Abstract
Measles virus (MV) is still an imposing threat to public health. The matrix (M) protein has been shown not only to function as a structure block in the assembled MV virions, but also to regulate viral RNA synthesis, playing an important role in MV's replication and assembly. In the present study, we generated a panel of IgG monoclonal antibodies (MAbs) against M protein and successfully obtained one IgA MAb (5H7) from the IgG panel. Employing the polarized Vero cells grown in the two-chamber transwell model, we investigated whether M-specific 5H7 IgA MAb could suppress MV's replication and assembly. The data presented indicate that, while failing to show the activities of traditional neutralization and immune exclusion, M-specific IgA MAb was able to effectively inhibit viral replication by intracellular neutralization (78%), supporting the notion that the M protein is important for MV assembly and replication and implying that the M protein was an effective target antigen. The data also showed that MV had a long entry and assembly phase during viral replication, providing an extended window for IgA intervention. The colocalization of M proteins and M-specific 5H7 IgA MAbs demonstrated that the intracellular neutralization was due to the direct binding of the M-specific 5H7 IgA MAbs to the M proteins. In summary, the present study has added another example showing that IgA antibodies targeting internal viral antigens could proactively participate in mucosal immune protection by intracellular neutralization and has provided evidence that M protein might be included as a target antigen in future MV vaccine design.
INTRODUCTION
Measles is a highly contagious and infectious disease that causes rash, respiratory symptoms, and fever and results in death due to severe complications, such as pneumonia and encephalitis (29). Measles was the third most common cause of death among children <5 years of age in developing countries (7). It was estimated that in 2008 alone there were 20 million cases of measles worldwide and 164,000 related deaths (32). Because of the success of the measles vaccine program, measles was declared to be eliminated from the United States in 2000 (15) and from the World Health Organization (WHO) Region of the Americas in 2002 (22). However, measles is one of the most communicable of all infectious diseases, exhibiting an extraordinary propensity to reach susceptible individuals even when they constitute only a small proportion of the population (7). It is no surprise that even in industrialized countries measles can be severe, with at least 1 case among every 1,000 proving fatal (8). From 2001 to 2008, a total of 557 confirmed cases of measles and 38 outbreaks were reported in the United States (23). Furthermore, some high-risk target groups (e.g., very young infants) cannot be effectively immunized with currently licensed measles vaccines, highlighting the need for development of new vaccines (24). Therefore, measles is still an imposing threat to public health, demanding vigorous research.
Measles virus (MV), the causative agent of measles, is an enveloped virus belonging to the genus Morbillivirus in the family Paramyxoviridae; it has a nonsegmented negative-strand RNA virus genome encoding six structural proteins, designated nucleoprotein (N), phosphoprotein (P), polymerase (L), matrix (M) protein, receptor binding hemagglutinin (H), and fusion (F) protein (9). The genome is encapsidated by the N protein, forming a nucleocapsid with helical symmetry (9). The M protein has been shown to be involved in the various aspects of MV replication. The M protein might play the role of a mediator and facilitator in virion assembly by associating with the inner surface of the plasma membrane (11, 26) and directly interacting with the cytoplasmic domain of the H and F glycoproteins (3, 4, 28, 31) and the ribonucleoprotein (RNP) complex (10, 30). The M protein also downregulates viral RNA synthesis by interacting with the nucleocapsid protein (14), and MV transcription levels were increased by a small interfering RNA against M mRNA (25). Moreover, the matrix protein also modulates the fusogenic capacity of the viral envelope glycoproteins and interacts with the glycoproteins for intrinsic sorting and apical virion release (21, 27).
During infection, MV, like most viruses, enters the body through mucosal surfaces, replicates within epithelial cells, and then spreads to surrounding cells and tissues (9). The mucosal immune system provides the initial immunological barrier against most viral infections, and immunoglobulin A (IgA) is thought to mediate specific defense functions, which mostly depend on polymeric immunoglobulin receptor (pIgR)-mediated endocytosis at the basolateral surfaces of epithelial cells and subsequent transcytosis (6, 16, 20). Mazanec et al. first demonstrated in polarized epithelial cells in vitro that IgA specific for the hemagglutinin-neuraminadase (HN) protein of Sendai virus inhibited viral replication by intracellular neutralization (19). More reports of IgA-mediated intracellular neutralization include the IgA antibodies specific against the hemagglutinin (HA) protein of influenza virus (18); the H, F, and N proteins of measles virus (34); the VP6 protein of rotavirus (5); and the gp160, Gag, and RT proteins of HIV (13, 33).
We have previously demonstrated in the measles virus model that IgA antibodies have multiple functions and that IgA antibodies specific for the H, F, and N proteins were effective in inhibiting viral replication via intracellular neutralization (34). Since the M protein plays a critical role in MV replication, we attempted to investigate whether IgA antibody specific against the M protein was able to inhibit MV replication and, if so, what the mechanisms were. Our results showed that the M protein was an effective target for IgA-mediated inhibition during viral replication. The importance and application of this study are also discussed.
MATERIALS AND METHODS
Cells and viruses.
Vero C1008 (ATCC CRL 1587) (Vero), an African green monkey kidney cell line, was obtained from the American Type Culture Collection (ATCC) (Manassas, Va.). Vero cells were grown in Dulbecco's modified Eagle medium supplemented with 10% inactivated fetal bovine serum and 1% penicillin-streptomycin. A modified Vero cell line expressing pIgR constitutively was designated Vero-IgR (12, 34) and was also cultured under the same conditions as for Vero cells.
The Edmonston strain of measles virus was obtained from the ATCC and propagated in Vero cells, and its titer was assessed by a plaque assay on Vero cell monolayers.
Animals.
Six- to 8-week-old female BALB/c mice were purchased from the Centre of Disease Control (CDC) of Hubei Province, China. All mice were handled according to the animal regulations of China.
Production and purification of monoclonal IgG and IgA antibodies.
A panel of murine monoclonal IgG antibodies against the matrix protein of MV was generated by the conventional fusion method using expressed matrix protein as an antigen. The full length of the M gene from the Edmonston strain of measles virus (3406 to 4871 of GenBank accession no. AF266288.2) was amplified by PCR with primers designed with cloning restriction enzyme sites introduced into the amplified M DNA fragments, and the resultant fragments were cloned into the pET30 plasmid vector (Invitrogen) according to the manufacturer's instructions. All plasmids contained a 6-His tag at the C termini of recombinant proteins. All recombinant plasmids were transformed into competent Escherichia coli BL21(DE3), selected, and then confirmed by DNA sequencing (Invitrogen). The fusion proteins were prepared and purified by affinity chromatography on an Ni-nitrilotriacetic acid (NTA) column (Qiagen). One IgA antibody (5H7-IgA) was successfully obtained by repeated cycles of limiting dilution and spontaneous isotype switching from one IgG antibody (5H7-IgG). 5H7-IgA and 5H7-IgG shared the same antigen specificity, as verified by competitive enzyme-linked immunosorbent assay (ELISA) (data not shown). A corresponding pair of murine anti-hemagglutinin IgA/IgG antibodies (16CD11-IgA and 16CD11-IgG) was described previously (34). One IgA monoclonal antibody (MAb) against HIV gp120 was a gift from Y. T. Huang (Case Western Reserve University, OH). All hybridoma cells were grown in RPMI 1640 with 10% inactivated fetal bovine serum and 1% penicillin-streptomycin.
Six- to 8-week-old female BALB/c mice were primed by celiac injection of paraffin 2 weeks before the production of ascites. Hybridoma cells were celiac injected into the primed mice, and ascites were drawn out after 8 to 14 days. SiO2 was used to remove lipid from the ascites, and then rProtein G Agarose was used to purify IgG antibodies and the MEP (mercapto-ethtyl-pyridine) HyperCel was used to purify IgA antibodies. The purity and specificity of the purified IgG and IgA antibodies were verified by SDS-PAGE, ELISA, Western blotting, and immunofluorescence staining (data not shown).
Measles virus replication and IgA transcytosis in a transwell system model.
The setup of the transwell system model followed our previous procedures (34). Briefly, Vero-IgR cells were cultured on tissue culture-treated 0.4-μm-pore-size Transwell polyester membranes (Costar Corp., Cambridge, MA). Polarization of monolayer cells was verified by monitoring the electrical resistance between the upper and lower chambers with a Millicell resistance system (Millipore Corp., Bedford, MA). Polarized Vero-IgR cell monolayers had resistances of 50 to 75 Ω per cm2 and were infected by measles virus at a multiplicity of infection (MOI) of 1; after removing the unattached viruses by three washes, the cells were cultured in fresh medium. Apical and basal supernatants and cell lysates were collected at 6, 12, 18, 24, 30, 36, 42, and 48 h postattachment, and virus titers were assessed by a plaque assay. Cell lysates were prepared by scraping the cells in medium, freeze-thawing them three times, and centrifuging them at 2,000 × g for 20 min to remove cellular debris.
The transcytosis of IgA antibodies across a polarized Vero or Vero-IgR monolayer was tested in the two-chamber transwell system by adding 20 μg of IgA or IgG antibody in 100 μl of medium into the basal chamber and quantifying the transported IgA antibodies in the apical chamber at 6, 12, 18, 24, 30, 36, 42, and 48 h after the application of antibodies (34).
Traditional virus neutralization.
About 100 PFU MV virions in 100 μl medium was mixed with 100 μl medium containing 3 or 10 μg of 5H7-IgA, 5H7-IgG, 16CD11-IgA, 16CD11-IgG, or irrelevant IgA or 100 μl medium alone and incubated at 37°C for 2 h. The mixtures were used to infect unpolarized Vero cell monolayers, and virus titers were determined by a plaque assay (34).
Blocking measles virus infection at the apical surface by IgA antibody (immune exclusion).
The apical chambers of the polarized Vero-IgR cells grown in transwells were each supplemented with 80 μg of 5H7-IgA, 5H7-IgG, 16CD11-IgA, 16CD11-IgG, or Irrelevant IgA (anti-HIVgp41) and then infected by MV at an MOI of 1. Medium alone was included as a control. The unadsorbed viruses were removed by three washes, followed by addition of fresh medium. Forty-eight hours after virus attachment, the apical and basal supernatants and cell lysates were collected, and virus titers were assessed by a plaque assay. Cell lysates were prepared by scraping the cells in medium, freeze-thawing them three times, and centrifuging them for 20 min at 2,000 × g to remove cellular debris.
Intracellular neutralization.
The two-chamber transwell system with Vero-IgR cells was set up as described above. The apical surfaces of the monolayer cells were infected with MV at an MOI of 1, and the unadsorbed viruses were removed after 2 h by washing three times followed by addition of fresh medium. Twenty micrograms of 5H7-IgA, 5H7-IgG, or irrelevant anti-HIV gp41 IgA was then added to each of the basal chambers. Medium alone was included as a negative control. The apical and basal supernatants and cell lysates were prepared 48 h after initial exposure. Virus titers were quantified by a plaque assay and expressed as PFU/well, and the percentage of virus reduction was calculated over the negative control.
Immunofluorescent staining and confocal microscopy.
The two-chamber transwell system with Vero-IgR cells was set up as described above. The polarized Vero-IgR cell monolayers grown on polyester membranes were either infected with measles virus at an MOI of 1 or mock infected via the apical surfaces. Two hours later, 20 μg of 5H7-IgA or 5H7-IgG antibody was added to the basal chambers. Irrelevant anti-HIVgp41 (D61-IgA) or medium alone was included as a control. At 24, 36, or 48 h after initial infection, the membrane-attached cells were fixed without disruption in 2% paraformaldehyde in phosphate-buffered saline (PBS) (pH 7.4), permeabilized with 0.1% Triton X-100 in PBS, and washed with PBS containing 1% bovine serum albumin. Two-color immunofluorescence was used to detect M protein and IgA or IgG antibodies simultaneously. IgA was localized with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgA (Southern Biotechnology Inc., Birmingham, AL). M protein was localized with another anti-M IgG MAb (8H3), followed by Alexa Fluor (R) 555 goat anti-mouse IgG (Invitrogen). A Zeiss model 510 laser scanning confocal microscope with a 63× (numerical aperture, 1.4) Planapochromat oil immersion objective lens (Zeiss, Thornwood, NY) was used for all experiments. Confocal images of FITC fluorescence were collected with a 488-nm excitation light from an argon laser, a 488-nm dichroic mirror, and a 500- to 550-nm band-pass barrier filter. Images of Alexa Fluor 555 (R) fluorescence were collected with 543-nm excitation from an He/Ne laser, a 543-nm dichroic mirror, and a 560-nm-long pass filter.
Statistical analysis.
Statistical analysis was performed with a two-tailed t test by using the computer-fitting program Prism (GraphPAD Software Inc., San Diego, CA). The 5% confidence limit was adopted as the criterion for statistical significance.
RESULTS
Temporal characteristics of measles virus replication in polarized epithelial cells in vitro in the two-chamber transwell model.
MV was used as a model virus to study the functions of IgA antibodies in our previous studies (34). While MV replication in polarized cells has been studied (1), the time course of MV replication in the polarized Vero cells has not been studied in detail yet. In order to gain more insight into MV replication in the polarized Vero cells, we first grew Vero-IgR cells into a polarized monolayer on the 0.4-μm-pore-size membrane in the two-chamber transwell model; infected the polarized monolayer with MV virions at the apical surface; and then collected apical and basal supernatants and cell lysates at 6, 12, 18, 24, 30, 36, 42 and 48 h after virus attachment. The virus titers in the collected samples were assessed by a plaque assay (data not shown). The replication of MV showed three distinct temporal phases: (i) an entry stage of 0 to 12 h with initial low titers from the original viruses and then the disappearance of any detectable virus titers, (ii) an assembly stage of 12 to 36 h without viruses released into the apical supernatants even though the infectious virions were first detected in cell lysates at 24 h, and (iii) a release stage of 36 to 48 h with the assembled viruses being released into the apical supernatants. The experiments were terminated at 48 h, when the monolayers started to lose their integrity due to infection. In addition, the virus titers in the apical supernatants were much lower than those of cell lysates, implying that the virus titers when cell lysates were used would not be significantly affected by the inclusion of the apical supernatants; therefore, some of the experiments assessed the virus titers of combined apical supernatant and cell lysate samples. Furthermore, in line with our previous studies (34), no virus titer was detected in the basal supernatants throughout the culture period. In summary, the replication of MV had distinctive temporal characteristics of long entry and assembly stages and showed low release of virions onto basolateral surfaces; these features might provide an extended window for intracellular neutralization by IgA antibodies.
Anti-M IgA and IgG MAbs have no traditional neutralization activity against measles virus.
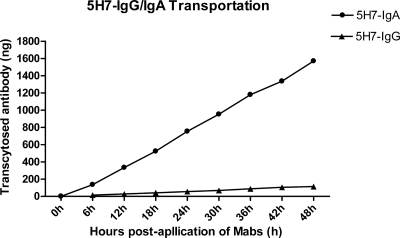
We first generated a panel of murine anti-M IgG monoclonal antibodies using the conventional fusion method. Among these, only one IgA antibody (5H7-IgA) was successfully obtained by limited dilution and screening of spontaneous isotype switch clones from one IgG MAb (5H7-IgG); they shared the same antigenic specificity, as verified by competitive ELISA and Western blotting (data not shown). Large preparations of 5H7-IgG and 5H7-IgA antibodies were made from ascites, and their purity was verified by SDS-PAGE (data not show). Furthermore, the transcytosis of 5H7-IgA and 5H7-IgG was tested across the polarized Vero or Vero-IgR cell monolayer in the transwell model. Both 5H7-IgG and 5H7-IgA showed no detectable transport in the polarized Vero monolayer (data not shown). In contrast, 5H7-IgA was readily transported across the Vero-IgR monolayer from the basolateral to the apical compartments in a time-dependent manner, but 5H7-IgG showed no detectable transport in the Vero-IgR monolayer (Fig. 1). This indicated that the transcytosis of 5H7-IgA was mediated by pIgR.
Fig. 1.
Transcytosis of 5H7-IgA and 5H7-IgG antibodies across polarized Vero-IgR monolayers. In the two-chamber transwell system with polarized Vero-IgR monolayers, 20 μg of 5H7-IgA or 5H7-IgG antibodies in 100 μl of medium was added to the basal chamber, and the transported IgA antibodies in the apical chamber were quantified at 6, 12, 18, 24, 30, 36, 42, and 48 h after the application of antibodies.
We next examined whether the anti-M MAb 5H7-IgA could reduce the infectivity of measles virus by traditional neutralization. MV virions with about 100 PFU were mixed with one of the antibodies 5H7-IgG (anti-M), 5H7-IgA (anti-M), 16CD11-IgG (anti-H), and 16CD11-IgA (anti-H) and one irrelevant IgA MAb (anti-HIVgp41) at a concentration of 15 μg/ml or 30 μg/ml; the irrelevant IgA MAb and medium alone were used as the negative controls. The mixtures were then applied to the Vero cell monolayers, and the plaques were counted (Table 1). As expected, the anti-H MAb pair (16CD11-IgA and 16CD11-IgG) showed significant neutralization activity, with 54 to 91% PFU reductions. In contrast, both anti-M MAbs (5H7-IgA and 5H7-IgG) failed to cause any significant PFU reduction, as did the irrelevant IgA MAb and medium alone. Therefore, anti-M IgA MAb (5H7-IgA) and IgG MAb (5H7-IgG) had no traditional neutralization activity against measles virus.
Table 1.
Traditional neutralization activities of anti-M and anti-H IgA and IgG MAbsa
| MAb | Specificity | 15 μg/ml MAb |
30 μg/ml MAb |
||
|---|---|---|---|---|---|
| Virus titer (PFU/well) | % Reduction of virusb | Virus titer (PFU/well) | % Reduction of virusb | ||
| 5H7-IgA | Anti-M | 34.00 ± 0.58 | 0.00 | 35.00 ± 2.31 | 0.00 |
| 5H7-IgG | Anti-M | 32.00 ± 0.58d | 4.48 | 36.50 ± 0.87 | 0.00 |
| 16CD11-IgA | Anti-H | 7.50 ± 0.29c | 77.61 | 3.00 ± 0.58c | 91.04 |
| 16CD11-IgG | Anti-H | 14.50 ± 0.87c | 56.72 | 13.00 ± 0.58c | 61.19 |
| Irrelevant IgA | Anti-HIV gp41 | 30.50 ± 1.44d | 8.96 | 31.00 ± 1.15d | 7.46 |
| No MAb | 33.50 ± 0.87 | 0.00 | 33.50 ± 0.87 | 0.00 | |
Experiments were repeated on at least two occasions with similar results. The results of a representative experiment are shown. The data are means ± standard errors of the mean (SEM) (n = 3).
Compared to no-antibody control.
Mean virus titers were significantly lower (P ≤ 0.0001) than those from cells exposed to no antibody.
Mean virus titers were not significantly lower (P ≥ 0.05) than those from cells exposed to no antibody.
Anti-M IgA and IgG MAbs failed to inhibit MV replication via immune exclusion.
Immune exclusion is one well-established mechanism by which antibodies inhibit viral replication (34). We tested whether anti-M IgA and IgG MAbs could inhibit MV replication via immune exclusion. In the two-chamber transwell model with Vero-IgR cell monolayers, the apical chamber was first supplemented with one of the antibodies 5H7-IgG (anti-M), 5H7-IgA (anti-M), 16CD11-IgG (anti-H), and 16CD11-IgA (anti-H) and one irrelevant IgA MAb (anti-HIVgp41) at a concentration of 80 μg/well and then with MV virions at an MOI of 1. After 2 h, the apical chamber was washed to remove unattached viruses and refilled with normal medium. Forty-eight hours later, the cell lysates and the apical and basal media were collected, and the virus titers were quantified by a plaque assay (Table 2). No significant virus reduction was observed in the groups of anti-M IgA (5H7-IgA) and IgG (5H7-IgG) treatments (P ≥ 0.05), while the anti-H IgA (16CD11-IgA) and IgG (16CD11-IgG) groups showed significant virus reduction (P ≤ 0.01). Therefore, both the anti-M IgA and IgG MAbs did not have immune exclusion activity.
Table 2.
Effects of blocking viral infection via immune exclusion by MAbs added at apical surfacesa
| MAb (80 μg in 480 μl) | Specificity | Virus titer (PFU/well) | % Reduction of virusb |
|---|---|---|---|
| 5H7-IgA | Anti-M | 9,750.00 ± 866.03d | 6.02 |
| 5H7-IgG | Anti-M | 8,000.00 ± 450.69d | 22.89 |
| 16CD11-IgA | Anti-H | 560.00 ± 45.48c | 94.60 |
| 16CD11-IgG | Anti-H | 1,437.50 ± 76.04c | 86.14 |
| Irrelevant IgA | Anti-HIVgp41 | 9,860.00 ± 54.06d | 4.96 |
| Medium alone | 10,375.00 ± 661.44 | 0.00 |
Experiments were repeated on at least three occasions with similar results. The results of a representative experiment are shown. The data are means ± SEM (n = 3).
Compared to no-antibody control.
Mean virus titers were significantly lower (P ≤ 0.01) than those from cells exposed to no antibody.
Mean virus titers were not significantly lower (P ≥ 0.05) than those from cells exposed to no antibody.
Anti-M IgA MAb inhibited MV replication via intracellular neutralization.
One unique feature of IgA antibodies is that in polarized cells they undergo transcytosis, during which IgA antibodies can interact with internal antigens inside the cells and thus exert inhibitory effects on viral replication (34). Since M protein is an internal antigen and is present in the cytoplasm during viral replication, we investigated whether the anti-M IgA MAb had intracellular-neutralization activity. In the two-chamber transwell model with Vero-IgR cell monolayers, the apical surfaces of the cells were infected with MV at an MOI of 1, and 2 h later, 5H7-IgA, 5H7-IgG, and irrelevant anti-HIVgp41 IgA antibodies were added to the basal chambers. Forty-eight hours later, the apical and basal supernatants and cell lysates were obtained, and their virus titers were quantified (Table 3). The viruses presented in the basal supernatants were none or minimal. For the sake of simple presentation, the virus titer is shown as the total number of viruses in a well. The results showed that 5H7-IgA was a highly effective inhibitor of MV replication; 5H7-IgA reduced the virus titer by 78.02% compared with the negative control (P ≤ 0.0001), while 5H7-IgG and irrelevant anti-HIVgp41 IgA failed to cause any reduction of virus titers (0%). Furthermore, none of the antibodies inhibited MV replication in Vero cells, further demonstrating that pIgR mediates the intracellular neutralization of IgA antibodies.
Table 3.
Intracellular neutralization of virus by anti-M IgA antibodya
| Cell and antibody (20 μg in 120 μl) | Vero-IgR |
Vero |
||
|---|---|---|---|---|
| Virus titer (PFU/well) | % Reduction of virusb | Virus titer (PFU/well) | % Reduction of virusb | |
| 5H7-IgA (anti-M) | 1,846.67 ± 153.22c | 78.02 | 9,208.67 ± 710.08 | 0.00 |
| 5H7-IgG (anti-M) | 8,470.00 ± 252.39 | 0.00 | 9,378.33 ± 309.49 | 0.00 |
| Irrelevant anti-HIVgp41 IgA | 7,840.00 ± 403.64d | 6.67 | 8,608.33 ± 457.88d | 3.62 |
| No MAb | 8,400.00 ± 378.58 | 0.00 | 8,930.33 ± 307.74 | 0.00 |
Experiments were repeated on at least three occasions with similar results. The results of a representative experiment are shown. The data are means ± SEM (n = 3).
Compared to no-antibody control.
Mean virus titers were significantly lower (P ≤ 0.0001) than those from cells exposed to no antibody.
Mean virus titers were not significantly lower (P ≥ 0.05) than those from cells exposed to no antibody.
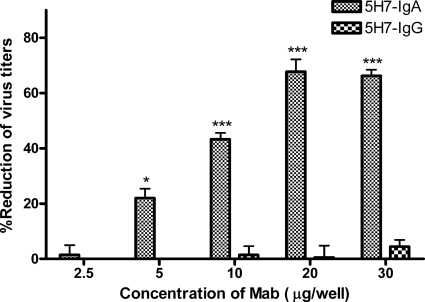
We next investigated whether 5H7-IgA had a dose-dependent effect. 5H7-IgA or 5H7-IgG antibody (2.5, 5, 10, 20, or 30 μg) was used in the intracellular neutralization assay as described above, and the percentages of virus reductions were summarized (Fig. 2). The results showed that the inhibitory effectiveness of 5H7-IgA was dose dependent in the range of 5 to 20 μg, while at a concentration of 2.5 μg, no inhibitory effect was detected, and at a concentration of 30 μg, the inhibitory effectiveness of 5H7-IgA seemed saturated.
Fig. 2.
Anti-M 5H7-IgA antibodies inhibited MV replication in a dose-dependent manner. Polarized Vero-IgR cells grown in transwells were infected by measles virus at an MOI of 1, and 2 h later, 2.5, 5, 10, 20, or 30 μg of 5H7-IgA or 5H7-IgG MAb was added to the basal chambers. Thirty hours after the MAbs were added, residual antibody was removed by washing the basal surface three times. Fresh medium was added, and 48 h after initial exposure to virus, apical and basal supernatants and cell lysates were collected and virus titers were assessed by plaque assay. The number of PFU per well and the percentage of virus reduction were calculated by comparison with no-antibody control. The percent reduction of virus was calculated over no-antibody control. *, virus titers were significantly lower (P ≤ 0.05) than those from cells exposed to no antibody; ***, P ≤ 0.001. The error bars indicate standard deviations.
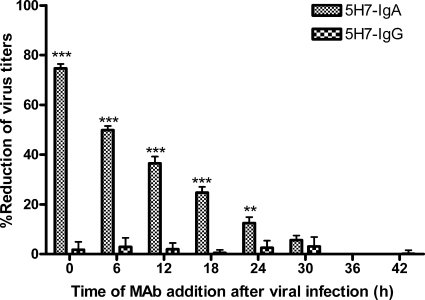
We have shown that MV replication progression had a long period of entry and assembly. Next, we investigated whether the inhibitory effectiveness of 5H7-IgA antibodies depended upon the timing of intervention during the MV replication progression. In the intracellular neutralization assay, 20 μg of 5H7-IgA or 5H7-IgG was added to basal chambers at 0, 6, 12, 18, 24, 30, 36, or 42 h after virus infection. Apical and basal supernatants and cell lysates were simultaneously collected 48 h after virus infection, and virus titers were assessed by plaque assay (Fig. 3). The results showed that the inhibitory effectiveness of 5H7-IgA decreased in correlation with the time when the antibodies were added. Interestingly, the addition of antibodies at 24 h after virus infection still caused about 10% reduction; this was the time when virions were first assembled. The results demonstrated that 5H7-IgA had an effective inhibitory effect on MV replication over a long window period and that the inhibitory effectiveness of 5H7-IgA depended upon the timing of intervention along the extent of the MV replication progression.
Fig. 3.
The inhibitory effectiveness of Anti-M 5H7-IgA antibodies depends upon the timing of intervention during MV replication progression. Polarized Vero-IgR cells grown in transwells were infected with measles virus at an MOI of 1 per cell. Twenty micrograms of 5H7-IgA or 5H7-IgG antibody was added to different basal chambers at 0, 6, 12, 18, 24, 30, 36, or 42 h after initial exposure to virus. Apical and basal supernatants and cell lysates were collected 48 h after initial exposure to virus, and virus titers were assessed by plaque assay and are presented as PFU per well. The percentage of virus reduction was calculated over no-antibody control. The percent reduction of virus was compared to no-antibody control. **, virus titers were significantly lower (P ≤ 0.01) than those from cells exposed to no antibody; ***, P ≤ 0.001. The error bars indicate standard deviations.
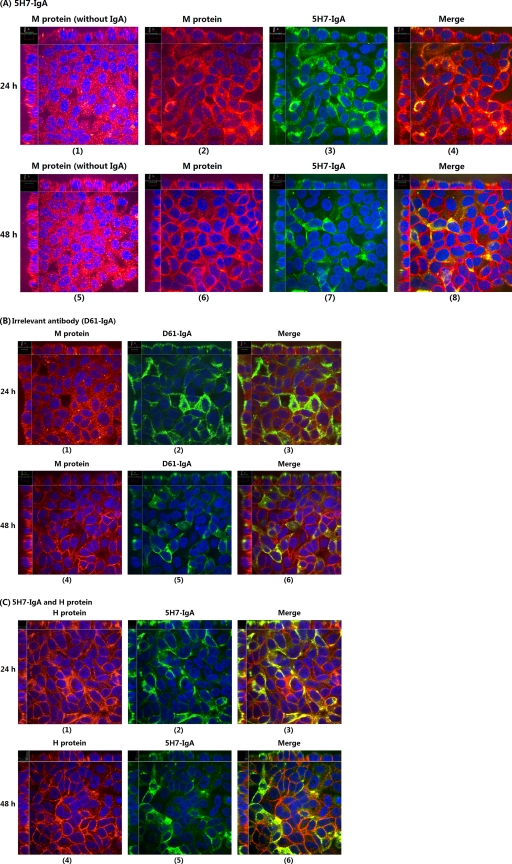
Anti-M IgA antibody colocalized intracellularly with MV M protein.
To shed light on the mechanisms by which anti-M 5H7-IgA antibodies inhibited MV replication, we performed a two-color immunofluorescent-staining assay specific for M protein and 5H7-IgA antibody. Following the normal intracellular neutralization assay, cell monolayers, together with the polyester membranes of transwells, were fixed 24 and 48 h postinfection (p.i.) and then immunofluorescently stained as described in Materials and Methods. The IgG-treated group did not show detectable staining (data not shown). The examples of confocal microscopic images of 5H7-IgA- or anti-HIVgp41-IgA-treated groups at 24 or 48 h (Fig. 4) reveal a few interesting features. First, M protein was expressed abundantly inside the polarized cells under all conditions; since we were unable to quantify the M protein expression levels in untreated or IgA-treated groups based on their fluorescence intensities, we cannot rule out the possibility that the addition of M-specific antibodies would affect M protein expression because M protein was shown to regulate viral RNA synthesis (14). Second, M proteins were mostly present in the cytoplasm at 24 h (Fig. 4A2 and B1), but more accumulated near the plasma membrane at 48 h (Fig. 4A6 and B4). Third, while irrelevant IgA was not colocalized with M protein (Fig. 4B3 and 6) and H protein was also not colocalized with 5H7 IgA antibodies (Fig. 4C3 and 6), M-specific IgA 5H7 antibodies were colocalized with M protein (Fig. 4A4). Notably, M-5H7 IgA colocalization was less evident at 48 h (Fig. 4A8); this might be due to the nature of transcytosis (inside, in the cytoplasm) and the depletion of M protein by M-specific IgA antibodies. Finally, the M-specific 5H7 IgA colocalization should result from their specific bindings, because the simultaneous presence of M proteins and irrelevant IgA antibodies in the cytoplasm did not show any colocalization and the H protein was not colocalized with the M-specific 5H7 IgA antibodies.
Fig. 4.
Confocal microscopic images from two-color immunofluorescent staining of M protein and IgA antibody. The polarized Vero-IgR cell monolayers grown on polyester membranes were either infected with measles virus at an MOI of 1 or mock infected via the apical surfaces. Two hours later, 20 μg of 5H7-IgA or irrelevant anti-HIV gp41 IgA MAb was added to the basal chambers. At 24 or 48 h after initial infection, the membrane-attached cells were fixed, permeabilized, and stained with Alexa Fluor (R) 555 goat anti-mouse IgG to detect M protein (or H protein) and FITC-conjugated goat anti-mouse IgA to detect IgA antibodies. (A) Staining of M protein and 5H7-IgA at 24 h (1 to 4) and 48 h (5 to 8) showing significant colocalization of M and 5H7-IgA. (B) Staining of M protein and anti-HIVgp41-IgA (D61-IgA) at 24 h (1 to 3) and 48 h (4 to 6) showing no colocalization of M and irrelevant IgA. (C) Staining of H protein and 5H7-IgA at 24 h (1 to 3) and 48 h (4 to 6) showing no colocalization of H and 5H7-IgA.
DISCUSSION
The present study investigated the effects of matrix protein-specific IgA antibodies on measles virus replication in the two-chamber transwell model; it represented our continuing efforts for better understanding of the functions and mechanisms of IgA antibodies in general and an extension of our persistent endeavor of looking for effective mucosal antigens for measles virus in particular. We first generated a panel of anti-M IgG MAbs and then successfully obtained one IgA (5H7) MAb from the IgG MAb panel. The M-specific IgA MAb was able to effectively inhibit MV replication by intracellular neutralization but failed to block viral infection by traditional neutralization or immune exclusion. We further provided data suggesting that the M-specific IgA MAb inhibited viral replication by directly binding to M protein because it colocalized with the M protein but not the H protein in the cytoplasm of epithelial cells.
The M protein plays important roles in measles virus assembly and replication. Electron microscopy studies showed that M protein underlies the viral envelope and forms electron-dense layers in electron micrographs (9). Molecular studies demonstrated that M protein regulates viral RNA synthesis (14) and interacts with the viral RNP complex (10, 30), as well as the cytoplasmic domains of viral envelope glycoproteins (3, 4, 28, 31). The importance of M protein in viral assembly was manifested by studies of a recombinant MV lacking the M protein (MV-ΔM) that had ∼250-fold-reduced viral titers compared with the parental virus (3) and studies of patients with subacute sclerosing panencephalitis (SSPE) that suggested that the inability of defective M proteins to associate with the viral nucleocapsid could be responsible for the low level of infectious viral particle production (10). Here, our results showed that even though the M-specific IgA MAb (5H7) failed to exhibit the activities of traditional neutralization and immune exclusion, it effectively inhibited MV replication in the polarized Vero-IgR cells by intracellular neutralization; this corroborated the notion that M protein is important for MV assembly and replication.
IgA antibodies are the major mediators of mucosal immune defenses. In addition to being able to directly bind to surface antigens of viral particles, IgA antibodies are unique in their capacity to bind to newly synthesized viral antigens inside cells during viral replication through pIgR-mediated transcytosis; this is known as intracellular neutralization (6, 16, 20). The antigens studied so far for IgA-mediated intracellular neutralization are in two groups: viral surface proteins, including the HN protein of Sendai virus (19), the HA protein of influenza virus (18), the H and F proteins of measles virus (34), and gp160 of HIV (13), and viral internal proteins, including the N protein of measles virus (34), the Gag and RT proteins of HIV (33), and the VP6 protein of rotavirus (5). The intracellular antivirus activities depend on the type of viral component, the reactive epitopes, the characteristics of a particular virus life cycle, and the opportunities for transcytosing IgA antibodies to meet their prospective targets (17). Compared with the excellent efficacy of IgA antibodies against viral surface antigens, IgA antibodies against internal viral proteins were much less effective, and the inhibitory effects of IgA antibodies against the Gag and RT proteins of HIV or the N protein of MV on virus titers were about 40% or 60%, respectively (33, 34). In contrast, the present study showed that M-specific IgA antibodies could cause an impressive 78% reduction of MV viral titers; the reason for this high efficacy might be due to the nature of M protein and/or the specificity of the antigenic epitope recognized by the M-specific IgA antibody. Regardless of the reasons, the results implied that M protein is a more effective antigen for mucosal immune protection. The studies of IgA antibodies against VP6 of rotavirus are worthy of special consideration; it was first reported that VP6-specific IgA MAbs were able to protect mice from viral infection in the “backpack tumor” model (2); then it was demonstrated in in vitro polarized epithelial cells that VP6-specific IgA MAbs inhibited viral production by 99% at a concentration of 5 μg/ml (5), seemingly explaining why VP6-specific IgA MAbs were effective in a mouse model, because the in vivo mouse model involved a continuous supply of antibodies with many viral replication cycles while the in vitro cell model was supplied once with one viral replication cycle. However, surprisingly, the same VP6-specific IgA MAbs were totally ineffective at a concentration of 2.5 μg/ml, and this critical concentration requirement for the efficacy of VP6-specific IgA MAbs was not explained (5). It highlights the complexity of the mechanisms by which IgA antibodies inhibit viral replication and demands vigorous studies to better understand the inhibitory effects of IgA antibodies.
The colocalization of IgA antibodies and newly synthesized antigens has been shown for intracellular neutralization (17). Our results clearly demonstrated that M proteins and M-specific IgA antibodies were colocalized, and the colocalization required the presence of M proteins in the cytoplasm, because the colocalization was less evident when the M proteins accumulated near the plasma membrane during the late stage of infection (Fig. 4A8). This was in accordance with our results showing that M-specific IgA antibodies worked more efficiently when they were added in the early stages of viral replication (Fig. 3). We also showed that MV had a long entry and assembly phase, providing an extended window for the IgA antibody interventions. Finally, the addition of M-specific IgA antibodies changed the distribution of M proteins inside the cells; whether the patterns of other viral proteins inside the cells are also affected by the addition of M-specific antibodies is still under investigation.
MV is still an imposing threat to public health, especially in developing countries and among the groups that cannot be effectively vaccinated. The present study demonstrated that M-specific IgA antibodies could effectively inhibit MV replication in polarized epithelial cells by intracellular neutralization, implying that M protein could be used as a target antigen in the design of a mucosal vaccine against MV.
ACKNOWLEDGMENTS
This work was financially supported by grants from the National Natural Science Foundation of China (no. 30471584 and 30670097) and the Ministry of Science and Technology of the People's Republic of China (973 Program, no. 2005CB522903; National Key R&D Program, no. 2007BAI28B04; National S&T Major Project on Major Infectious Diseases, no. 2008ZX10001-010 and 2008ZX10001-015).
We sincerely acknowledge George Dacai Liu for his critical revision of and comments on the manuscript.
Footnotes
Published ahead of print on 24 August 2011.
REFERENCES
- 1. Blau D. M., Compans R. W. 1995. Entry and release of measles virus are polarized in epithelial cells. Virology 210:91–99 [DOI] [PubMed] [Google Scholar]
- 2. Burns J. W., Siadat-Pajouh M., Krishnaney A. A., Greenberg H. B. 1996. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science 272:104–107 [DOI] [PubMed] [Google Scholar]
- 3. Cathomen T., et al. 1998. A matrix-less measles virus is infectious and elicits extensive cell fusion: consequences for propagation in the brain. EMBO J. 17:3899–3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cathomen T., Naim H. Y., Cattaneo R. 1998. Measles viruses with altered envelope protein cytoplasmic tails gain cell fusion competence. J. Virol. 72:1224–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corthésy B., et al. 2006. Rotavirus anti-VP6 secretory immunoglobulin A contributes to protection via intracellular neutralization but not via immune exclusion. J. Virol. 80:10692–10699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corthesy B., Kraehenbuhl J. P. 1999. Antibody-mediated protection of mucosal surfaces. Curr. Top. Microbiol. Immunol. 236:93–111 [DOI] [PubMed] [Google Scholar]
- 7. Duke T., Mgone C. S. 2003. Measles: not just another viral exanthem. Lancet 361:763–773 [DOI] [PubMed] [Google Scholar]
- 8. Gindler J., et al. 2004. Acute measles mortality in the United States, 1987-2002. J. Infect. Dis. 189(Suppl. 1):S69–S77 [DOI] [PubMed] [Google Scholar]
- 9. Griffin D. E. (ed.). 2007. Measles virus, 5th ed Lippincott Williams and Wilkins, Philadelphia, PA [Google Scholar]
- 10. Hirano A., Ayata M., Wang A. H., Wong T. C. 1993. Functional analysis of matrix proteins expressed from cloned genes of measles virus variants that cause subacute sclerosing panencephalitis reveals a common defect in nucleocapsid binding. J. Virol. 67:1848–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hirano A., Wang A. H., Gombart A. F., Wong T. C. 1992. The matrix proteins of neurovirulent subacute sclerosing panencephalitis virus and its acute measles virus progenitor are functionally different. Proc. Natl. Acad. Sci. U. S. A. 89:8745–8749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang Y. T., et al. 1999. Replication and budding of simian immunodeficiency virus in polarized epithelial cells. Virology 257:24–34 [DOI] [PubMed] [Google Scholar]
- 13. Huang Y. T., et al. 2005. Intraepithelial cell neutralization of HIV-1 replication by IgA. J. Immunol. 174:4828–4835 [DOI] [PubMed] [Google Scholar]
- 14. Iwasaki M., et al. 2009. The matrix protein of measles virus regulates viral RNA synthesis and assembly by interacting with the nucleocapsid protein. J. Virol. 83:10374–10383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Katz S. L., Hinman A. R. 2004. Summary and conclusions: measles elimination meeting, 16-17 March 2000. J. Infect. Dis. 189(Suppl. 1):S43–S47 [DOI] [PubMed] [Google Scholar]
- 16. Lamm M. E. 1997. Interaction of antigens and antibodies at mucosal surfaces. Annu. Rev. Microbiol. 51:311–340 [DOI] [PubMed] [Google Scholar]
- 17. Lamm M. E. (ed.). 2007. Protection of mucosal epithelia by IgA: intracellular neutralization and excretion of antigens. Springer Science and Business Media LLC, New York, NY [Google Scholar]
- 18. Mazanec M. B., Coudret C. L., Fletcher D. R. 1995. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J. Virol. 69:1339–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mazanec M. B., Kaetzel C. S., Lamm M. E., Fletcher D., Nedrud J. G. 1992. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc. Natl. Acad. Sci. U. S. A. 89:6901–6905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mazanec M. B., Nedrud J. G., Kaetzel C. S., Lamm M. E. 1993. A three-tiered view of the role of IgA in mucosal defense. Immunol. Today 14:430–435 [DOI] [PubMed] [Google Scholar]
- 21. Naim H. Y., Ehler E., Billeter M. A. 2000. Measles virus matrix protein specifies apical virus release and glycoprotein sorting in epithelial cells. EMBO J. 19:3576–3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pan American Health Organization, Area of Family and Community Health, I. U. P 2003. Absence of transmission of the D9 measles virus in the region of the Americas, November 2002-May 2003. Epidemiol. Bull. 24:24. [PubMed] [Google Scholar]
- 23. Parker Fiebelkorn A., et al. 2010. Measles in the United States during the postelimination era. J. Infect. Dis. 202:1520–1528 [DOI] [PubMed] [Google Scholar]
- 24. Pasetti M. F., et al. 2007. Heterologous prime-boost strategy to immunize very young infants against measles: pre-clinical studies in rhesus macaques. Clin. Pharmacol. Ther. 82:672–685 [DOI] [PubMed] [Google Scholar]
- 25. Reuter T., Weissbrich B., Schneider-Schaulies S., Schneider-Schaulies J. 2006. RNA interference with measles virus N, P, and L mRNAs efficiently prevents and with matrix protein mRNA enhances viral transcription. J. Virol. 80:5951–5957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Riedl P., Moll M., Klenk H. D., Maisner A. 2002. Measles virus matrix protein is not cotransported with the viral glycoproteins but requires virus infection for efficient surface targeting. Virus Res. 83:1–12 [DOI] [PubMed] [Google Scholar]
- 27. Runkler N., Pohl C., Schneider-Schaulies S., Klenk H. D., Maisner A. 2007. Measles virus nucleocapsid transport to the plasma membrane requires stable expression and surface accumulation of the viral matrix protein. Cell Microbiol. 9:1203–1214 [DOI] [PubMed] [Google Scholar]
- 28. Spielhofer P., et al. 1998. Chimeric measles viruses with a foreign envelope. J. Virol. 72:2150–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Strebel P. M., Papania M. J., Dayan G. H., Halsey N. A. (ed.). 2008. Measles vaccine. Elsevier, Philadelphia [Google Scholar]
- 30. Suryanarayana K., Baczko K., ter Meulen V., Wagner R. R. 1994. Transcription inhibition and other properties of matrix proteins expressed by M genes cloned from measles viruses and diseased human brain tissue. J. Virol. 68:1532–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tahara M., Takeda M., Yanagi Y. 2007. Altered interaction of the matrix protein with the cytoplasmic tail of hemagglutinin modulates measles virus growth by affecting virus assembly and cell-cell fusion. J. Virol. 81:6827–6836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. WHO 2010, posting date Measles fact sheet. World Health Organization http://www.who.int/mediacenter/factsheets/fs286/en/index.html [Google Scholar]
- 33. Wright A., Yan H., Lamm M. E., Huang Y. T. 2006. Immunoglobulin A antibodies against internal HIV-1 proteins neutralize HIV-1 replication inside epithelial cells. Virology 356:165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yan H., Lamm M. E., Bjorling E., Huang Y. T. 2002. Multiple functions of immunoglobulin A in mucosal defense against viruses: an in vitro measles virus model. J. Virol. 76:10972–10979 [DOI] [PMC free article] [PubMed] [Google Scholar]