J. L. Nájera, V. Jiménez, J. García-Arriaza, A. C. Guardo, I. Pérez, V. Diaz-Brito, M. Sanchez Condes, N. Gonzólez, A. Alvarez, J. Alcamí, J. L. Jiménez, J. Pich, J. A. Arnaiz, M. J. Maleno, A. León, M. A. Muñoz-Fernández, P. Liljeström, J. Weber, G. Pantaleo, J. M. Gatell, M. Plana, and M. Esteban, unpublished data). No related serious adverse events occurred during the study, indicating that MVA-B was safe and well tolerated.
Vaccine-induced T cell responses.
Vaccine-induced T cell responses were assessed in 16 volunteers by ICS assay after the stimulation of freshly isolated PBMCs with a panel of 450 HIV-1 peptides (15-mers overlapping by 11 amino acids) grouped in three pools: Env (121 peptides), Gag (105 peptides), and GPN (224 peptides). The peptides encompassed the Env, Gag, Pol, and Nef proteins of HIV-1 and were designed based on the sequences of the immunogens expressed by MVA-B.
The response rates at weeks 6, 18, and 48 were determined for each T cell population based on the percentage of antigen-specific IFN-γ-positive cells. Cumulative analysis of the data demonstrated that MVA-B induced HIV-1-specific T cell responses that were balanced and significantly different from those determined in the placebo group (P = 0.04) (Table 1). CD4+ and CD8+ T cell responses to any HIV-1 peptide pool at any time postvaccination were detected in 69.2% (9/13) and 92.3% (12/13) of the vaccines, respectively. The CD4+ T cell responses were predominantly Env directed (Env, 69.2%; Gag, 15.4%; and GPN, 7.7%), whereas the CD8+ T cell responses were similarly distributed against the three peptide pools (Env, 61.5%; Gag, 69.2%; and GPN, 69.2%).
Table 1.
Vaccine responsiveness based on IFN-γ-positive ICS assay across the RISVAC02 study
| Vaccination group | Antigen | No. of responders/no. of total volunteers (%)a |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Wk 6 (2 wks after 2nd dose) |
Wk 18 (2 wks after 3rd dose) |
Wk 48 (30 wks after 3rd dose) |
Cumulative (any time after vaccination) |
||||||
| CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | ||
| MVA-B (108 PFU) | Env | 7/12 (58.3) | 4/12 (33.3) | 3/13 (23.1) | 5/13 (38.5) | 5/13 (38.5) | 5/13 (38.5) | 9/13 (69.2) | 8/13 (61.5) |
| Gag | 1/12 (8.3) | 3/12 (25.0) | 0/13 (0) | 5/13 (38.5) | 1/13 (7.7) | 6/13 (46.1) | 2/13 (15.4) | 9/13 (69.2) | |
| GPN | 0/12 (0) | 3/12 (25.0) | 0/13 (0) | 4/13 (30.8) | 1/13 (7.7) | 5/13 (38.5) | 1/13 (7.7) | 9/13 (69.2) | |
| Any | 7/12 (58.3) | 6/12 (50.0) | 3/13 (23.1) | 8/13 (61.5) | 5/13 (38.5) | 9/13 (69.2) | 9/13 (69.2) | 12/13 (92.3)c | |
| Placebo | Env | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) |
| Gag | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) | |
| GPN | 1/3b (33.3) | 1/3b (33.3) | 0/3 (0) | 0/3 (0) | 1/3b (33.3) | 0/3 (0) | 1/3b (33.3) | 1/3b (33.3) | |
| Any | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0/3 (0)c | |
An ICS was considered positive if the percentages of IFN-γ-positve cells in the stimulated samples were 3 times more than the values obtained in the unstimulated controls and if the background-subtracted magnitudes were higher than 0.02%. One volunteer at week 6 did not have data. Cumulative analysis represents a positive response at any time point postvaccination.
One placebo recipient was excluded for the cumulative analysis due to the reactivity against the GPN pool at baseline and at subsequent time points. For this reason, the GPN pool was excluded in the comparison of the cumulative responses between vaccinees and placebo groups.
The differences among cumulative proportions between vaccinees and placebo groups have been tested by comparing two binomial distributions (implemented by the R function prop.test).
The assessment of vaccine-induced T cell responses at different time points, determined as the rate of CD4+ and/or CD8+ responses to any HIV-1 antigen, indicated that the proportion of responders after 2 doses of MVA-B (week 6) was similar to that obtained after the third dose of MVA-B vaccination (week 18) (75% versus 69.2%) and was sustained by 32 weeks after the last immunization (84.6% at week 48) (Fig. 1B). The mean values for the total HIV-1 responses (Env, Gag, and GPN) in each T cell population are shown in Fig. 1C. For CD4+ T cells, both the magnitude and response rates peaked after 2 MVA-B doses, declining with time. The response rates to any antigen decreased from 58.3% at week 6 to 23.1% at week 18 and to 38.5% at week 48 (Table 1). For CD8+ T cells, magnitudes of responses and response rates were both higher than for CD4+ T cells, especially at weeks 18 and 48. The magnitudes of the responses remained similar during the study, as did response rates to any antigen (50% at week 6; 61.5% at week 18, and 69.2% at week 48) (Table 1). There was no significant correlation between the magnitude of the response for CD4+ and CD8+ T cells in individuals.
The CD4+ T cell response was essentially directed against 1 HIV-1 peptide pool (Env) at all time points assayed, with occasional recognition of 2 antigens (Env and Gag), whereas the CD8+ T cell response was broad and evenly distributed to 1, 2, or 3 HIV-1 peptide pools (Fig. 1D).
The cross-sectional responsiveness per antigen showed that Env response was mediated by both CD4 and CD8 T cell subsets, whereas the Gag and GPN responses were mediated mainly by the CD8 T cell population (Fig. 1E).
Functional profile of vaccine-induced CD4 and CD8 T cell responses.
The profiles of vaccine-induced CD4 and CD8 T cell responses were analyzed in those volunteers with IFN-γ-positive ICS responses. The polychromatic ICS assays were performed on fresh PBMCs 2 weeks after injection of the second (week 6) and third (week 18) dose of MVA-B vaccine. The panel of T cell functions analyzed included IL-2, TNF-α, MIP1β, and IFN-γ secretion. For each subpopulation, the background, as detected in the unstimulated control sample, was subtracted. Only responses exceeding a predefined threshold level after background subtraction were considered.
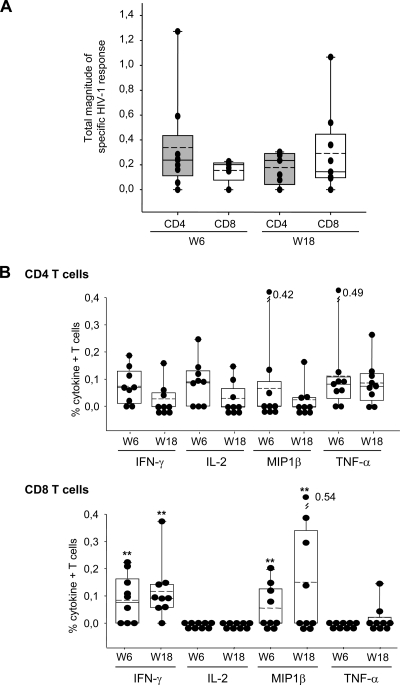
The mean values for the total responses (Env, Gag, and GPN) in each T cell population, considering the frequencies of all the cytokines, are represented in Fig. 2A. The magnitudes of the total HIV-1-specific responses were similar for both populations at the two time points. Among the cytokine-producing CD8+ T cells, IFN-γ and MIP1β predominate at both week 6 and week 18, whereas no single cytokine prevails in the CD4+ T cells at any time assayed (Fig. 2B). Representative functional profiles of vaccine-induced CD4 and CD8 T cell responses were shown for one of the responders at week 18 (see Fig. S1 in the supplemental material).
Fig. 2.
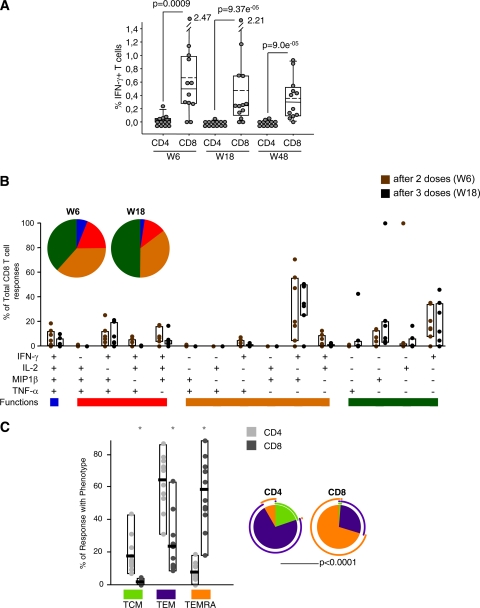
Vaccine-induced T cell responses at primary immunogenicity endpoints (weeks 6 and 18). (A) Magnitude of HIV-1-specific CD4+ and CD8+ T cells after two and three doses of MVA-B. The mean values for the total responses (Env, Gag, and GPN) in each T cell population are shown. The box plots show the distribution of responses in positive responders only. The boxes indicate the median (solid line), mean (dashed line), and interquartile range (IQR). (B) Percentages of HIV-1-specific T cells secreting cytokines in the CD4 and CD8 T cell subsets. The box plots show the distribution of responses in positive responders only. The boxes indicate the median (solid line), mean (dashed line), and IQR. Data points represent the sum of the frequencies obtained against the Env, Gag, and GPN peptide pools. All data are background subtracted. **, P values of <0.005 determined using the Wilcoxon rank sum test with continuity correction, comparing the secretions of the different cytokines at the same time points.
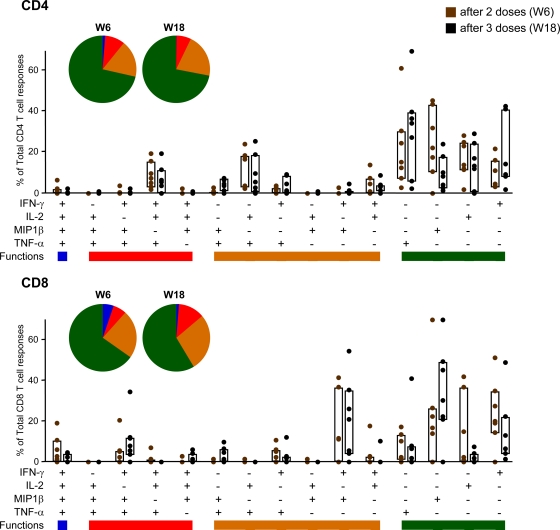
The quality of a T cell response can be characterized in part by the pattern of cytokine production. On the basis of the analysis of IL-2, TNF-α, MIP1β, and IFN-γ secretion, 15 distinct HIV-1-specific CD4+ and CD8+ T cell populations were identified (Fig. 3). Vaccine-induced CD4+ T cell responses at weeks 6 and 18 were represented mainly by cells expressing 1 function, although about 25% of CD4+ T cells exhibit two or three functions. In contrast to CD4+ T cells, vaccine-induced CD8+ T cells were more polyfunctional, with about 45% of vaccine-induced HIV-1-specific CD8+ T cells exhibiting more than one function (Fig. 3). In both subsets, there were no changes in the polyfunctional profile after the third dose of MVA-B.
Fig. 3.
Functional profile of vaccine-induced CD4 and CD8 T cells. The results shown are generated from the determinations in responders at weeks 6 and 18. All the possible combinations of the responses are shown on the x axis, whereas the percentage of the functionally distinct cell populations within the total CD4 and CD8 T cell populations are shown on the y axis. Responses are grouped and color coded on the basis of the number of functions. The bars correspond to the individual data points and interquartile ranges (IQR) after 2 (W6) or 3 (W18) doses of MVA-B. The pie charts showed the average proportion of the CD4 or CD8 vaccine-specific T cell responses according to the functions.
To determine whether polyfunctionality is a feature of an individual or of responses to particular antigens, we performed a two-way analysis of variance (ANOVA) (response as a function of the patient and the antigen) of the responses for CD8+ and CD4+ T cells after 2 (week 6) and 3 (week 18) doses. We found that all patients responded similarly (except one individual who is particularly polyfunctionally responsive for CD8+ after 2 doses). At week 6, we found significant differences (P < 0.05) between the polyfunctional response of CD8+ T cells to Env, Gag, and GPN and the polyfunctional response to MVA. Similarly, the polyfunctional response of CD4+ T cells to Env was significantly larger than that to GPN. The rest of the responses were not significantly different. At week 18, we did not find any difference between the polyfunctional responses of individuals and antigens at the level of CD8+ T cells. Moreover, when we determined if the magnitude or breadth of the response correlates with the polyfunctionality, we found only a positive correlation (0.78) between breadth and CD8+ polyfunctionality after 2 doses (week 6). Otherwise, these variables are not correlated (with Pearson's correlation coefficient test).
Phenotypic profile of long-lived memory HIV-1-specific T cell responses.
Phenotypic analysis of long-lived memory vaccine-induced T cell responses was carried out at 32 weeks after the last MVA-B immunization (week 48) by polychromatic ICS assay. Fresh PBMCs were stimulated with the HIV-1 peptide pools Env, Gag, and GPN for 6 h and stained with specific antibodies to identify T cell lineage (CD3, CD4, and CD8), responding cells (IL-2 and IFN-γ), as well as memory stages (CD45RA and CCR7).
At this time point, the HIV-1-specific response was mediated mainly by CD8+ T cells, although in 3 out of 11 responders (27.3%), it was mediated by both CD4+ and CD8+ T cells. A total of 55.6% of the responders at week 48 had specific CD8+ T cells against 2 or 3 pools, correlating with the individuals that secrete more IFN-γ (P < 0.05).
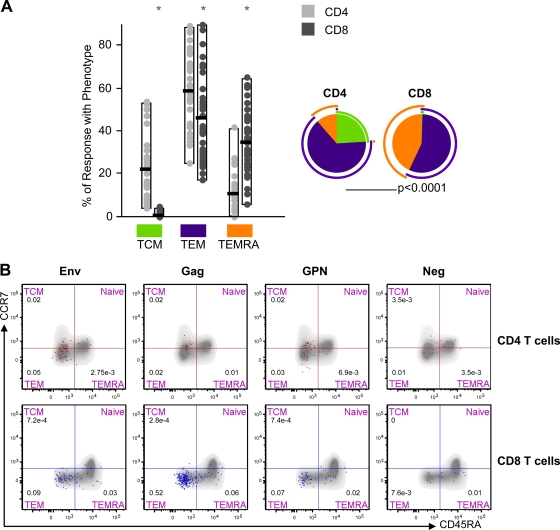
Since previous studies have shown that CD45RA and CCR7 define functionally distinct populations of memory antigen-specific T cells (2, 19, 37), we characterized the differentiation stages of the responding CD4 and CD8 T cells into central memory (TCM; CD45RA− CCR7+), effector memory (TEM; CD45RA− CCR7−), or terminally differentiated effector memory (TEMRA; CD45RA+ CCR7−) populations. For each vaccine, we summed the totality ([single IL-2 plus dual IL-2]/[IFN-γ plus single IFN-γ]) of Env-, Gag-, and GPN-specific T cell responses and determined for CD4 and CD8 T cell subsets the percentages of the specific responses with the TCM, TEM, or TEMRA phenotype (Fig. 4). The HIV-1-specific CD4+ T cell responses were distributed mainly within the TCM and TEM cell populations, whereas the CD8+ T cell responses were distributed mainly within the TEM and TEMRA cell populations (Fig. 4A). In both CD4 and CD8 T cell subsets, the higher numbers of cytokine-secreting cells were found within the TEM cell population. Figure 4B shows representative phenotypic profiles of long-lived memory HIV-1-specific T cells in one of the volunteers.
Fig. 4.
Phenotype of long-lived memory vaccine-induced T cell responses. (A) Distribution of HIV-1 antigen-specific T cells at week 48 based on CCR7 expression in combination with CD45RA. The bars correspond to the individual data points and interquartile ranges (IQR) of the CD4+ and CD8+ T cell responses against Env, Gag, and GPN with phenotype central memory (TCM; CD45RA− CCR7+), effector memory (TEM; CD45RA− CCR7−), or terminally differentiated effector memory (TEMRA; CD45RA+ CCR7−). The pie charts showed the average proportion of the CD4+ or CD8+ vaccine-specific T cell responses according to the memory phenotype. *, distributions that are different from the CD4 T cell subset at P values of <0.05 (Student's t test). All data are background subtracted. (B) Representative phenotypic profiles of long-lived memory HIV-1-specific CD4 and CD8 T cells. Fresh PBMCs obtained from the responder volunteers at week 48 were stimulated with Env, Gag, or GPN peptide pools. The red dots indicate antigen-specific (IL-2 and IFN-γ) vaccine-induced CD4+ T cells, and blue dots indicate antigen-specific (IL-2 and IFN-γ) vaccine-induced CD8+ T cells, both overlaid on the total T cell subsets (gray). Neg, background values in unstimulated cells.
Antivector T cell responses.
Vaccine-induced antivector T cell responses were assessed by ICS assay after the stimulation of freshly isolated PBMCs with autologous cells infected with MVA. The response rates at weeks 6, 18, and 48 were determined for each T cell population based on the percentages of MVA-specific IFN-γ-positive cells by following the same criteria described above. The analysis of antivector T cell responses at different time points, determined as the rate of CD4 and/or CD8+ responses to MVA-infected cells, indicated that the proportion of responders after 2 doses of MVA-B (week 6) was similar to that obtained after the third MVA-B vaccination (week 18) (83.3% versus 84.6%) and remained unchanged over time (91.7% at week 48) (see Fig. S2A in the supplemental material). There was no correlation between vector and HIV-1 antigen responses. None of the placebo recipients had a positive response against the vector. The responses were induced mostly by the CD8 T cells (Fig. 5 A) and were highly polyfunctional, with about 70% of MVA-specific CD8+ T cells displaying more than one function (Fig. 5B). The magnitudes and polyfunctionality of antivector CD8 T cell responses were maintained after the third dose of MVA-B. Representative functional profiles of the anti-MVA responses in one of the volunteers at week 18 are shown in Fig. S2B in the supplemental material. Although antivector CD8+ T cell responses appeared to be more polyfunctional than responses to the HIV-1 antigens, we have to take into consideration that the different assay systems used (one stimulated with peptide pools, the other with virus-infected cells) might influence the results and do not allow the direct comparison between the polyfunctional degree against the vector and that against the HIV-1 antigens.
Fig. 5.
Antivector-induced T cell responses across the study. (A) Percentages of CD4+ and CD8+ T cells producing IFN-γ against MVA-infected cells as measured by ICS at the different time points. The box plots show the distribution of responses in positive responders at weeks 6 and 18. The boxes indicate the median (solid line), mean (dashed line), and interquartile range (IQR). All data are background subtracted. P values for significant differences were determined using the Wilcoxon rank sum test with continuity correction and are represented. (B) Functional profile of MVA-specific CD8 T cells. The results shown are generated from the determinations in all the responders. All the possible combinations of the responses are shown on the x axis, whereas the percentages of the functionally distinct cell populations within the total CD8 T cell populations are shown on the y axis. Responses are grouped and color coded on the basis of the number of functions. The bars correspond to the individual data points and IQR after 2 (week 6 [W6]) or 3 (W18) doses of MVA-B. The pie charts show the average proportion of the MVA-specific CD8+ T cell responses according to the functions. (C) Phenotype of long-lived memory MVA-specific T cell responses. Distribution of MVA-specific T cells at week 48 based on CCR7 expression in combination with CD45RA. The bars correspond to the individual data points and IQR of the CD4+ and CD8+ T cell responses against MVA-infected cells with phenotype central memory (TCM; CD45RA− CCR7+), effector memory (TEM; CD45RA− CCR7−), or terminal effector memory (TEMRA; CD45RA+ CCR7−). The pie charts show the average proportions of the CD4+ or CD8+ MVA-specific T cell responses according to the memory phenotype. *, distributions that are different from the CD4 T cell subset at P values of <0.05 (Student's t test). All data are background subtracted.
To define whether strong responses to the vector at earlier times reduce the benefit of boosting for the HIV-1 antigens, we analyzed, using Pearson's correlation coefficient test, whether the antivector response at week 6 affects the anti-HIV-1 response at week 18, and we found that strong responses to the vector at earlier times do not reduce the benefit of boosting for the HIV-1 antigens (Pearson's coefficient, 0.348).
At week 48, the totality ([single IL-2 plus dual IL-2]/[IFN-γ plus single IFN-γ]) of MVA-specific CD4+ T responses was distributed mainly within the TEM cell population, whereas the CD8+ T cell responses were distributed mainly within the TEMRA cell population (Fig. 5C). Representative phenotypic profiles of long-lived memory MVA-specific T cells are shown in one of the volunteers (see Fig. S2C in the supplemental material).
DISCUSSION
At present, it remains unclear which elements of the immune system need to be stimulated to provide protection against HIV-1 infection and to improve viral control in already HIV-1-infected individuals. For this reason, HIV/AIDS vaccine development is currently directed toward the quantitative and qualitative improvements of vaccine-induced immune responses through the use of novel vectors administered either alone or in prime-boost heterologous combination. The modest efficacy and low-level immune responses of the RV144 Thai phase III trial based on the poxvirus vector ALVAC in combination with the protein gp120 (35) suggest that improved poxvirus vectors may be effective components of a realistic strategy for vaccination against HIV-1 infection.
We have previously described the generation and characterization of the MVA-B vaccine candidate against HIV/AIDS (11). MVA-B used alone, or in combination with DNA vectors expressing the same HIV-1 antigens, was able to induce in mice robust, polyfunctional, and durable T cell HIV-1-specific responses (8, 11). In macaques, a similar MVA construct expressing Env (gp120 from simian-human immunodeficiency virus strain 89.6P [SHIV89.6P]) and Gag-Pol-Nef (from simian immunodeficiency virus isolate mac239 [SIVmac239]) induced strong, specific CD4+ and CD8+ T cell immune responses with a bias for CD8+ and high protection after challenge with SHIV89.6P (28). Furthermore, expression of HIV-1 antigens from MVA-B selectively induced in human monocyte-derived dendritic cells (moDCs) the expression of different cellular genes that might act as regulators of immune responses to HIV-1 antigens (14), and MVA-B-infected moDCs cocultured with autologous T lymphocytes induced a highly functional HIV-1-specific CD8+ T cell response, including proliferation, secretion of IFN-γ, IL-2, TNF-α, MIP1β, MIP1α, RANTES, and IL-6, and strong cytotoxic activity against autologous HIV-1-infected CD4+ T lymphocytes (3). Based on these previous results, MVA-B was approved in Spain for a phase I clinical trial in healthy volunteers (RISVAC02).
The primary aim of this study was to characterize in detail the magnitude, breadth phenotype, function, and type of memory T cell responses induced by the recombinant MVA-B vaccine in participants enrolled in the RISVAC02 clinical trial. The availability of fresh PBMCs from 16 volunteers obtained at different times postimmunization made it possible to analyze directly the T cell profile in all of these samples, thus ensuring minimal loss of T cell functions. The analysis of the vaccine-induced T cell responses was performed by polychromatic ICS assay from PBMCs stimulated with a panel of peptide pools encompassing Env, Gag, Pol, and Nef HIV-1 antigens from clade B included in the MVA-B vector. Although the IFN-γ enzyme-linked immunosorbent spot assay (ELISPOT) is the best standardized assay used internationally for measuring HIV-1 vaccine-induced immune responses (1, 5, 13), it provided limited information on a spectrum of cytokine/chemokine profiles. To overcome the limitation of evaluating a single cytokine, novel techniques, such as the polychromatic ICS assay, are becoming increasingly more stringent in assessing HIV-1-specific immune responses in different clinical settings (4, 10, 16, 22). This assay provides simultaneous information on multiple markers measured at the single cell level, allowing a detailed characterization of the vaccine-specific T cell responses.
Here, we demonstrate that the vaccination regimen based on 3 doses of 108 PFU of MVA-B given intramuscularly is highly immunogenic, induces high frequencies of HIV-1-specific CD4+ and CD8+ T cells which are polyfunctional and have broad IFN-γ ICS reactivity, and, more importantly, induces long-lasting T cell immunity, activating a specific subset of memory T cell populations. The majority (12 out of 13 [92.3%]) of the volunteers immunized with MVA-B had a positive HIV-1-specific T cell response at any time postvaccination, detected by IFN-γ ICS assay. While direct comparison of overall response rates between MVA recombinants tested in clinical trials has to be taken with caution due to differences in the HIV-1-expressing cassette of the vectors, simple comparison with other stand-alone MVA-based HIV-1 vaccine products revealed that MVA-B appears to be an immunogen that is as good as or even better than MVA-CMDR (84.6%) (4), more immunogenic than MVA62 (43%) (10), and substantially more immunogenic than MVA-HIVA (0%) (21, 32). Other studies using the same immunization regimen, but with higher doses of MVA products, had reported similar or even lower response rates than those reported here. The use of ADMVA (40) and TBC-M4 (34) at 2.5-fold-higher doses than MVA-B gave response rates of 62% and 100%, respectively. Furthermore, after a third dose of 109 PFU of MVA-HIV, a response rate of 41.4% was reported (22). MVA-B was also more immunogenic than the related attenuated poxvirus vector NYVAC-C used in homologous combination (16, 26). Overall, the response rates assigned to MVA-B in comparison with other MVA-HIV-related vaccines provided strong support for the potential benefit of this vector as an HIV/AIDS vaccine candidate.
Considering the consensus that for an HIV/AIDS vaccine to be effective it should aim to trigger specific T cell immune responses with an immunogenic profile of a high frequency of CD4+ and CD8+ T cells and be polyfunctional and durable, the immunogenic characteristics of MVA-B described in this work fulfill these criteria. The HIV-1-specific T cell responses induced by the MVA-B vaccine were balanced, with CD4+ and CD8+ T cell responses detected in 69.2% and 92.3% of the vaccinees, respectively. The CD4+ T cell response peaked and then declined after the second dose of MVA-B and was directed almost entirely to Env, whereas the CD8+ T cell response slightly increased over time and was more evenly distributed between Env, Gag, and GPN antigens. These results were in line with the preclinical evaluation of MVA-B in mice (8, 11) and also with the results obtained in macaques by using an analogous MVA vaccine expressing gp120 from SHIV89.6P and Gag-Pol-Nef from SIVmac239 (28) but differed from studies by others that suggest that MVA-vectored constructs expressing multigenic products induced primarily a CD4+ T cell response (4, 10, 22). Using flow cytometry-based assays, Currier et al. reported that the Env antigen was consistently the predominant target of the cellular immune response, and CD4+ T cells were the most frequently detected responder cell type when using 108 PFU of MVA-CMDR (4). Using MVA62 in a homologous regimen, a 2.4-fold excess of CD4+ over CD8+ T cell responses was reported, with strong bias toward Gag (10). More recently, it was reported that after 3 doses of 109 PFU of MVA-HIV, there was a 3-fold excess of CD4+ over CD8+ responses, with the CD4+ T cell response more frequently directed at Gag than Env, and the CD8+ T cell response was directed entirely at Env (22). The divergences observed between the studies described above and our study must be attributed to the delivery format and the nature of the HIV-1 antigens expressed by the different vaccine candidates. The MVA-CMDR and MVA62 share similar features. In both recombinants, the truncated gp160 env gene was inserted into the deletion II, whereas the modified gag-pol gene was inserted into the deletion III. In addition, both viruses expressed the Env protein on the surface of the infected cells, while Gag and Pol antigens are produced as noninfectious viruslike particles (VLPs) (6, 10). On the other hand, MVA-HIV represents a mix of two different MVA recombinants, one expressing the structural env and gag genes and the other expressing the regulatory tat, rev, nef, and reverse transcriptase (RT) genes, all at different loci (22). Our MVA-B vaccine has inserted in the single viral TK locus the env and gag-pol-nef genes, and both are expressed in infected cells simultaneously, with the monomeric gp120 Env protein as a cell-released product, and Gag-Pol-Nef (GPN) as an intracellular polyprotein. The better stimulation of CD4 T cells in the previous studies might be related with the preferential activation of the exogenous pathway of antigen presentation by secreted products as VLPs or Env protein. In fact, in our study, almost all of the vaccine-induced CD4+ T cell response was directed against Env. The MVA-B-induced T cell responses against Gag and GPN antigens were mediated mainly by CD8+ T cells, and this might be related with the activation of the intrinsic pathway of antigen presentation by the Gag-Pol-Nef intracellular polyprotein. As we have reported, both the expression of gp120 and GPN by MVA-B on moDCs had an effect on host cellular functions. In fact, expression of HIV-1 proteins from moDCs infected with MVA-B induced the expression of cytokines, cytokine receptors, chemokine receptors, and molecules involved in antigen uptake and processing, including major histocompatibility complex (MHC) genes, whose products might act as regulators of immune responses to HIV-1 antigens (14). Moreover, MVA-B infection of moDCs stimulate a strong HIV-1 immune response, induced mainly by CD8+ T cell proliferation together with high secretion of CD8+ polyfunctional-related cytokines (3). Thus, the preferential induction of CD8+ T cells by MVA-B might be related to the intrinsic innate vector effects on target cells.
In addition to T cell responses, MVA-B also elicited a strong and durable Env-specific humoral response. Binding antibodies against HIV-1 gp160 from the LAV isolate were detected in 45.8% of the volunteers after the second MVA-B dose, while nearly all recipients (95.8%) tested positive by enzyme-linked immunosorbent assay (ELISA) after the third MVA-B dose. At 32 weeks after the last immunization, 72.7% of the vaccinees had detectable levels of anti-Env antibodies (F. García et al., unpublished). These results are comparable to the previous studies reported by Currier et al. (4) and Goepfert et al. (10) in which the anti-Env antibody responses peaked after the third dose of MVA-CMDR (90%) and MVA62 (86%).
In our vaccination scheme with MVA-B, the last boost was needed to enhance humoral HIV-1-specific responses in vaccinees and might also be important for increasing and maintaining the anti-Gag and anti-GPN CD8+ T cell responses. The ICS data correlated with the immune responses detected by IFN-γ ELISPOT in all volunteers included in the RISVAC02 study, whereas at early times the higher responses were detected against Env, and after the third dose of MVA-B these responses were against Gag and GPN (F. García et al., unpublished). Similar remarks were reported in the MVA62 study, although the specific responses were lost 6 months after the last dose (10). The induction of Gag responses in vaccinees could be favorable for a vaccine, since in the natural HIV-1 infection it has been reported that Gag-specific CD8+ T cell responses are associated with better control of HIV/AIDS disease in individuals with chronic HIV-1 infection (7, 9, 23). The HIV-1-specific CD4+ and CD8+ T cell responses induced by the MVA-B vaccine were polyfunctional, and both T cell subsets maintained similar functional profiles after 2 or 3 doses of the MVA-B vaccine. In this regard, several studies performed in the setting of HIV-1 infection have shown that polyfunctional T cell responses are associated with better clinical outcomes and protection from disease progression (20, 31, 33).
A critical component of the effectiveness of vaccines is their ability to induce long-lasting immunity. Here, we observed that 84.6% of volunteers have HIV-1-specific T cell responses at week 48. This response rate is higher than that reported in other studies using multigenic vaccines, such as MVA-CMDR (about 60%) (4), MVA62 (8%) (10), or TBC-M4, which although reporting 100% of responders after the third dose, point out that only few vaccinated individuals exhibited long-lasting responses (34). The T cell responses at week 48 were balanced and do not differ with the response obtained 2 weeks after the third booster. In our volunteers, the vaccine-induced CD4+ T cell populations had mainly TCM (CD45RA− CCR7+) or TEM (CD45RA− CCR7−) phenotypes, which correspond to cells with effector functions but also those with the ability to secrete IL-2 and endowed with proliferation capacity (2, 17, 19, 37). In the case of CD8+ T cells, the memory phenotypes were either TEM (CD45RA− CCR7−) or TEMRA (CD45RA+ CCR7−). The presence of both memory populations at 8 months after the last vaccination is an important consideration, since they have been implicated in the control of different virus infections. The presence of CD45RA+ CCR7− CD8 T cells has been found in controlled chronic virus infections, such as cytomegalovirus (CMV) and Epstein-Barr virus (EBV) (2, 18, 42), and a correlation between the percentage of this cell population and virus control has also been shown in HIV-1 infection (31). Moreover, the relevance of the effector memory T cells on the early control of highly pathogenic SIV was recently described (15).
As did others (33), we observed that the MVA-B vaccine also induced specific antivector immune responses mediated mainly by the CD8 T cells. The responses were highly polyfunctional, with about 70% of MVA-specific CD8+ T cells displaying more than one function. Significantly, the magnitude and polyfunctionality of antivector CD8+ T cell responses were maintained after the third dose of MVA-B and were durable, with a phenotype related with advanced stages of differentiation. The antivector memory responses were predominantly of TEM phenotype for CD4+ T cells and of TEMRA phenotype for CD8+ T cells.
In conclusion, this study revealed a number of significant findings on the immune profile of the MVA-B vector as an HIV/AIDS vaccine based on ICS data from human PBMCs. First, the vector MVA-B given alone is highly immunogenic, as over 90% of recipients responded to the vaccine; second, MVA-B induces broad HIV-1-specific T cell responses, comprising both CD4 and CD8 T cells, which were balanced after the third dose; third, the HIV-1-specific immune responses triggered by MVA-B were polyfunctional; fourth, MVA-B responses were maintained at least for 1 year in 85% of vaccinees, with HIV-1-specific memory T cells being of TEM and TEMRA phenotypes for CD8+ T cells; fifth, the antivector responses were largely polyfunctional, with a predominance of memory CD8+ T cells of TEMRA phenotype. This immune profile fulfils immune requirements as a promising HIV/AIDS vaccine candidate and supports the use of the MVA-B product into larger clinical trials, alone or combined with other HIV-1 immunogens, like DNA or proteins. Undoubtedly, the immune value of the MVA-B vaccine to impact the outcome of HIV-1 infection can be tested only in an efficacy trial.
Supplementary Material
ACKNOWLEDGMENTS
This investigation was supported by grants from FIPSE-360731/09, the Ministry of Science and Innovation (SAF2008-02036), and Foundation Botín of Spain (to M.E.).
We are grateful to EuroVacc Foundation for peptide pools of HIV-1 antigens from clade B. We thank Alexandre Harari for critical advice.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 24 August 2011.
REFERENCES
- 1. Bull M., et al. 2007. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J. Immunol. Methods 322:57–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Champagne P., et al. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410:106–111 [DOI] [PubMed] [Google Scholar]
- 3. Climent N., et al. Dendritic cells exposed to MVA-based HIV-1 vaccine induce highly functional HIV-1-specific CD8 T cell responses in HIV-1-infected individuals. PLoS One 6:e19644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Currier J. R., et al. 2010. Phase I safety and immunogenicity evaluation of MVA-CMDR, a multigenic, recombinant modified vaccinia Ankara-HIV-1 vaccine candidate. PLoS One 5:e13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dubey S., et al. 2007. Detection of HIV vaccine-induced cell-mediated immunity in HIV-seronegative clinical trial participants using an optimized and validated enzyme-linked immunospot assay. J. Acquir. Immune Defic. Syndr. 45:20–27 [DOI] [PubMed] [Google Scholar]
- 6. Earl P. L., et al. 2009. Design and evaluation of multi-gene, multi-clade HIV-1 MVA vaccines. Vaccine 27:5885–5895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frahm N., et al. 2004. Consistent cytotoxic-T-lymphocyte targeting of immunodominant regions in human immunodeficiency virus across multiple ethnicities. J. Virol. 78:2187–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garcia-Arriaza J., Najera J. L., Gomez C. E., Sorzano C. O., Esteban M. 2010. Immunogenic profiling in mice of a HIV/AIDS vaccine candidate (MVA-B) expressing four HIV-1 antigens and potentiation by specific gene deletions. PLoS One 5:e12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geldmacher C., et al. 2007. CD8 T-cell recognition of multiple epitopes within specific Gag regions is associated with maintenance of a low steady-state viremia in human immunodeficiency virus type 1-seropositive patients. J. Virol. 81:2440–2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goepfert P. A., et al. 2011. Phase 1 safety and immunogenicity testing of DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J. Infect. Dis. 203:610–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gomez C. E., et al. 2007. Head-to-head comparison on the immunogenicity of two HIV/AIDS vaccine candidates based on the attenuated poxvirus strains MVA and NYVAC coexpressing in a single locus the HIV-1BX08 gp120 and HIV-1(IIIB) Gag-Pol-Nef proteins of clade B. Vaccine 25:2863–2885 [DOI] [PubMed] [Google Scholar]
- 12. Gomez C. E., Najera J. L., Krupa M., Esteban M. 2008. The poxvirus vectors MVA and NYVAC as gene delivery systems for vaccination against infectious diseases and cancer. Curr. Gene Ther. 8:97–120 [DOI] [PubMed] [Google Scholar]
- 13. Gotch F., Holmes H., Imami N. 2005. The importance of standardisation of laboratory evaluations in HIV vaccine trials. Microbes Infect. 7:1424–1432 [DOI] [PubMed] [Google Scholar]
- 14. Guerra S., et al. 2010. Selective induction of host genes by MVA-B, a candidate vaccine against HIV/AIDS. J. Virol. 84:8141–8152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hansen S. G., et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473:523–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harari A., et al. 2008. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J. Exp. Med. 205:63–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harari A., et al. 2006. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol. Rev. 211:236–254 [DOI] [PubMed] [Google Scholar]
- 18. Harari A., Vallelian F., Meylan P. R., Pantaleo G. 2005. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J. Immunol. 174:1037–1045 [DOI] [PubMed] [Google Scholar]
- 19. Harari A., Vallelian F., Pantaleo G. 2004. Phenotypic heterogeneity of antigen-specific CD4 T cells under different conditions of antigen persistence and antigen load. Eur. J. Immunol. 34:3525–3533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heeney J. L., Plotkin S. A. 2006. Immunological correlates of protection from HIV infection and disease. Nat. Immunol. 7:1281–1284 [DOI] [PubMed] [Google Scholar]
- 21. Jaoko W., et al. 2008. Safety and immunogenicity of recombinant low-dosage HIV-1 A vaccine candidates vectored by plasmid pTHr DNA or modified vaccinia virus Ankara (MVA) in humans in East Africa. Vaccine 26:2788–2795 [DOI] [PubMed] [Google Scholar]
- 22. Keefer M. C., et al. 2011. A phase I trial of preventive HIV vaccination with heterologous poxviral-vectors containing matching HIV-1 inserts in healthy HIV-uninfected subjects. Vaccine 29:1948–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kiepiela P., et al. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13:46–53 [DOI] [PubMed] [Google Scholar]
- 24. Kutscher S., et al. 2010. MVA-nef induces HIV-1-specific polyfunctional and proliferative T-cell responses revealed by the combination of short- and long-term immune assays. Gene Ther. 17:1372–1383 [DOI] [PubMed] [Google Scholar]
- 25. Mascola J. R., Montefiori D. C. 2010. The role of antibodies in HIV vaccines. Annu. Rev. Immunol. 28:413–444 [DOI] [PubMed] [Google Scholar]
- 26. McCormack S., et al. 2008. EV02: a phase I trial to compare the safety and immunogenicity of HIV DNA-C prime-NYVAC-C boost to NYVAC-C alone. Vaccine 26:3162–3174 [DOI] [PubMed] [Google Scholar]
- 27. McElrath M. J., Haynes B. F. 2010. Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity 33:542–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mooij P., et al. 2008. Differential CD4+ versus CD8+ T-cell responses elicited by different poxvirus-based human immunodeficiency virus type 1 vaccine candidates provide comparable efficacies in primates. J. Virol. 82:2975–2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Najera J. L., Gomez C. E., Garcia-Arriaza J., Sorzano C. O., Esteban M. 2010. Insertion of vaccinia virus C7L host range gene into NYVAC-B genome potentiates immune responses against HIV-1 antigens. PLoS One 5:e11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pantaleo G., Esteban M., Jacobs B., Tartaglia J. 2010. Poxvirus vector-based HIV vaccines. Curr. Opin. HIV AIDS 5:391–396 [DOI] [PubMed] [Google Scholar]
- 31. Pantaleo G., Koup R. A. 2004. Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat. Med. 10:806–810 [DOI] [PubMed] [Google Scholar]
- 32. Peters B. S., et al. 2007. Studies of a prophylactic HIV-1 vaccine candidate based on modified vaccinia virus Ankara (MVA) with and without DNA priming: effects of dosage and route on safety and immunogenicity. Vaccine 25:2120–2127 [DOI] [PubMed] [Google Scholar]
- 33. Precopio M. L., et al. 2007. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J. Exp. Med. 204:1405–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ramanathan V. D., et al. 2009. A phase 1 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 subtype C-modified vaccinia Ankara virus vaccine candidate in Indian volunteers. AIDS Res. Hum. Retroviruses 25:1107–1116 [DOI] [PubMed] [Google Scholar]
- 35. Rerks-Ngarm S., et al. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220 [DOI] [PubMed] [Google Scholar]
- 36. Ross A. L., Brave A., Scarlatti G., Manrique A., Buonaguro L. 2010. Progress towards development of an HIV vaccine: report of the AIDS Vaccine 2009 Conference. Lancet Infect. Dis. 10:305–316 [DOI] [PubMed] [Google Scholar]
- 37. Sallusto F., Geginat J., Lanzavecchia A. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22:745–763 [DOI] [PubMed] [Google Scholar]
- 38. Sandstrom E., et al. 2008. Broad immunogenicity of a multigene, multiclade HIV-1 DNA vaccine boosted with heterologous HIV-1 recombinant modified vaccinia virus Ankara. J. Infect. Dis. 198:1482–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sutter G., Staib C. 2003. Vaccinia vectors as candidate vaccines: the development of modified vaccinia virus Ankara for antigen delivery. Curr. Drug Targets Infect. Disord. 3:263–271 [DOI] [PubMed] [Google Scholar]
- 40. Vasan S., et al. 2010. Phase 1 safety and immunogenicity evaluation of ADMVA, a multigenic, modified vaccinia Ankara-HIV-1 B′/C candidate vaccine. PLoS One 5:e8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wilson E. 1927. Probable inference, the law of succession, and statistical inference. J. Am. Stat. Assoc. 22:209–212 [Google Scholar]
- 42. Zimmerli S. C., et al. 2005. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl. Acad. Sci. U. S. A. 102:7239–7244 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.