Abstract
The Apobec3 family of cytidine deaminases can inhibit the replication of retroviruses and retrotransposons. Human and chimpanzee genomes encode seven Apobec3 paralogs; of these, Apobec3DE has the greatest sequence divergence between humans and chimpanzees. Here we show that even though human and chimpanzee Apobec3DEs are very divergent, the two orthologs similarly restrict long terminal repeat (LTR) and non-LTR retrotransposons (MusD and Alu, respectively). However, chimpanzee Apobec3DE also potently restricts two lentiviruses, human immunodeficiency virus type 1 (HIV-1) and the simian immunodeficiency virus (SIV) that infects African green monkeys (SIVagmTAN), unlike human Apobec3DE, which has poor antiviral activity against these same viruses. This difference between human and chimpanzee Apobec3DE in the ability to restrict retroviruses is not due to different levels of Apobec3DE protein incorporation into virions but rather to the ability of Apobec3DE to deaminate the viral genome in target cells. We further show that Apobec3DE rapidly evolved in chimpanzee ancestors approximately 2 to 6 million years ago and that this evolution drove the increased breadth of chimpanzee Apobec3DE antiviral activity to its current high activity against some lentiviruses. Despite a difference in target specificities between human and chimpanzee Apobec3DE, Apobec3DE is likely to currently play a role in host defense against retroelements in both species.
INTRODUCTION
Apobec3 proteins are cytidine deaminases that inhibit the replication of retroelements during reverse transcription (reviewed in reference 26). During a retroviral infection, Apobec3 proteins are incorporated into virions, from a producer cell and prevent infection of subsequent target cells. Apobec3 proteins have been shown to act in deaminase-dependent and -independent manners (reviewed in reference 20). Due to paralogous expansions of the Apobec3 family, humans and chimpanzees have a cluster of seven Apobec3 genes (Apobec3A through Apobec3H) (21, 37). The human Apobec3 paralogs may have adapted to particular retroelements since they vary in their antiviral strength and target specificity. For instance, human Apobec3A restricts retrotransposons but not retroviruses (8–10), and human Apobec3G has been reported to restrict human immunodeficiency virus type 1 (HIV-1) more potently than any other human Apobec3 (6, 32, 34).
Genetic conflict between primates and the pathogens that infect them has led to the rapid evolution of many host defense factors, including the Apobec3 gene family (41). Several members of the Apobec3 family, including Apobec3B, -C, -DE, -F, -G, and -H, are highly divergent between humans and chimpanzees (37, 41). The Apobec3 gene with the highest human-chimpanzee divergence is Apobec3DE (41), but analyses to date have not determined whether this rapid evolution occurred in the human lineage, the chimpanzee lineage, or both. Previous studies have shown that human Apobec3DE has weak activity against HIV-1 and other retroviruses (12, 43) and moderately restricts the nonautonomous Alu retrotransposon (46). There are conflicting reports about whether or not Apobec3DE restricts the non-long terminal repeat (LTR) retrotransposon LINE-1 (35, 45). In addition to weak activity, human Apobec3DE has been reported to have relatively low protein expression compared to other Apobec3 proteins during transient transfection (12, 35, 46). However, many studies have excluded Apobec3DE from their assays due to confusion surrounding its initial classification as two separate genes (Apobec3D and Apobec3E), one of which was incorrectly assigned as a pseudogene due to sequencing errors (21). Thus, despite its strong signal of adaptive evolution, a clear role for human Apobec3DE in host defense has not yet been identified.
Viral restriction by Apobec3s can be overcome by lentiviruses encoding a Vif protein, such as HIV-1, by causing the degradation of Apobec3s in producer cells via the proteasome (26). The sensitivity of Apobec3 proteins to Vif antagonism has been shown to be species specific. For instance, human Apobec3G is sensitive to Vif from HIV-1 but not simian immunodeficiency virus (SIV) that infects African green monkeys (SIVagmTAN) and Apobec3G from African green monkeys is sensitive to Vif from SIVagmTAN but not HIV-1 (7, 28, 42, 48). However, Apobec3 specificities in modern primates were more likely to have been driven by genetic “arms races” against ancient viruses (called paleoviruses) rather than against modern viruses (15, 41). Thus, while human Apobec3DE has weak antiviral activity against HIV-1, it may restrict ancient retroelements, and another lineage of Apobec3DE may have an entirely different specificity. For this reason, and because human and chimpanzee Apobec3DE sequences are very divergent, we wished to determine whether orthologs of Apobec3DE may have differential antiviral activities.
In this study, we discover that human and chimpanzee orthologs of Apobec3DE share the ability to inhibit a broad range of retrotransposons, including Alu elements, suggesting that they may play a potential current role in host defense against these elements. Strikingly, chimpanzee Apobec3DE can also potently restrict some lentiviruses, including HIV-1 and SIVagmTAN, but not HIV-2. On the other hand, human Apobec3DE has only poor activity against HIV-1 and SIVagmTAN. We find that, despite being highly incorporated into virions, human Apobec3DE lacks the ability to hypermutate HIV-1 genomes, while chimpanzee Apobec3DE induces high levels of hypermutation in HIV-1. Through evolutionary analyses, we find that chimpanzee Apobec3DE evolved very rapidly in chimpanzee-bonobo ancestors, including within the domain responsible for differential antiviral activity. The specific adaptation of chimpanzee Apobec3DE to lentiviruses together with its rapid evolution approximately 2 to 6 million years ago suggests that Apobec3DE was selected to acquire new functions in the defense of chimpanzees against an ancient retroelement.
MATERIALS AND METHODS
Apobec3 sequences.
Human Apobec3DE was amplified from a testes cDNA library (Invitrogen). This sequence is identical to the sequence in the GenBank database (NM_152426.3). Chimpanzee (Pan troglodytes) Apobec3DE was amplified by reverse transcriptase (RT) PCR of RNA isolated from fibroblasts (Coriell AG06939) followed by nested PCR. The PCR fragment was subcloned into a TOPO vector (Invitrogen) for sequencing. This sequence differs from the NCBI database entry for the predicted chimpanzee sequence (XM_525657.2) across the entire first exon. The sequences for bonobo (Pan paniscus) Apobec3DE exons 1 to 7 and gibbon (Nomascus leucogenys) Apobec3DE exons 1 and 2 were obtained by amplifying genomic DNA isolated from fibroblasts (Coriell AG05253 and PR01037) using hominoid-specific Apobec3DE genomic DNA primers. The sequences for rhesus macaque (Macaca mulatta), African green monkey (Cercopithecus aethiops), and Patas monkey (Erythrocebus patas) Apobec3DE exons 1 to 7 were obtained by amplifying genomic DNA isolated from fibroblasts (Coriell AG07098 and AG06254) or cell lines (Vero) using Old World monkey-specific Apobec3DE genomic DNA primers. PCR products were sequenced directly. The sequence for rhesus macaque Apobec3DE differs from the GenBank entry (XM_002798351.1) by 7 nucleotides. The sequences for gorilla (Gorilla gorilla) Apobec3DE and gibbon Apobec3DE (exons 2 to 7) were amplified from RNA isolated from fibroblasts (Coriell NG05251) using 3′ rapid amplification of cDNA ends (3′ RACE) followed by nested PCR and subcloned into the pGEM-T Easy vector (Promega) for sequencing. cDNA was synthesized using the oligo(dT)17-Adaptor primer described in reference 40. Gorilla Apobec3F was amplified from RNA by One-Step RT-PCR (Qiagen). All primer sequences are available upon request. Sequences have been deposited in GenBank under the accession numbers JN247640 to JN247649. Additional sequences for Apobec3F were obtained from the GenBank database: human Apobec3F (NM_145298.5), chimpanzee Apobec3F (XM_525658.2), and rhesus macaque Apobec3F (NM_001042373.1). The sequence for African green monkey Apobec3F was provided by Vinay Pathak.
Apobec3 sequence analysis.
Apobec3 sequences were analyzed with the Phylogeny.fr web service (13) using default settings, including maximum likelihood (ML) phylogeny construction with an approximate likelihood-ratio test (aLRT) for branch support. Recombination breakpoints were identified using the GARD method with an HKY95 nucleotide substitution model on the Datamonkey web server (23). Evolutionary analyses of the phylogeny were performed with the CODEML program in the PAML (phylogenetic analysis by maximum likelihood) software package (50). Global dN/dS ratios for the branches were calculated using a free-ratio model. We calculated the log-likelihood ratios of data under different NSsites models (model 1 versus model 2 and model 7 versus model 8). For both cases, a model of positive selection gave a better fit (P < 0.001). A Bayes empirical bayes analysis identified amino acid residues with a high posterior probability (>0.95) of evolving under positive selection (51).
Apobec3 plasmids.
Human Apobec3A, Apobec3G, and Apobec3DE and chimpanzee Apobec3DE were cloned into the expression vector pCS2. A hemagglutinin (HA) tag and Kozak sequence were added to the N terminus of A3A and A3G, and an HA tag was added to the C terminus of Apobec3DE proteins. Untagged Apobec3DE constructs were also made. Human/chimpanzee chimeras 1 and 2 were made by ligating BamHI fragments. Human/chimpanzee chimeras 3 and 4 and human and chimpanzee Apobec3DE catalytic mutants were constructed by overlapping PCR products.
Cell lines, transfections, and Western blot analysis.
293T, CRFK, HeLa, and HeLa-HA cells were maintained in Dulbecco's modified Eagle medium (DMEM)–1% penicillin-streptomycin (Pen/Strep)–10% bovine growth serum (BGS) at 37°C in a CO2 incubator. SupT1 cells were maintained similarly in RPMI-1% Pen/Strep-10% fetal bovine serum (FBS). Transfections were performed with TransIT-LT1 transfection reagent (Mirus Bio) at a reagent/plasmid DNA ratio of 2:1. Cell and viral lysates were harvested 48 h after transfection. Cells were lysed in NP-40-doc buffer (1% NP-40, 0.2% sodium deoxycholate, 0.12 M NaCl, 20 mM Tris [pH 8.0], 2.4 mM dithiothreitol [DTT]) with protease inhibitors (Roche) and spun for 1 min at 16,000 × g, and supernatants were quantified by Bradford assay. Virus was quantified by p24 gag enzyme-linked immunosorbent assay (ELISA) (Advanced BioScience), and 20 ng virions was spun down at 16,000 × g for 45 min and lysed in protein loading dye (125 mM Tris [pH 6.8], 4% SDS, 20% glycerol, 10% β-mercaptoethanol, 6 M urea, bromophenol blue). Lysates were resolved by 10% SDS-PAGE, transferred to polyvinylidene difluoride (PVDF) membranes, and probed with anti-HA (Santa Cruz Biotechnology) or anti-actin (Sigma) antibodies. Of note, lysates containing human Apobec3DE that were prepared without urea did not fully denature the protein, preventing its appropriate migration through the gel. This requirement for urea in protein preparations was not observed for chimpanzee Apobec3DE.
Retrotransposition assays.
MusD, LINE-1, and Alu assays were performed as described previously (14, 36, 39, 47) with some modifications. Cells were plated in 6-well plates at 8 × 104 cells/well, and 24 h later, cells were transfected in triplicate. For MusD assays, 0.25 μg neoTNF-marked MusD plasmid (39) and 0.6 μg Apobec or empty plasmid were transfected into HeLa cells. For L1 assays, 0.1 μg LINE-1 plasmid (JM101/L1.3 [33, 47]) and 0.6 μg Apobec or empty plasmid were transfected into HeLa cells. For Alu assays, 1 μg Alu plasmid (AluneoTET [14] or Alu-Ya5-eab, Alu-Yc1-eab, Alu-Y-eab, Alu-Sx-eab, and Alu-Jo-eab [5]), 0.3 μg Orf2p (pCep5′UTRORF2Δneo [2]), and 0.45 μg Apobec or empty plasmid were transfected into HeLa-HA cells. For testing the nonspecific inhibition of neomycin-resistant colony formation, 0.25 μg LXSN plasmid (30) and 0.6 μg Apobec or empty plasmid were transfected into HeLa cells. Three days after transfection, cells were selected in G418 for 10 to 12 days. For MusD, LINE-1, and LXSN assays, cells were split into 10-cm2 plates at the time of selection into multiple dilutions. Colonies were stained with crystal violet and counted manually.
Viral infectivity assays.
Single-round murine leukemia virus (MLV), HIV-1, HIV-2, and SIVagmTAN infectivity assays were performed as described previously (49). To produce vesicular stomatitis virus G protein (VSV-G)-pseudotyped MLV, 4.2 × 106 293T cells were plated in a 12-well plate, and, 24 h later, cotransfected with 0.5 μg gag-pol expression plasmids (pCS2-mGP [49]), 0.1 μg tat (pCMVtat), 0.5 μg murine retrovirus-based vector encoding luciferase (pLNC-luc [49]), 0.25 μg L-VSV-G (3), and 0.5 μg Apobec or empty plasmid. To produce VSV-G-pseudotyped HIV-1, HIV-2, and SIVagmTAN, 3 × 106 293T cells were plated in a 6-well plate, and 24 h later, cotransfected with 0.6 μg lentiviral vector encoding luciferase or enhanced green fluorescent protein (EGFP) in the place of the nef gene (pLai3ΔenvLuc2 [49], pLai3ΔenvLuc2Δvif [37], pROD9ΔenvEGFP, pRODΔenvEGFPΔvif, pSIVagmTANΔenvΔvprLuc [29], or pSIVagmTANΔenvΔvprLucΔvif [37]), 0.2 μg L-VSV-G, and 0.2 μg Apobec or empty plasmid. For HIV-1 assays, medium was changed to DMEM-1% FBS 24 h before harvesting virions. All viruses were harvested 48 h after transfection and filtered through a 0.2-μm filter. For MLV and HIV-2 infections, equivalent volumes of virus were used to infect 8 × 105 CRFK cells or 1 × 106 SupT1 cells, respectively, in a 12-well plate in the presence of 20 μg/ml DEAE-dextran, followed by a 30-min spinoculation at room temperature. For HIV-1 and SIVagmTAN infections, virus equivalent to two nanograms of p24 gag or four nanograms of p27 gag, respectively, was used to infect 4 × 106 SupT1 cells in a 96-well plate in the presence of 20 μg/ml DEAE-dextran. For MLV, HIV-1, and SIVagmTAN infections, 48 h after infection, cells from triplicate infections were lysed in 100 μl of cell culture lysis buffer (Promega) or Bright-Glo luciferase assay reagent (Promega) and read on a luminometer. For HIV-2 infections, 48 h after infection, cells from duplicate infections were fixed and analyzed for GFP expression using fluorescence-activated cell sorting.
Pulse-chase analysis.
Pulse-chase and immunoprecipitations were performed essentially as described previously (36). Briefly, 1.25 × 106 293T cells were plated on 6-cm2 poly-l-lysine plates (Becton Dickinson) and transfected with 5 μg human or chimpanzee Apobec3DE plasmids. Cells were pulsed with 200 μCi EasyTag EXPRE35S35S protein label (PerkinElmer) per plate and chased for 0 min, 30 min, 1 h, and 20 h. Cells were scraped into 500 μl NP-40-doc lysis buffer, and lysates were spun for 1 min at 16,000 × g. Lysates were precleared with protein G-Sepharose beads, and Apobec3DE proteins were immunoprecipitated with beads preconjugated to anti-HA antibody (Santa Cruz Biotechnology). Immunoprecipitated proteins were resolved by 10% SDS-PAGE, and the gel was imaged using a Typhoon Trio imager (Amersham Biosciences).
Detection of G-to-A hypermutation.
VSV-G-pseudotyped HIV-1Δvif containing Apobec3 was produced in 293T cells. Twenty-four nanograms of virus was used to infect 5 × 105 SupT1 cells in 6-well plates. Twenty-four hours after infection, extrachromosomal DNA was harvested using a plasmid isolation kit (Roche). DNA was treated with DpnI for 1 h at 37°C to eliminate plasmid DNA carryover from the initial transfection. A 431-bp fragment of HIV-1 pol was amplified using the previously described forward primer pol1721 and reverse primer pol2152 (37), subcloned into the pGEM-T Easy vector (Promega), and sequenced.
Nucleotide sequence accession numbers.
Sequences have been deposited in GenBank under the accession numbers JN247640 to JN247649.
RESULTS
Human and chimpanzee Apobec3DE restrict retrotransposons.
The sequences for human and chimpanzee Apobec3DE are very divergent (41) and differ by 35 amino acids, suggesting that they may have differential antiviral activity. Previous reports on the activity of human Apobec3DE against retrotransposons have varied, suggesting that it has weak to strong effects (35, 45, 46). No previous study has examined chimpanzee Apobec3DE. Therefore, we characterized the breadth of Apobec3DE restriction by testing the ability of human and chimpanzee Apobec3DE to inhibit several types of retrotransposons. We cotransfected Apobec3DE plasmids with LTR and non-LTR retrotransposons that express neomycin resistance after retrotransposition (14, 39, 47). After selection, neomycin-resistant colonies were counted as a measure of retrotransposition. Human Apobec3G and Apobec3A were used as positive controls in these experiments, as they have previously been shown to restrict retrotransposons. A Western blot shows similar expression levels of human Apobec3G, human Apobec3A, human Apobec3DE, and chimpanzee Apobec3DE when transiently transfected (Fig. 1A).
Fig. 1.
Human and chimpanzee Apobec3DE restrict retrotransposons. Human and chimpanzee Apobec3DE were expressed in retrotransposition assays to determine their antiviral activity. Retrotransposition activity is normalized to 100% in the absence of Apobec3 (none). (A) Western blot analysis of protein levels of human Apobec3G (hA3G), human Apobec3A (hA3A), human Apobec3DE (hA3DE), and chimpanzee Apobec3DE (cA3DE) during transient transfection. (B) Retrotransposition of MusD. Human Apobec3G was used as a positive control. (C) Retrotransposition of the prototypic Alu element. Human Apobec3A was used as a positive control. (D) Retrotransposition of LINE-1. Human Apobec3G was used as a positive control. Experiments were performed at least 3 times, and results from one representative experiment are shown. Error bars represent the standard deviation of triplicate transfections within one experiment.
We found that human and chimpanzee Apobec3DE are strong inhibitors of specific retrotransposons. The most potent activity of human and chimpanzee Apobec3DE was against the LTR retrotransposon MusD (25- and 175-fold restriction, respectively; Fig. 1B). Also, both human and chimpanzee Apobec3DE restricted the prototypic Alu element cloned by Dewannieux et al. (14) by 35- and 25-fold, respectively (Fig. 1C). However, neither chimpanzee nor human Apobec3DE had a strong effect on the non-LTR retrotransposon LINE-1 (3-fold, Fig. 1D), as previously shown for human Apobec3DE (45).
Because different Alu subfamilies have been active in humans compared to chimpanzees in the past several million years (19, 31), human and chimpanzee Apobec3DE may have specialized to differentially restrict species-specific Alu elements. For example, humans have higher levels of Alu-Ya5 and Alu-S subfamily insertions than do chimpanzees, and both species have recent Alu-Y and -Yc1 insertions as well as remnants of ancient Alu-J elements (5, 19, 31). Therefore, we tested human and chimpanzee Apobec3DE against the consensus Alu-Y, -S, and -J subfamilies (5). We found that human and chimpanzee Apobec3DE restricted all Alu subfamily members tested by 80 to 95% (Fig. 2A). We also tested the ability of human Apobec3G to inhibit Alu elements to determine whether the ability to restrict all Alu subfamilies was gained specifically by Apobec3DE. Human Apobec3G also restricted all Alu elements tested, indicating that restriction of multiple Alu subfamilies is not unique to Apobec3DE. To determine whether human Apobec3DE can nonspecifically inhibit foreign DNA, as has been previously reported (44), we cotransfected human Apobec3DE with a plasmid that expresses neomycin resistance. We observed no difference in the numbers of neomycin-resistant colonies formed in the presence and in the absence of human Apobec3DE (Fig. 2B). Thus, Apobec3DE specifically inhibits MusD and Alu retrotransposition. Despite the sequence divergence between human and chimpanzee Apobec3DE, both are able to restrict MusD and multiple Alu subfamilies, suggesting that this is a conserved and important function of Apobec3DE that is retained despite the sequence divergence between human and chimpanzee Apobec3DE genes. Moreover, this emphasizes that the diversification of Alu elements was not the primary driver of the evolution of human and chimpanzee Apobec3DE.
Fig. 2.
Human and chimpanzee Apobec3DE restrict multiple Alu subfamilies. Human and chimpanzee Apobec3DE were expressed in retrotransposition assays to determine their antiviral activity. Retrotransposition activity is normalized to 100% in the absence of Apobec3 (none). (A) Retrotransposition of consensus Alu subfamilies in the absence or presence of human Apobec3G (hA3G), human Apobec3DE (hA3DE), or chimpanzee Apobec3DE (hA3DE). (B) Percentage of nonretroviral neomycin-resistant colonies formed in the absence or presence of human Apobec3DE. Error bars represent the standard deviation of triplicate transfections within one experiment.
Chimpanzee, but not human, Apobec3DE restricts lentiviruses.
We further characterized the breadth of Apobec3DE restriction by testing the ability of human and chimpanzee orthologs to inhibit a range of retroviruses, including three different primate lentiviruses and one gammaretrovirus. We assessed the ability of human and chimpanzee Apobec3DE to inhibit MLV, HIV-1, HIV-2, and SIVagmTAN in the presence (wild type [WT]) or absence of Vif (Δvif) using single-round infectivity assays. We cotransfected Apobec3DE with proviral constructs and VSV-G to produce pseudotyped virions, and equivalent amounts of virions were used to infect SupT1 cells.
We found that neither human nor chimpanzee Apobec3DE restricted MLV or HIV-2Δvif, unlike human Apobec3G, which restricted both MLV and HIV-2Δvif (Fig. 3A and B). However, we found a marked difference between human and chimpanzee Apobec3DE in their ability to restrict other retroviruses. Chimpanzee Apobec3DE restricted HIV-1Δvif and SIVagmTANΔvif (20- and 40-fold, respectively, Fig. 3C and D) almost as potently as human Apobec3G, whereas human Apobec3DE only weakly restricted HIV-1Δvif and SIVagmTANΔvif (3-fold and 6-fold, respectively). This difference between human and chimpanzee Apobec3DE in their activity against HIV-1Δvif and SIVagmTANΔvif is significant (P < 0.05). Clearly, chimpanzee Apobec3DE, but not human Apobec3DE, can potently restrict certain lentiviruses.
Fig. 3.
Restriction of retroviruses by human and chimpanzee Apobec3DE. Human Apobec3DE (hA3DE) and chimpanzee Apobec3DE (cA3DE) were expressed in single-round infectivity assays to determine antiviral activity. Infections in the presence of human Apobec3G (hA3G) were used as a positive control. Infectivity is normalized to 100% in the absence of Apobec3 (none). (A) Infectivity of MLV. (B) Infectivity of HIV-2 without Vif (HIV-2Δvif) and of HIV-2 containing Vif (HIV-2 WT). (C) Infectivity of HIV-1 without Vif (HIVΔvif) and HIV-1 containing Vif (HIV WT). (D) Infectivity of SIVagmTAN without Vif (SIVagmTANΔvif) and SIVagmTAN containing Vif (SIVagmTAN WT). Experiments were performed at least 2 times, and results from one representative experiment are shown. Error bars represent the standard deviation of triplicate infections within one experiment. P values were calculated using paired two-tailed Student's t test.
Because human and chimpanzee Apobec3DE have differential antiviral activities against HIV-1Δvif and SIVagmTANΔvif, we hypothesized that they may also be differentially sensitive to neutralization by HIV-1 and SIVagmTAN Vif proteins. However, infectivity of HIV-1 was recovered in the presence of HIV-1 Vif for all Apobec3s tested (Fig. 3C), and infectivity of SIVagmTAN was only partially recovered by SIVagmTAN Vif for all Apobec3s tested (Fig. 3D). Therefore, human and chimpanzee Apobec3DE are similar in their sensitivity to Vif proteins. These data suggest that the divergence between human and chimpanzee Apobec3DE, which allowed chimpanzee Apobec3DE to target a much broader range of retroelements than human Apobec3DE, was not driven by antagonism by Vif.
Apobec3DE is incorporated into virions, and protein levels are decreased by HIV-1 Vif.
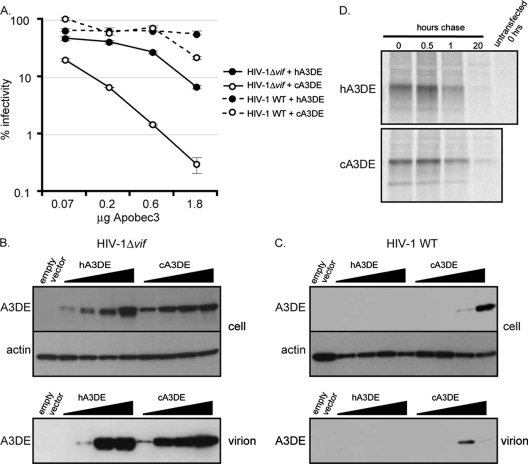
We wished to determine the basis for the differences in antiviral activity between human and chimpanzee Apobec3DE. We tested a gradient of human and chimpanzee Apobec3DE levels against HIV-1 and examined, in parallel, their cellular and virion-incorporated protein levels. HIV-1Δvif virions containing increasing amounts of chimpanzee Apobec3DE lost infectivity in a dose-dependent manner (Fig. 4A). However, HIV-1Δvif virions containing increasing amounts of human Apobec3DE remained more infectious at all doses tested (Fig. 4A). At the highest concentration of Apobec3DE, chimpanzee Apobec3DE restricted HIV-1Δvif 20 times more potently than human Apobec3DE. Western blots of cellular lysates show similar protein levels of human and chimpanzee Apobec3DE (Fig. 4B, top), indicating that differential protein expression is not the cause of the differential restriction phenotypes. In addition, we examined viral lysates to determine the levels of Apobec3DE incorporated into virions and found that both human and chimpanzee Apobec3DE are incorporated into virions at high levels (Fig. 4B, bottom). We did not find human Apobec3DE in the supernatant of cells transfected without the viral vector, indicating that human Apobec3DE is indeed contained within infectious particles (data not shown). Thus, neither limits on cellular expression nor virion incorporation underlies the differences in antiviral activity between human and chimpanzee Apobec3DE. We also performed a pulse-chase analysis of Apobec3DE in 293T cells and showed that human Apobec3DE has a half-life similar to that of chimpanzee Apobec3DE (Fig. 4D). From these data, we can conclude that the intrinsic stability of the human Apobec3DE protein is intact and is not responsible for its weak restriction of HIV-1Δvif.
Fig. 4.
Human and chimpanzee Apobec3DE are incorporated into HIV-1Δvif virions. The infectivity of HIV with and without Vif was assessed in the presence of increasing amounts of Apobec3DE. (A) Threefold dilutions of human and chimpanzee Apobec3DE were expressed in single-round infectivity assays. Infections are represented as a percentage of the infectivity of HIV-1 without Apobec3, which was set to 100%. Filled circles show infections in the presence of human Apobec3DE (hA3DE), and open circles show infections in the presence of chimpanzee Apobec3DE (cA3DE). Solid lines show infections with HIV-1 without Vif (HIV-1Δvif), and dashed lines show infections with HIV-1 containing Vif (HIV-1 WT). The experiment was performed at least 3 times, and results from one representative experiment are shown. Error bars represent the standard deviation of triplicate infections within one experiment. (B) Western blot analysis of Apobec3DE protein levels in the absence of Vif. (Top) Cell lysates from HIV-1Δvif infections. (Bottom) Viral lysates from HIV-1Δvif infections. (C) Western blot analysis of Apobec3DE protein levels in the presence of Vif. (Top) Cell lysates from infections with HIV-1 containing Vif. (Bottom) Viral lysates from infections with HIV-1 containing Vif. Apobec3DE levels are increasing from left to right on each blot. Apobec3DE was detected with an anti-HA antibody. Actin was used as a loading control for cellular lysates. Virions were normalized by quantification of p24 gag levels before lysis. (D) Pulse-chase analysis of Apobec3DE proteins. Human and chimpanzee Apobec3DE were radiolabeled for 30 min and chased for 0 min, 30 min, 1 h, and 20 h. Proteins were immunoprecipitated using an anti-HA antibody and resolved by SDS-PAGE. Autoradiography was used to visualize the gel.
We also found that HIV-1 Vif can antagonize increasing amounts of both human and chimpanzee Apobec3DE (Fig. 4A). Additionally, cellular and viral levels of Apobec3DE protein drop dramatically in the presence of Vif (Fig. 4C). Consistent with previous models of Vif action (reviewed in reference 26), our findings suggest that HIV-1 Vif antagonizes Apobec3DE by targeting it for degradation in the cell. Thus, despite the differential ability of human and chimpanzee Apobec3DE to restrict HIV-1Δvif, human and chimpanzee Apobec3DEs are incorporated into virions, and protein levels are decreased by HIV-1 Vif, indicating that at least some of their functional interactions with lentiviral proteins must be conserved.
The C terminus of Apobec3DE determines antiviral activity.
To determine which region of chimpanzee Apobec3DE confers its ability to restrict lentiviruses, we made several chimeras of human and chimpanzee Apobec3DE (Fig. 5A). Swapping the last 131 amino acids, differing by 10 residues, of chimpanzee Apobec3DE with the corresponding residues of human Apobec3DE (human/chimpanzee 1) leads to a loss of the ability of chimpanzee Apobec3DE to restrict HIV-1Δvif (Fig. 5B). Moreover, the reverse swap (human/chimpanzee 2) allowed human Apobec3DE to restrict HIV-1Δvif (P < 0.01). These chimeras led to the identification of the C terminus of the protein as responsible for its antiviral activity. Interestingly, the human/chimpanzee 2 chimera can restrict HIV-1Δvif even more strongly than chimpanzee Apobec3DE (P < 0.01), suggesting that an interaction with the N terminus of human Apobec3DE enhances restriction. By making additional chimeras, we found that swapping a 34-amino-acid patch, differing by 5 residues, within the C terminus of human Apobec3DE (human/chimpanzee 4) is sufficient for human Apobec3DE to gain a level of restriction of HIV-1Δvif equal to that of chimpanzee Apobec3DE. In support of this finding, one of these residues (position 320) has recently been shown to affect the antiviral activity of several Apobec3s, including Apobec3DE (11). Therefore, this patch in the C terminus of Apobec3DE is essential for restriction of HIV-1, and additional residues across Apobec3DE also contribute to antiviral activity. These data show that the C terminus of Apobec3DE determines the target specificity of Apobec3DE and suggest that sequence divergence in this region is especially relevant to antiviral activity.
Fig. 5.
C terminus of Apobec3DE determines its ability to restrict HIV-1Δvif. (A) (Top) Schematic of human/chimpanzee (h/c) Apobec3DE chimeras used in infectivity assays. (Bottom) Western blot analysis of cellular levels of Apobec3DE chimeras. Apobec3DE was detected with an anti-HA antibody. Actin was used as a loading control. (B) Infectivity of HIV-1Δvif in the presence of human/chimpanzee chimeras. Infections are represented as a percentage of the infectivity of HIV-1 without Apobec3 (none), which was set to 100%. Experiments were performed at least 3 times, and results from one representative experiment are shown. Error bars represent the standard deviation of triplicate infections within one experiment. P values were calculated using two-tailed Student's t test.
Inefficient hypermutation of viral genomes by human Apobec3DE.
Human Apobec3DE is incorporated into HIV-1Δvif virions and yet does not restrict viral replication (Fig. 4). Because Apobec3s can restrict viruses by inducing G-to-A hypermutation of the viral genome, we asked whether human Apobec3DE induces hypermutation of HIV-1. We infected SupT1 cells with HIV-1Δvif containing human or chimpanzee Apobec3DE and measured G-to-A hypermutation of reverse-transcribed viral genomes.
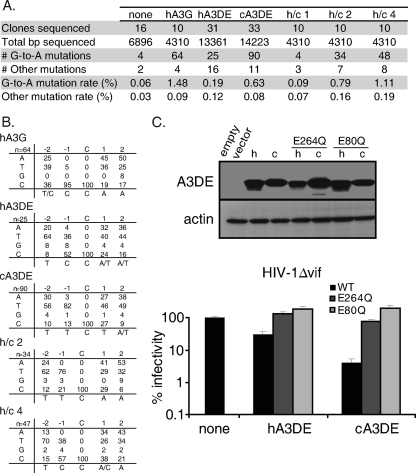
We found that viral genomes had a higher rate of G-to-A hypermutation when viruses contained chimpanzee Apobec3DE than when viruses contained human Apobec3DE (0.63% and 0.19%, respectively, Fig. 6A). Furthermore, we tested the human/chimpanzee Apobec3DE chimeras from Fig. 5 for their ability to induce hypermutation of the viral genome, and the levels of hypermutation correlate well with their antiviral activity in single-round infectivity assays. For instance, human/chimpanzee 1, which has reduced levels of HIV-1 restriction, has a very low rate of G-to-A hypermutation (0.09%), whereas human/chimpanzee 2 and human/chimpanzee 4, which have increased levels of restriction, induce high rates of G-to-A hypermutation (0.79% and 1.11%, respectively). As a positive control, genomes were collected from cells infected with virus containing human Apobec3G, and these sequences had very high levels of G-to-A hypermutation (1.48%), as expected. Thus, while human Apobec3DE can deaminate cytidines during infection, it induces hypermutation of the HIV-1 genome much less frequently than chimpanzee Apobec3DE does, and this difference in hypermutation is determined by the residues identified in Fig. 5 as important for antiviral activity.
Fig. 6.
Chimpanzee Apobec3DE induces higher levels of hypermutation than human Apobec3DE does during viral infection. (A) HIV-1 genomes were sequenced from HIV-1Δvif infections performed in the absence of Apobec3 (none) or in the presence of human Apobec3G (hA3G), human Apobec3DE (hA3DE), chimpanzee Apobec3DE (cA3DE), or human/chimpanzee chimeras, and the G-to-A mutation rate of viral genomes was calculated. The rate of other mutations was also calculated. Combined data from three independent experiments are shown. (B) The nucleotide context of G-to-A mutations was characterized. The percentage of each nucleotide occurring at the −2, −1, +1, and +2 positions of deaminated cytidines is shown, with the most common nucleotide listed below each position. The number of mutations examined is noted in the top left corner of each grid. (C) (Top) Western blot analysis of cellular levels of human (h) and chimpanzee (c) Apobec3DE catalytic mutants. Apobec3DE was detected with an anti-HA antibody. Actin was used as a loading control. (Bottom) Infectivity of HIV-1Δvif in the presence of wild-type Apobec3DE (WT) or Apobec3DE catalytic mutants (E264Q and E80Q). Infections are represented as a percentage of the infectivity of HIV-1 without Apobec3 (none), which was set to 100%. Experiments were performed at least 2 times, and results from one representative experiment are shown. Error bars represent the standard deviation of triplicate infections within one experiment.
The sequence context of the mutated cytidines was also examined to identify target motifs for Apobec3DE deamination. We calculated the frequency of each nucleotide at the −2, −1, +1, and +2 positions of the deaminated cytidines identified in Fig. 6A. As has been previously shown, cytidines deaminated by human Apobec3G were found most frequently with a cytidine in the −1 position, referred to as a CC motif (Fig. 6B). We found that human Apobec3DE also preferentially deaminated cytidines in a CC motif or, slightly less frequently, with a thymine in the −1 position, referred to as a TC motif. Chimpanzee Apobec3DE clearly preferentially targeted cytidines in a TC context. Human/chimpanzee chimera 2, which contains the C terminus of chimpanzee Apobec3DE, also shared this TC target motif. Interestingly, human/chimpanzee 4, which contains only 5 residues from chimpanzee Apobec3DE, deaminated cytidines in a context more similar to human Apobec3DE (TC and CC motifs). This suggests that the target motif of Apobec3DE is determined by residues in the C terminus outside these 5 residues. Together, these data show that human and chimpanzee Apobec3DE target cytidines in slightly different contexts and at very different rates during infection.
To determine whether the high levels of G-to-A hypermutation induced by chimpanzee Apobec3DE contribute to its restriction of HIV-1, we evaluated the dependence of Apobec3DE restriction on its cytidine deaminase catalytic activity. We created catalytic mutants of human and chimpanzee Apobec3DE by changing a single glutamate to a glutamine within the HxE(x)27PCxxC catalytic domain (25, 27). Because Apobec3DE contains two cytidine deaminase domains, we mutated each domain individually (E80Q and E264Q). We also constructed double catalytic mutants, but these mutants had very low or undetectable protein expression levels (data not shown). We found that the catalytic mutants of Apobec3DE lost the ability to restrict HIV-1Δvif (Fig. 6C, shaded bars), showing that cytidine deamination is necessary for Apobec3DE restriction of HIV-1. Together, these data suggest that human Apobec3DE has weak antiviral activity against HIV-1, despite being incorporated into virions, due to lower levels of cytidine deamination of the HIV-1 genome.
Rapid evolution of Apobec3DE in chimpanzees.
Due to the differential antiviral activity found here for human and chimpanzee Apobec3DE, we were interested in whether this functional divergence was driven by positive selection. In previous evolutionary analyses of Apobec3 genes, pairwise comparisons of human and chimpanzee divergence found a high ratio of the nonsynonymous mutation rate (dN) to the synonymous mutation rate (dS) for Apobec3DE (called dN/dS) (41). Since pairwise comparisons do not indicate which species is rapidly evolving, we performed a more detailed evolutionary analysis of Apobec3DE to determine whether human or chimpanzee Apobec3DE experienced a selective pressure.
We collected Apobec3DE sequences from additional hominoids (bonobo, gorilla, and gibbon) and Old World monkeys (rhesus macaque, African green monkey, and Patas monkey). We amplified most Apobec3DE sequences from genomic DNA. However, bacterial artificial chromosome (BAC) sequences containing the Apobec3 locus from gorilla (accession number FP245429), gibbon (ADFV01024628), and orangutan (AC206461) genomes appeared to be missing the 3′ half or all of Apobec3DE. Since we were unsure whether this represented true deletions of sequences or assembly errors, we designed primers in the 5′ portion of the gene and used 3′ RACE to amplify gorilla and gibbon Apobec3DE. We were unable to amplify orangutan Apobec3DE in this way as no BAC clones containing Apobec3DE sequences were identified to design effective primers. Because of the large amount of homology between Apobec3DE and Apobec3F, we also collected sequences for Apobec3F to distinguish the paralogs. Nucleotide sequences for Apobec3DE and Apobec3F were aligned, and a maximum likelihood (ML) phylogeny was constructed (Fig. 7A). The sequences that we obtained for Apobec3DE and Apobec3F cluster with known sequences and are clearly distinct. The phylogenies of Apobec3DE and Apobec3F follow the predicted species tree with high statistical support, except for the gorilla-chimpanzee-human trichotomy, which could not be resolved with these sequences. We evaluated an alignment of Apobec3DE sequences for evidence of recombination using the GARD program (23) and found no evidence for recombination in Apobec3DE.
Fig. 7.
Apobec3DE evolved rapidly in chimpanzee ancestors. Sequences obtained by PCR in this study, as described in Materials and Methods, were analyzed. (A) A maximum-likelihood phylogeny based on primate Apobec3DE and Apobec3F sequences is shown. Statistical support, calculated as aLRT values, is shown for each node. (B) Positive selection analysis of Apobec3DE. Two phylogenies of Apobec3DE, one with the human ortholog as an outgroup to gorilla, chimpanzee, and bonobo Apobec3DE (right) and another with gorilla Apobec3DE as an outgroup to human, chimpanzee, and bonobo Apobec3DE (left), were analyzed. Global dN/dS ratios are indicated above each branch. The numbers of nonsynonymous and synonymous changes along each branch are indicated below each branch in parentheses (NS:S). dN/dS values greater than 1 are indicative of positive selection. (C) Codons in Apobec3DE under positive selection. An alignment of human and chimpanzee Apobec3DE protein sequences is shown with chimpanzee-specific changes highlighted in black. Residues having a high probability of being under positive selection across all primates are marked with asterisks. Residues with BEB values of >0.95 are marked with a single asterisk, and residues with BEB values of >0.99 are marked with two asterisks. The C-terminal domain in Apobec3DE that was tested for antiviral activity is underlined, with the minimal 5 residues that are required for antiviral activity underlined twice.
To determine the rate of evolution of Apobec3DE along each primate lineage, we used phylogenetic analysis by maximum likelihood (PAML) and performed a free-ratio model to calculate the dN/dS ratio for each branch. Because the gorilla-chimpanzee-human trichotomy was ambiguous for Apobec3DE, we performed these analyses using two phylogenies of Apobec3DE—the gene phylogeny obtained by ML with human Apobec3DE as an outgroup to gorilla, chimpanzee, and bonobo Apobec3DE (Fig. 7B, right) and a species phylogeny with gorilla Apobec3DE as an outgroup to human, chimpanzee, and bonobo Apobec3DE (Fig. 7B, left). For both input trees, we found that a model of codon evolution under positive selection (M2 and M8) fit our data more strongly than a model of codon evolution under neutral selection (M1 and M7, P < 0.001, Table 1). Also, the dN/dS values along each lineage are similar between the two trees. We found that the chimpanzee-bonobo ancestor of Apobec3DE has a dN/dS value much greater than 1 (6.6 or 6.5), indicative of rapid evolution in this lineage between 2 and 6 million years ago. This represents the most intense episode of positive selection among all primate Apobec3DEs and, indeed, among all Apobec3s (37, 41). The human Apobec3DE lineage exhibits lower dN/dS values (0.9 or 2.3). More than twice as many amino acids changed in chimpanzee Apobec3DE as in human Apobec3DE since their divergence (25 or 26 compared to 11 or 10, respectively). To assess the dN/dS ratio of the chimpanzee-bonobo ancestor of Apobec3DE, we compared a model where dN/dS was fixed at 1 along this branch to a model where dN/dS was allowed to be greater than 1 along this branch. The second model fit our data more strongly (P < 0.05), confirming positive selection along this lineage. The 25 sites that unambiguously changed in the chimpanzee lineage were identified using the CODEML marginal reconstruction of the human-chimpanzee ancestral sequences from both phylogenies (residues highlighted in Fig. 7C). Strikingly, all of the residues identified in Fig. 5 that alter Apobec3DE antiviral activity evolved in the chimpanzee lineage, rather than in the human lineage. This shows that the increase in antiviral activity within chimpanzee Apobec3DE was driven by positive selection since separation from the human-chimpanzee common ancestor.
Table 1.
Likelihood ratio tests for positive selection in Apobec3DE
| Treea | Codon modelb | M1 vs M2c | M7 vs M8d | Chimpanzee-bonobo ancestore |
|---|---|---|---|---|
| A (h [g {c, b}]) | F3 × 4 | P < 0.001 | P < 0.001 | P < 0.05 |
| F61 | P < 0.001 | P < 0.001 | P < 0.05 | |
| B (g [h {c, b}]) | F3 × 4 | P < 0.001 | P < 0.001 | P < 0.05 |
| F61 | P < 0.001 | P < 0.001 | P < 0.05 |
Tree A was obtained with Apobec3DE gene sequences. In tree A, human Apobec3DE (h) is an outgroup to gorilla (g), chimpanzee (c), and bonobo (b) Apobec3DE. Tree B follows species phylogeny. In tree B, gorilla Apobec3DE is an outgroup to human, chimpanzee, and bonobo Apobec3DE.
Two different models of codon frequencies (F3 × 4 and F61) were used for analyses.
Likelihood ratio tests were performed by comparing model 1 (two-state, dN/dS > 1 disallowed) to model 2 (two-state, dN/dS > 1 allowed). P values of <0.05 indicate that a model of selection (M2) provides a better fit of the data.
Likelihood ratio tests were performed by comparing model 7 (beta distribution, dN/dS > 1 disallowed) to model 8 (beta distribution, dN/dS > 1 allowed). P values of <0.05 indicate that a model of selection (M8) provides a better fit of the data.
Likelihood ratio tests were performed by comparing a model where dN/dS = 1 to a model where dN/dS > 1 for the chimpanzee-bonobo ancestor. P values of <0.05 indicate that a model of selection (dN/dS > 1) provides a better fit of the data.
We also identified individual codons in Apobec3DE under positive selection across primates. By using a Bayes empirical Bayes (BEB) analysis, we found that 15 residues have a high posterior probability of dN/dS > 1 using both phylogenies (*, BEB > 0.95; **, BEB > 0.99; Fig. 7C). Interestingly, many of the positively selected codons cluster in the C terminus of Apobec3DE, which contains residues important for viral restriction. Our analysis of the evolution of Apobec3DE indicates that selection has acted both across primates and very potently in the chimpanzee lineage. Further, this evolution is targeted to the domain of Apobec3DE that determines viral specificity, suggesting that positive selection may have driven changes in the antiviral activity of other primates' Apobec3DE in addition to broadening the target range of chimpanzee Apobec3DE.
DISCUSSION
In this study, we investigated the role and diversification of the primate gene Apobec3DE in host defense. We found that the human and chimpanzee orthologs of Apobec3DE are able to strongly inhibit Alu and MusD retrotransposition. Chimpanzee Apobec3DE is also able to restrict the infectivity of HIV-1 and SIVagmTAN in the absence of Vif, while human Apobec3DE has weak activity against all retroviruses tested. We show that human and chimpanzee Apobec3DE have similar cellular and virion-associated protein levels but differentially hypermutate HIV-1 genomes. Further, we show that Apobec3DE has evolved very rapidly in chimpanzee ancestors and in the domain responsible for antiviral activity. This suggests that a selective pressure existing between 2 and 6 million years ago drove the adaptation of antiviral activity of chimpanzee Apobec3DE.
The C terminus of Apobec3DE contains the residues responsible for the differential activity of human and chimpanzee Apobec3DE (Fig. 5). We mapped this region to a patch between residues 311 and 345 of Apobec3DE containing five amino acid differences. These data correlate very well with recently published data showing that human Apobec3DE, which has weak activity against HIV-1, has much higher antiviral activity when the cysteine residue at position 320 is changed to a tyrosine (notably, the residue found in chimpanzee Apobec3DE) (11). Additionally, many residues in the C terminus of Apobec3DE are under positive selection (Fig. 7), suggesting that this domain of Apobec3DE has repeatedly changed throughout evolutionary history, perhaps because it forms an interaction with a viral factor.
The reason that human Apobec3DE does not induce hypermutation of lentiviruses is unclear, as the human Apobec3DE protein is efficiently packaged into virions (Fig. 4) and can restrict other retroelements (Fig. 1 and 2). Potentially, the human Apobec3DE protein may have low levels of enzymatic deaminase activity compared to chimpanzee Apobec3DE; however, we were unable to address this because, in an in vitro gel-based deaminase assay, we were unable to detect deaminase activity of either human or chimpanzee Apobec3DE (data not shown). Alternatively, human Apobec3DE may contain catalytic activity but be improperly targeted within the virion, such as is proposed for human Apobec3A (1, 18), rendering it unable to access the viral genome appropriately. Because of the strong denaturants required to resolve human Apobec3DE by SDS-PAGE (see Materials and Methods), we know that the human Apobec3DE protein has some biochemical differences from chimpanzee Apobec3DE, perhaps preventing it from binding necessary viral factors.
It is possible that human Apobec3DE lost antiviral activity since divergence with chimpanzees due to a lack of selective pressure; however, human Apobec3DE can inhibit Alu elements that have been active in human ancestors for millions of years (Fig. 2) (reviewed in reference 4), and this interaction likely exerted some selective pressure on Apobec3DE. In fact, human Apobec3DE is likely to have a current role in cell intrinsic immunity, as it can restrict the activity of retrotransposons that are still active in the human population (Fig. 1). Even though other human Apobec3s can restrict Alu elements, human Apobec3DE may have an expression profile that differs from other Apobec3s, thereby retaining its selective pressure and leading to its conservation as a defense factor across primates. In support of a potential role in host defense, other studies have found broad Apobec3DE mRNA expression in many tissues (12, 22, 38), and Apobec3DE transcript levels have been shown to be moderately induced by interferon (IFN) treatment, especially in monocyte-derived macrophages (22, 38). Identifying the tissue tropisms of human and chimpanzee Apobec3DE proteins may further explain their differences in targets and selective pressures. Additionally, human Apobec3DE may have gained activities against a pathogen not tested here, which would exert an additional selective pressure.
The most likely scenario is that chimpanzee Apobec3DE gained antiviral activity since its divergence from human Apobec3DE. There are no changes in the C terminus of Apobec3DE in the human lineage, suggesting that the human-chimpanzee ancestor of Apobec3DE would function most similarly to human Apobec3DE and would not have the ability to restrict lentiviruses that are similar to the ones tested here. Because chimpanzee Apobec3DE has evolved antiviral activity only against some lentiviruses rather than against other retroviruses or additional retrotransposons (Fig. 1 to 3), the selection on chimpanzee Apobec3DE would most likely have been an ancient lentivirus. A Vif-like protein is also unlikely to have been the viral factor driving selection on chimpanzee Apobec3DE because both human and chimpanzee Apobec3DE are sensitive to antagonism by lentiviral Vif proteins. The identity of this paleolentivirus is unknown because modern primate lentiviruses are too young to have driven the rapid evolution of chimpanzee Apobec3DE, which we showed in Fig. 7 occurred 2 to 6 million years ago (15, 41). However, since endogenous lentiviral sequences identified in lemur genomes show that lentiviruses have circulated in prosimians for millions of years (16, 17), it is likely that ancient lentiviruses also infected other primates millions of years ago. Since chimpanzee Apobec3DE can restrict modern-day lentiviruses, we can infer that an extinct lentivirus must have infected chimpanzee ancestors 2 to 6 million years ago and was responsible for the selection of Apobec3DE along the chimpanzee lineage since its divergence from the human-chimpanzee common ancestor. Thus, we have identified the possible consequences of an ancient lentivirus infection of chimpanzees, as well as a potential role for Apobec3DE in host defense in modern hominids.
ACKNOWLEDGMENTS
We thank Masahiro Yamashita for the pRODΔenvEGFP plasmid and Scott Devine for plasmids containing consensus Alu-Ya5, -Yc1, -Y, -Sx, and -Jo elements. We thank the FHCRC Genetic Analysis core, Adam Geballe, and Stephanie Child for advice on the pulse-chase analysis; Jaisri Lingappa for advice on the deaminase assay; and Alex Compton, Melody Li, and Semih Tareen for comments on the manuscript.
This work was supported by NIH grant R01 AI30937 (M.E.) and an NSF Career grant (H.S.M.). H.S.M. is an Early-Career Scientist of the Howard Hughes Medical Institute. N.K.D. was supported by an NSF Graduate Research Fellowship.
Footnotes
Published ahead of print on 10 August 2011.
REFERENCES
- 1. Aguiar R. S., Lovsin N., Tanuri A., Peterlin B. M. 2008. Vpr.A3A chimera inhibits HIV replication. J. Biol. Chem. 283:2518–2525 [DOI] [PubMed] [Google Scholar]
- 2. Alisch R. S., Garcia-Perez J. L., Muotri A. R., Gage F. H., Moran J. V. 2006. Unconventional translation of mammalian LINE-1 retrotransposons. Genes Dev. 20:210–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bartz S. R., Vodicka M. A. 1997. Production of high-titer human immunodeficiency virus type 1 pseudotyped with vesicular stomatitis virus glycoprotein. Methods 12:337–342 [DOI] [PubMed] [Google Scholar]
- 4. Batzer M. A., Deininger P. L. 2002. Alu repeats and human genomic diversity. Nat. Rev. Genet. 3:370–379 [DOI] [PubMed] [Google Scholar]
- 5. Bennett E. A., et al. 2008. Active Alu retrotransposons in the human genome. Genome Res. 18:1875–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bishop K. N., et al. 2004. Cytidine deamination of retroviral DNA by diverse APOBEC proteins. Curr. Biol. 14:1392–1396 [DOI] [PubMed] [Google Scholar]
- 7. Bogerd H. P., Doehle B. P., Wiegand H. L., Cullen B. R. 2004. A single amino acid difference in the host APOBEC3G protein controls the primate species specificity of HIV type 1 virion infectivity factor. Proc. Natl. Acad. Sci. U. S. A. 101:3770–3774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bogerd H. P., Wiegand H. L., Doehle B. P., Lueders K. K., Cullen B. R. 2006. APOBEC3A and APOBEC3B are potent inhibitors of LTR-retrotransposon function in human cells. Nucleic Acids Res. 34:89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bogerd H. P., et al. 2006. Cellular inhibitors of long interspersed element 1 and Alu retrotransposition. Proc. Natl. Acad. Sci. U. S. A. 103:8780–8785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen H., et al. 2006. APOBEC3A is a potent inhibitor of adeno-associated virus and retrotransposons. Curr. Biol. 16:480–485 [DOI] [PubMed] [Google Scholar]
- 11. Dang Y., et al. 2011. Identification of a single amino acid required for APOBEC3 antiretroviral cytidine deaminase activity. J. Virol. 85:5691–5695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dang Y., Wang X., Esselman W. J., Zheng Y. H. 2006. Identification of APOBEC3DE as another antiretroviral factor from the human APOBEC family. J. Virol. 80:10522–10533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dereeper A., et al. 2008. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36:W465–W469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dewannieux M., Esnault C., Heidmann T. 2003. LINE-mediated retrotransposition of marked Alu sequences. Nat. Genet. 35:41–48 [DOI] [PubMed] [Google Scholar]
- 15. Emerman M., Malik H. S. 2010. Paleovirology—modern consequences of ancient viruses. PLoS Biol. 8:e1000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gifford R. J., et al. 2008. A transitional endogenous lentivirus from the genome of a basal primate and implications for lentivirus evolution. Proc. Natl. Acad. Sci. U. S. A. 105:20362–20367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gilbert C., Maxfield D. G., Goodman S. M., Feschotte C. 2009. Parallel germline infiltration of a lentivirus in two Malagasy lemurs. PLoS Genet. 5:e1000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goila-Gaur R., Khan M. A., Miyagi E., Kao S., Strebel K. 2007. Targeting APOBEC3A to the viral nucleoprotein complex confers antiviral activity. Retrovirology 4:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hedges D. J., et al. 2004. Differential alu mobilization and polymorphism among the human and chimpanzee lineages. Genome Res. 14:1068–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holmes R. K., Malim M. H., Bishop K. N. 2007. APOBEC-mediated viral restriction: not simply editing? Trends Biochem. Sci. 32:118–128 [DOI] [PubMed] [Google Scholar]
- 21. Jarmuz A., et al. 2002. An anthropoid-specific locus of orphan C to U RNA-editing enzymes on chromosome 22. Genomics 79:285–296 [DOI] [PubMed] [Google Scholar]
- 22. Koning F. A., et al. 2009. Defining APOBEC3 expression patterns in human tissues and hematopoietic cell subsets. J. Virol. 83:9474–9485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kosakovsky Pond S. L., Posada D., Gravenor M. B., Woelk C. H., Frost S. D. 2006. Automated phylogenetic detection of recombination using a genetic algorithm. Mol. Biol. Evol. 23:1891–1901 [DOI] [PubMed] [Google Scholar]
- 24. Li M. M., Wu L. I., Emerman M. 2010. The range of human APOBEC3H sensitivity to lentiviral Vif proteins. J. Virol. 84:88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. MacGinnitie A. J., Anant S., Davidson N. O. 1995. Mutagenesis of apobec-1, the catalytic subunit of the mammalian apolipoprotein B mRNA editing enzyme, reveals distinct domains that mediate cytosine nucleoside deaminase, RNA binding, and RNA editing activity. J. Biol. Chem. 270:14768–14775 [PubMed] [Google Scholar]
- 26. Malim M. H., Emerman M. 2008. HIV-1 accessory proteins—ensuring viral survival in a hostile environment. Cell Host Microbe 3:388–398 [DOI] [PubMed] [Google Scholar]
- 27. Mangeat B., et al. 2003. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature 424:99–103 [DOI] [PubMed] [Google Scholar]
- 28. Mangeat B., Turelli P., Liao S., Trono D. 2004. A single amino acid determinant governs the species-specific sensitivity of APOBEC3G to Vif action. J. Biol. Chem. 279:14481–14483 [DOI] [PubMed] [Google Scholar]
- 29. Mariani R., et al. 2003. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell 114:21–31 [DOI] [PubMed] [Google Scholar]
- 30. Miller A. D., Rosman G. J. 1989. Improved retroviral vectors for gene transfer and expression. Biotechniques 7:980–990 [PMC free article] [PubMed] [Google Scholar]
- 31. Mills R. E., et al. 2006. Recently mobilized transposons in the human and chimpanzee genomes. Am. J. Hum. Genet. 78:671–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miyagi E., et al. 2010. Stably expressed APOBEC3F has negligible antiviral activity. J. Virol. 84:11067–11075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moran J. V., DeBerardinis R. J., Kazazian H. H., Jr 1999. Exon shuffling by L1 retrotransposition. Science 283:1530–1534 [DOI] [PubMed] [Google Scholar]
- 34. Mulder L. C., et al. 2010. Moderate influence of human APOBEC3F on HIV-1 replication in primary lymphocytes. J. Virol. 84:9613–9617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Niewiadomska A. M., et al. 2007. Differential inhibition of long interspersed element 1 by APOBEC3 does not correlate with high-molecular-mass-complex formation or P-body association. J. Virol. 81:9577–9583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. OhAinle M., Kerns J. A., Li M. M., Malik H. S., Emerman M. 2008. Antiretroelement activity of APOBEC3H was lost twice in recent human evolution. Cell Host Microbe 4:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. OhAinle M., Kerns J. A., Malik H. S., Emerman M. 2006. Adaptive evolution and antiviral activity of the conserved mammalian cytidine deaminase APOBEC3H. J. Virol. 80:3853–3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Refsland E. W., et al. 2010. Quantitative profiling of the full APOBEC3 mRNA repertoire in lymphocytes and tissues: implications for HIV-1 restriction. Nucleic Acids Res. 38:4274–4284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ribet D., Dewannieux M., Heidmann T. 2004. An active murine transposon family pair: retrotransposition of “master” MusD copies and ETn trans-mobilization. Genome Res. 14:2261–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sambrook J., Russell D. W. 2006. Rapid amplification of 3′ cDNA ends (3′-RACE). Cold Spring Harb. Protoc. doi:10.1101/pdb.prot3865 [DOI] [PubMed] [Google Scholar]
- 41. Sawyer S. L., Emerman M., Malik H. S. 2004. Ancient adaptive evolution of the primate antiviral DNA-editing enzyme APOBEC3G. PLoS Biol. 2:E275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schrofelbauer B., Chen D., Landau N. R. 2004. A single amino acid of APOBEC3G controls its species-specific interaction with virion infectivity factor (Vif). Proc. Natl. Acad. Sci. U. S. A. 101:3927–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smith J. L., Pathak V. K. 2010. Identification of specific determinants of human APOBEC3F, APOBEC3C, and APOBEC3DE and African green monkey APOBEC3F that interact with HIV-1 Vif. J. Virol. 84:12599–12608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stenglein M. D., Burns M. B., Li M., Lengyel J., Harris R. S. 2010. APOBEC3 proteins mediate the clearance of foreign DNA from human cells. Nat. Struct. Mol. Biol. 17:222–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stenglein M. D., Harris R. S. 2006. APOBEC3B and APOBEC3F inhibit L1 retrotransposition by a DNA deamination-independent mechanism. J. Biol. Chem. 281:16837–16841 [DOI] [PubMed] [Google Scholar]
- 46. Tan L., Sarkis P. T., Wang T., Tian C., Yu X. F. 2009. Sole copy of Z2-type human cytidine deaminase APOBEC3H has inhibitory activity against retrotransposons and HIV-1. FASEB J. 23:279–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wei W., et al. 2001. Human L1 retrotransposition: cis preference versus trans complementation. Mol. Cell. Biol. 21:1429–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xu H., et al. 2004. A single amino acid substitution in human APOBEC3G antiretroviral enzyme confers resistance to HIV-1 virion infectivity factor-induced depletion. Proc. Natl. Acad. Sci. U. S. A. 101:5652–5657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yamashita M., Emerman M. 2004. Capsid is a dominant determinant of retrovirus infectivity in nondividing cells. J. Virol. 78:5670–5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang Z. 2007. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24:1586–1591 [DOI] [PubMed] [Google Scholar]
- 51. Yang Z., Wong W. S., Nielsen R. 2005. Bayes empirical bayes inference of amino acid sites under positive selection. Mol. Biol. Evol. 22:1107–1118 [DOI] [PubMed] [Google Scholar]