Abstract
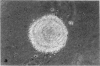
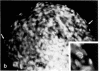
Normal human fibroblasts, considered to be entirely anchorage dependent for proliferation, have been grown in methylcellulose medium. The most important factor required for growth in suspension appears to be the use of high levels of serum and hydrocortisone. Newborn foreskin or fetal lung fibroblasts form colonies as large as 0.5 mm in diameter after 3 wk, with a colony-forming efficiency as high as 70%. Mouse 3T3 cells that do not form colonies in standard assays for anchorage-independent growth also grow under these conditions. Colony formation results after inoculation of as few as 100 cells per 60-mm dish, and metaphase cells have been visualized with a fluorescent DNA stain, showing that colony formation is due to division rather than aggregation. Fibroblasts recovered from suspension and grown as monolayers retain a diploid karyotype and normal shape, do not form tumors upon injection into nude mice, and become senescent. Thus, the trait of anchorage-independent growth in vitro is clearly possessed by normal human fibroblasts and can be expressed under the proper conditions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colburn N. H., Bruegge W. F., Bates J. R., Gray R. H., Rossen J. D., Kelsey W. H., Shimada T. Correlation of anchorage-independent growth with tumorigenicity of chemically transformed mouse epidermal cells. Cancer Res. 1978 Mar;38(3):624–634. [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Freedman V. H., Shin S. I. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974 Dec;3(4):355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H., McDougall J. K., Chen L. B. In vitro traits of adenovirus-transformed cell lines and their relevance to tumorigenicity in nude mice. Cell. 1977 Apr;10(4):669–678. doi: 10.1016/0092-8674(77)90100-3. [DOI] [PubMed] [Google Scholar]
- Hamburger A. W., Salmon S. E. Primary bioassay of human tumor stem cells. Science. 1977 Jul 29;197(4302):461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Laug W. E., Gardner A., Nye C. A., Fink L. M., Benedict W. F. In vitro correlates of transformation in C3H/10T1/2 clone 8 mouse cells. Cancer Res. 1976 Aug;36(8):2863–2867. [PubMed] [Google Scholar]
- Kahn P., Shin S. I. Cellular tumorigenicity in nude mice. Test of associations among loss of cell-surface fibronectin, anchorage independence, and tumor-forming ability. J Cell Biol. 1979 Jul;82(1):1–16. doi: 10.1083/jcb.82.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelovich L., Bias N. E. Tumor promoter induces loss of anchorage dependence in human skin fibroblasts from individuals genetically predisposed to cancer. Exp Cell Biol. 1980;48(3):207–217. doi: 10.1159/000162988. [DOI] [PubMed] [Google Scholar]
- Laug W. E., Tokes Z. A., Benedict W. F., Sorgente N. Anchorage independent growth and plasminogen activator production by bovine endothelial cells. J Cell Biol. 1980 Feb;84(2):281–293. doi: 10.1083/jcb.84.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Marshall C. J. Expression of the transformed phenotype and tumorigenicity in somatic cell hybrids. J Cell Sci. 1979 Oct;39:319–327. doi: 10.1242/jcs.39.1.319. [DOI] [PubMed] [Google Scholar]
- McKeehan W. L., Hamilton W. G., Ham R. G. Selenium is an essential trace nutrient for growth of WI-38 diploid human fibroblasts. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2023–2027. doi: 10.1073/pnas.73.6.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeehan W. L., McKeehan K. A., Hammond S. L., Ham R. G. Improved medium for clonal growth of human diploid fibroblasts at low concentrations of serum protein. In Vitro. 1977 Jul;13(7):399–416. doi: 10.1007/BF02615100. [DOI] [PubMed] [Google Scholar]
- Paul D. Growth control in HeLa cells by serum and anchorage. Exp Cell Res. 1978 Jul;114(2):435–438. doi: 10.1016/0014-4827(78)90503-7. [DOI] [PubMed] [Google Scholar]
- Peehl D. M., Ham R. G. Growth and differentiation of human keratinocytes without a feeder layer or conditioned medium. In Vitro. 1980 Jun;16(6):516–525. doi: 10.1007/BF02626465. [DOI] [PubMed] [Google Scholar]
- Pollack R., Risser R., Conlon S., Rifkin D. Plasminogen activator production accompanies loss of anchorage regulation in transformation of primary rat embryo cells by simian virus 40. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4792–4796. doi: 10.1073/pnas.71.12.4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. C., Newman C., Williamson D. H. A simple cytochemical technique for demonstration of DNA in cells infected with mycoplasmas and viruses. Nature. 1975 Feb 6;253(5491):461–462. doi: 10.1038/253461a0. [DOI] [PubMed] [Google Scholar]
- Shin S. I., Freedman V. H., Risser R., Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanbredge E. J., Boulger L. R., Franks C. R., Garrett J. A., Reeson D. E., Bishop D., Perkins F. T. Optimal conditions for the growth of malignant human and animal cell populations in immunosuppressed mice. Cancer Res. 1975 Aug;35(8):2203–2212. [PubMed] [Google Scholar]
- Stanbridge E. J. Suppression of malignancy in human cells. Nature. 1976 Mar 4;260(5546):17–20. doi: 10.1038/260017a0. [DOI] [PubMed] [Google Scholar]
- Stanbridge E. J., Wilkinson J. Analysis of malignancy in human cells: malignant and transformed phenotypes are under separate genetic control. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1466–1469. doi: 10.1073/pnas.75.3.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanbridge E. J., Wilkinson J. Dissociation of anchorage independence form tumorigenicity in human cell hybrids. Int J Cancer. 1980 Jul 15;26(1):1–8. doi: 10.1002/ijc.2910260102. [DOI] [PubMed] [Google Scholar]
- Stiles C. D., Desmond W., Chuman L. M., Sato G., Saier M. H., Jr Relationship of cell growth behavior in vitro to tumorigenicity in athymic nude mice. Cancer Res. 1976 Sep;36(9 PT1):3300–3305. [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E. Growth factors produced by sarcoma virus-transformed cells. Cancer Res. 1978 Nov;38(11 Pt 2):4147–4154. [PubMed] [Google Scholar]
- Weissman B., Stanbridge E. J. Characterization of ouabain resistant, hypoxanthine phosphoribosyl transferase deficient human cells and their usefulness as a general method for the production of human cell hybrids. Cytogenet Cell Genet. 1980;28(4):227–239. doi: 10.1159/000131536. [DOI] [PubMed] [Google Scholar]