Abstract
Innate and adaptive immunity play important protective roles by combating herpes simplex virus 1 (HSV-1) infection. Transforming growth factor β (TGF-β) is a key negative cytokine regulator of both innate and adaptive immune responses. Yet, it is unknown whether TGF-β signaling in either immune compartment impacts HSV-1 replication and latency. We undertook genetic approaches to address these issues by infecting two different dominant negative TGF-β receptor type II transgenic mouse lines. These mice have specific TGF-β signaling blockades in either T cells or innate cells. Mice were ocularly infected with HSV-1 to evaluate the effects of restricted innate or adaptive TGF-β signaling during acute and latent infections. Limiting innate cell but not T cell TGF-β signaling reduced virus replication in the eyes of infected mice. On the other hand, blocking TGF-β signaling in either innate cells or T cells resulted in decreased latency in the trigeminal ganglia of infected mice. Furthermore, inhibiting TGF-β signaling in T cells reduced cell lysis and leukocyte infiltration in corneas and trigeminal ganglia during primary HSV-1 infection of mice. These findings strongly suggest that TGF-β signaling, which generally functions to dampen immune responses, results in increased HSV-1 latency.
INTRODUCTION
Transforming growth factor β (TGF-β) is a pleiotropic cytokine that is present on most cell types and acts as a switch to regulate processes such as immune function, cell proliferation, and differentiation (14, 67, 68). Mammalian TGF-β consists of three nonredundant isoforms (β1, β2, and β3), which all share a high level of homology (14, 43). TGF-β signaling begins with high-affinity binding to a type II Ser/Thr kinase receptor known as TGF-β RII (13, 68). This receptor then phosphorylates and activates a second Ser/Thr kinase receptor, TGF-β RI (13, 75). Once activated, this heteromeric complex recruits the receptor-associated Smad (r-Smad) proteins 2 and 3 (Smad2/3) and together with a co-Smad (Smad4) translocates to the nucleus to mediate transcription of a vast array of genes (39).
Previously, it was shown that TGF-β1 knockout mice survive only to 3 to 4 weeks of age (35), while the absence of TGF-β2 or -β3 is embryonic lethal (30, 55, 59). To overcome developmental problems associated with the generation of TGF-β knockout mice and to specifically limit TGF-β signaling to immune cells, transgenic mice were generated using a dominant negative TGF-β RII (DNR) under innate cell (CD11c) or T cell (CD4) immune-specific promoters (27, 36). Placement of the DNR transgene under the control of the CD11c promoter (CD11cdnTGF-RII) results in a specific TGF-β signaling blockade in innate immune cells (36), while CD4 promoter control (CD4dnTGF-RII) leads to TGF-β signaling inhibition in CD4+ and CD8+ T cells (27). Thus, in CD11cdnTGF-RII mice, the innate immune cells do not respond to TGF-β ligands, while T cells do not respond to TGF-β in CD4dnTGF-RII mice.
Inhibitory molecules such as TGF-β and programmed death ligand 1 (PD-L1) are involved in tumor resistance to immunity (61). For example, it was demonstrated that CD4+ T cells in Hodgkin lymphoma are under the inhibitory influence of both TGF-β and the PD-L1 receptor, programmed death 1 (PD-1) (11). Cooperativity between these pathways has also been reported; specifically, TGF-β is able to upregulate PD-1 expression on T cells in vitro (51). Both PD-1 and PD-L1 are markers of T cell exhaustion (8, 12, 48). In the context of herpes simplex virus 1 (HSV-1) infection, we recently reported increased HSV-1 latency in the trigeminal ganglia (TG) of latently infected mice that correlated with increased expression of PD-1 and PD-L1 (3). We also showed that immunization with different HSV-1 antigens can alter T cell exhaustion in latently infected mice (5), although the relationship between HSV-1 latency and T cell exhaustion is not well understood. Nonetheless, the above studies led us to hypothesize potential interplay between PD-1/PD-L1 and TGF-β in the context of HSV-1 infectivity and reactivation after latency.
It was previously shown that upregulation of TGF-β increases susceptibility to HSV-1 infection in mice (34). However, due to the presence of TGF-β receptors on most cell types, those authors were unable to determine which cellular subset(s) was responsible for the effect of TGF-β on HSV-1 infection. To determine if TGF-β signaling played an immune cell type-specific role in controlling HSV-1 infection, we infected both CD11cdnTGF-RII and CD4dnTGF-RII mice (27, 36, 37). Our findings indicate that blocking TGF-β signaling in either innate cells or T cells reduces latency and reactivation in mice ocularly infected with HSV-1, as determined by latency-associated transcript (LAT) expression levels and TG explant reactivation. These differences were not due to increased apoptosis, cell death, or leukocyte infiltration during primary infection in the eyes and TG of CD4dnTGF-RII mice. Thus, TGF-β affects HSV-1 latency via actions on both innate and adaptive immune compartments.
MATERIALS AND METHODS
Virus, mice, and infection.
Plaque-purified wild-type (WT) HSV-1 strain McKrae was grown in rabbit skin (RS) cell monolayers in minimal essential medium (MEM) containing 5% fetal calf serum (FCS). Methods utilized were as previously described (49, 52).
CD11cdnTGF-RII and CD4dnTGF-RII transgenic mice that express a dominant negative form of TGF-β RII under the control of either the CD11c (36) or CD4 promoter (27), respectively, were bred in-house. Both strains of mice were backcrossed at least 12 times onto a C57BL/6 background. As such, WT C57BL/6 mice were purchased from Jackson Laboratories and used as controls. All animal procedures adhered to the Association for Research in Vision and Ophthalmology (ARVO) statement for the Use of Animals in Ophthalmic and Vision Research and according to institutional animal care and use guidelines.
Mice were infected ocularly with 2 × 105 PFU of the McKrae HSV-1 strain. Virus was suspended in 2 μl of tissue culture medium and administered as an eye drop without prior corneal scarification.
Titration of virus in tears and trigeminal ganglia.
Transgenic and WT C57BL/6 mice were infected as described above. Tear films were collected from both eyes of either 5 or 10 mice per group at various times, using a Dacron-tipped swab (19). Each swab was placed in 0.5 ml of tissue culture medium and squeezed, and the amount of virus was determined by a standard plaque assay on RS cells. In a subset of infected mice on days 3 and 5 postinfection (PI), 3 mice per group were euthanized, and individual TG were isolated for a total 6 TG per group per time point. Individual TG were homogenized, cellular debris was removed, and viral titer in supernatants was measured by standard plaque assay on RS cells as described previously (20, 24).
Monitoring eye disease.
The severity of blepharitis and corneal scaring (CS) was scored in a masked fashion by examination with a slit lamp biomicroscope following the addition of 1% fluorescein as eye drops. Disease was scored on a 0 to 4 scale (0 = no disease and 1 = 25%, 2 = 50%, 3 = 75%, and 4 = 100% involvement) as we described previously (19).
In vitro explant reactivation assay.
Mice were sacrificed at day 30 postinfection, and individual TG were removed and cultured in tissue culture medium. Aliquots of medium were removed from each culture daily for up to 12 days and plated on indicator cells (RS cells) to assay for the appearance of reactivated virus as previously described (44). As the medium from the explanted TG cultures was plated daily, the time at which reactivated virus first appeared in the explanted TG cultures could be determined.
Fluorescence-activated cell staining of isolated cells from cornea and TG.
Corneas or TG from 3 mice per group were harvested on days 3 and 5 PI. Tissues were digested in a phosphate-buffered saline (PBS) solution containing collagenase type I (3 mg/ml; Sigma-Aldrich, St. Louis, MO) and incubated for 2 h at 37°C with trituration approximately every 30 min, according to our previously described methodology (47). The recovered cells were washed and stained for necrosis with 7-amino-actinomycin-D (7-ADD), for apoptosis with phycoerythrin (PE)-annexin V, and for leukocytes for PE–Cy7–anti-CD45 antibodies together for 1 h. Following fixation, cells were washed twice in BD-Perm/Wash, resuspended in 4% paraformaldehyde, and analyzed using multicolor fluorescence-activated cell sorting (FACS) five-laser LSR II instrumentation (Applied Biosystems, Foster City, CA).
RNA extraction, cDNA synthesis, and TaqMan RT-PCR.
Individual TG from mice that survived ocular infection were collected at day 30 postinfection and immersed in RNAlater stabilization reagent and stored at −80°C until processing. Tissue processing, total RNA extraction, and RNA yield were carried out as we have described previously (45, 46). Primer-probe sets consisted of two unlabeled PCR primers and the 6-carboxyfluorescein (FAM) dye-labeled TaqMan MGB probe formulated into a single mixture. The assays used in this study were as follows: (i) CD4, ABI assay identifier Mm00442754_m1, amplicon length of 72 bp; (ii) CD8 (α chain), ABI assay identifier Mn01182108_m1, amplicon length of 67 bp; (iii) PD-1 (also known as CD279), ABI identifier Mm00435532_m1, amplicon length of 65 bp; (iv) Tim-3, ABI identifier Mm00454540_m1, amplicon length of 98 bp; (v) interleukin-21 (IL-21), ABI Mm00517640_m1, amplicon length of 67 bp; (vi) IL-2, ABI assay identifier Mm00434256_m1, amplicon length of 82 bp; (vii) gamma interferon (IFN-γ), ABI assay identifier Mm00801778_m1, amplicon length of 101 bp; and (viii) tumor necrosis factor alpha (TNF-α), ABI identifier Mm00443258_m1, amplicon length of 81 bp. The custom-made primer and probe sets for LAT were forward primer 5′-GGGTGGGCTCGTGTTACAG-3′, reverse primer 5′-GGACGGGTAAGTAACAGAGTCTCTA-3′, and probe 5′-FAM-ACACCAGCCCGTTCTTT-3′, with an amplicon length of 81 bp, corresponding to LAT nucleotides (nt) 119553 to 119634. Quantitative real-time PCR (qRT-PCR) was performed using an ABI PRISM 7900HT sequence detection system (Applied Biosystems, Foster City, CA) in 384-well plates as we described previously (4, 5). GAPDH (glyceraldehyde 3-phosphate dehydrogenase) RNA was used for the normalization of transcripts.
Statistical analysis.
Student's t test, analysis of variance (ANOVA), and chi-squared tests were performed using the computer program Instat (GraphPad, San Diego, CA). Results were considered statistically significant when the P value was <0.05.
RESULTS
Effect of TGF-β immune signaling on ocular viral clearance, eye disease, and survival in HSV-1-infected mice.
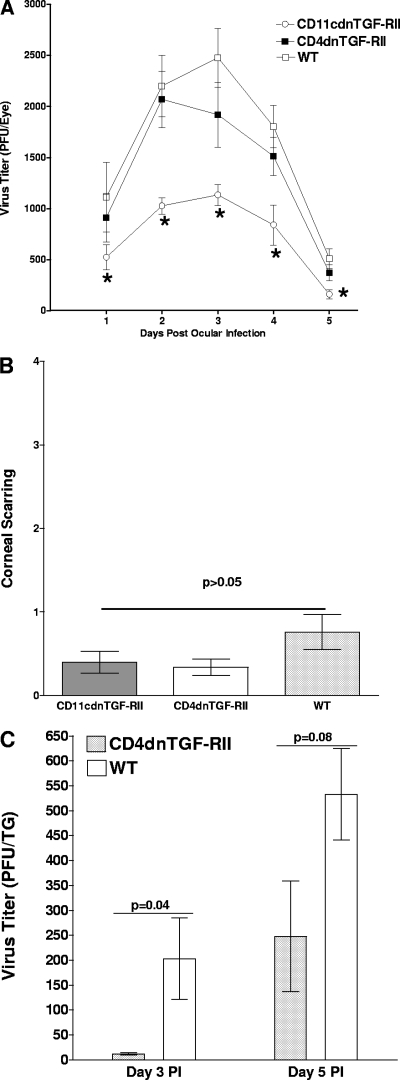
A total of 32 CD4dnTGF-RII (in four separate experiments), 21 CD11cdnTGF-RII (in three separate experiments), and 31 WT C57BL/6 (four experiments) mice were ocularly infected with 2 × 105 PFU/eye of HSV-1 strain McKrae. Tear films from 20 eyes/group were collected daily on day 1 to day 5 post-ocular infection, and the amount of virus in each eye was determined by standard plaque assay. No significant differences were detected between CD4dnTGF-RII and WT groups, while CD11cdnTGF-RII mice had significantly lower levels of virus replication than both the CD4dnTGF-RII and WT groups from days 1 to 5 post-ocular infection (Fig. 1A). Thus, the absence of TGF-β signaling in innate cells but not in T cells resulted in enhanced HSV-1 clearance from the eyes of CD11cdnTGF-RII mice.
Fig. 1.
Effect of TGF-β immune signaling absence on virus replication and corneal scarring (CS) in ocularly infected mice. (A) Virus titers in mouse eyes following ocular infection. CD11cdnTGF-RII, CD4dnTGF-RII, and WT mice were infected ocularly, and the presence of HSV-1 was monitored daily as described in Materials and Methods. For each time point, the virus titer (y axis) represents the average of the titers from 20 eyes. The error bars represent the standard errors of the means (SEM). Asterisks indicate significance (P < 0.05) when titers are compared between groups by Student's t test. (B) CS following ocular infection. CS in ocularly infected mice was measured on day 28 post-ocular infection. CS (y axis) represents the average disease from 64 eyes in CD4dnTGF-RII, 40 eyes in CD4dnTGF-RII, and 58 eyes in WT mice. The error bars indicate the SEM. (C) TG viral titer. CD4dnTGF-RII and WT mice were ocularly infected as described above, and the amount of infectious HSV-1 in the TG was determined on days 3 and 5 PI as described in Materials and Methods. For each time point, the virus titer (y axis) represents the average (±SEM) of the titers from 6 TG per time point.
Upon examination of eyes for corneal scarring (CS) at day 30 postinfection, there was an apparent trend toward reduction in both transgenic lines versus the WT, although this did not reach statistical significance between groups (Fig. 1B; P > 0.05, Student's t test). Thus, blocking TGF-β signaling in either immune compartment clearly did not lead to immunopathogenesis or exacerbated CS and perhaps even further reduced already modest HSV-1-induced corneal pathology present in WT mice. Finally, survival of mice in each group following ocular infection with HSV-1 strain McKrae was determined at day 30 postinfection. All (32 of 32) of the mice in the CD4dnTGF-RII group, 20 of 21 in the CD11cdnTGF-RII group, and 29 of 31 in the WT group survived ocular infection (data not shown). These differences were statistically insignificant (P > 0.05; Fisher's exact test). Overall, our results suggest that the absence of TGF-β signaling did not affect CS or survival but did reduce virus replication in the eyes of CD11cdnTGF-RII mice.
To determine the effect of TGF-RII signaling deficiency on viral titers in the TG, 6 mice per group were infected ocularly as described above. On days 3 and 5 PI, TG were harvested and individually homogenized, and standard plaque assays were performed as described in Materials and Methods. On day 3 PI, TG from CD4dnTGF-RII mice had less infectious virus than those from WT mice (Fig. 1C; P = 0.04, Student's t test). However, by day 5 PI, the differences were not statistically significant (Fig. 1C; P = 0.08). Thus, in contrast to the virus replication in the eyes of CD4dnTGFRII mice, on day 3 (but not day 5) PI, less virus was detected in the TG of infected mice than in the TG of WT mice, suggesting that a different mechanism may be involved in virus clearance in the eyes compared to that in TG.
Absence of TGF-β signaling in immune cells reduces the establishment of HSV-1 latency.
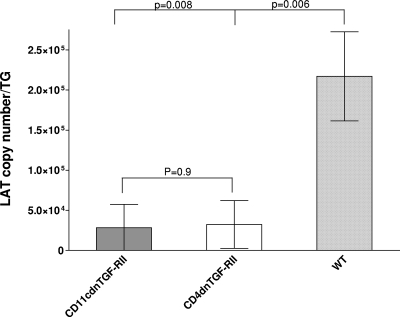
In contrast to lytic infection, during latency the latency-associated transcript (LAT) RNA species is the only gene product that is consistently detected in abundance after HSV-1 infection (16, 57, 63, 70). In general, the level of LAT expression directly correlates with the incidence of recurrence and subsequent induction of eye disease (4, 5, 44, 47). To determine if the absence of TGF-β signaling altered latency, LAT expression was measured in TG from the three groups of mice described above on day 30 post-ocular infection. TaqMan RT-PCR was performed on individual TG RNAs, and LAT copy number was determined for each group. Results indicated that the absence of TGF-β signaling in both CD4dnTGF-RII and CD11cdnTGF-RII mouse groups reduced the level of LAT by at least 7-fold in relation to that of their WT counterparts (Fig. 2; P < 0.01 for each comparison). Thus, the absence of TGF-β signaling in either the innate or adaptive immune compartments significantly reduced the amount of LAT in latently infected TG.
Fig. 2.
Blockade of TGF-β immune signaling reduces LAT in the TG of latently infected mice. TG from CD11cdnTGF-RII, CD4dnTGF-RII, and WT mice were isolated on day 30 postinfection, and quantitative TaqMan RT-PCR was performed on total RNA per individual mouse TG as described in Materials and Methods. In each experiment, an estimated relative copy number of HSV-1 LAT was calculated using standard curves generated from pGem-5317. Briefly, the DNA template was serially diluted 10-fold such that 5 μl contained 103 to 1011 copies of LAT DNA and was then subjected to TaqMan PCR with the same set of primers. By comparing the normalized threshold cycle of each sample to the threshold cycle of the standard, the copy number for each reaction was determined. GAPDH expression was used to normalize the relative expression of LAT in TGs. Each time point represents the mean ± SEM from 20 TG for CD11cdnTGF-RII, 15 TG for CD4dnTGF-RII, and 20 TG for WT mice.
Effect of the absence of TGF-β immune signaling on in vitro reactivation of latent virus.
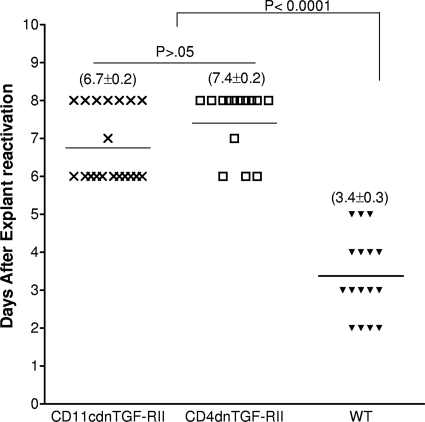
The RT-PCR analyses described above suggested that the absence of TGF-β signaling in immune cells reduced LAT expression in the TG of latently infected mice. We therefore investigated whether this reduction in the level of LAT correlated with amelioration of latent virus reactivation. To test this, TG were harvested from the three groups of mice on day 30 postinfection, and the kinetics of virus reactivation were measured in explanted TG. The average reactivation times (±standard deviations) were 6.7 ± 0.2 days for the CD11cdnTGF-RII group and 7.4 ± 0.2 days for the CD4dnTGF-RII group, while the average for WT mice was 3.4 ± 0.3 days (Fig. 3). These results indicate that the absence of TGF-β immune signaling results in a significant (P < 0.0001) reduction in reactivation of latent virus. This result can possibly be explained by lower virus load in the TG of latently infected mice, which is consistent with the effects of TGF-β signaling on the level of LAT as measured by RT-PCR and as shown in Fig. 2. Alternatively, reduced explant reactivation in the two transgenic mouse lines could be due to the lack of TGF-β signaling (i) inhibiting explant-induced reactivation, (ii) reducing the establishment of latency in TG and resulting in slower explant-induced reactivation, or (iii) a combination of both mechanisms.
Fig. 3.
Effect of TGF-β signaling inhibition on kinetics of induced reactivation in explanted TG from latently infected mice. Thirty days postinfection, when latency is already established, individual TG were obtained from CD11cdnTGF-RII, CD4dnTGF-RII, or WT mice. Each individual TG was incubated in 1.5 ml of tissue culture medium at 37°C. A 100-μl aliquot was removed from each culture daily for 10 days and used to infect RS cell monolayers. The RS cells were monitored daily for the appearance of cytopathic effect (CPE) for 5 days to determine the time of first appearance of reactivated virus from each TG. The results are plotted as the numbers of TG that reactivated daily. Numbers indicate the average time (±SEM) that the TG from each group first showed CPE. For the CD11cdnTGF-RII mice, 20 TG from 10 mice were used; for the CD4dnTGF-RII mice, 15 TG from 8 mice were used; for the WT group, 16 TG from 8 mice were used.
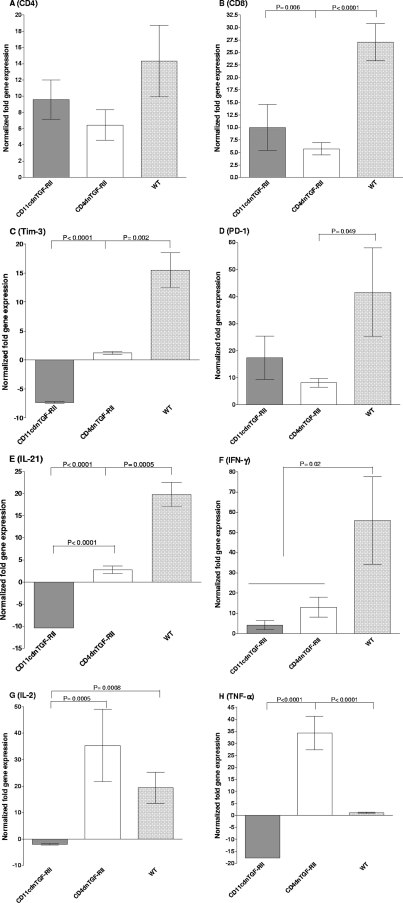
TGF-β immune signaling blockade alters exhaustion markers in the TG of latently infected mice.
A positive correlation was previously reported between the presence of TGF-β and the upregulation of PD-1 and PD-L1 (9, 47, 55). Recently, we showed functional significance of PD-1 and PD-L1 in increasing HSV-1 latency, since PD-1- and PD-L1-deficient mice had reduced latency (3). We sought to investigate if reduced latency in the absence of TGF-β immune signaling modified T cell exhaustion markers and related cytokines in the TG of mice latently infected with HSV-1. Therefore, we utilized qRT-PCR to assay relative levels of several RNAs in TG extracts for T cell subtypes (CD4, CD8), markers of exhaustion (PD-1, Tim-3, interleukin 21 [IL-21]), and cytokines whose levels may be altered by exhaustion (IL-2, gamma interferon [IFN-γ], tumor necrosis factor alpha [TNF-α]). These results are presented as fold increases (or decreases) versus baseline RNA levels in the TG from uninfected mice (Fig. 4). We did not detect differences in CD4 expression between the WT or the CD4dnTGF-RII and CD11cdnTGF-RII groups (Fig. 4A), although we did note a significant decrease in CD8 transcript levels in CD4dnTGF-RII and CD11cdnTGF-RII mice compared to those of WT mice (Fig. 4B; P < 0.0001 and P < 0.01, respectively). There was also a significant decrease in Tim-3 RNA abundance in both CD4dnTGF-RII and CD11cdnTGF-RII mice compared to that of WT mice (Fig. 4C; P < 0.01 and P < 0.0001, respectively). The exhaustion marker PD-1 was significantly reduced in CD4dnTGF-RII mice compared to that of WT mice (P = 0.049), and a nonsignificant trend was noted for the CD11cdnTGF-RII mice (Fig. 4D; P > 0.05). Expression of IL-21 was significantly reduced in both CD4dnTGF-RII and CD11cdnTGF-RII mice compared to that of WT animals (Fig. 4E; P < 0.001 and P < 0.0001, respectively). This trend was also observed for IFN-γ, where we observed decreased expression for CD11cdnTGF-RII mice compared to that of WT mice (Fig. 4F; P < 0.05). Increased expression of both IL-2 (Fig. 4G) and TNF-α (Fig. 4H) was observed in CD11cdnTGF-RII over both CD11cdnTGF-RII and wild-type mice (P < 0.001 and P < 0.0001, respectively). These results suggest that the TGF-β signaling blockade in both innate and adaptive immune compartments decreases HSV-1 latency. This appears to be the result of the blockade increasing immune resistance by reducing expression of exhaustion markers (Tim-3, PD-1, and IL-21), which may directly or indirectly increase IL-2 and TNF-α expression in CD11cdnTGF-RII and CD4dnTGF-RII mice after HSV-1 infection.
Fig. 4.
Effect of TGF-β immune signaling on the expression of key host factors in the TG of latently infected mice. TG from latently infected mice were individually isolated on day 30 postinfection, and quantitative RT-PCR was performed using total RNA as described in Materials and Methods. CD4, CD8, Tim-3, PD-1, IL-21, IFN-γ, IL-2, or TNF-α expression in naive mice was used as a baseline control to estimate relative expression of each transcript in the TG of latently infected mice. GAPDH expression was used to normalize relative expression of each transcript. Each data point represents the mean ± SEM from 20 TG for CD11cdnTGF-RII, 15 TG for CD4dnTGF-RII, and 20 TG for WT mice. (A) CD4; (B) CD8; (C) Tim-3; (D) PD-1; (E) IL-21; (F) IFN-γ; (G) IL-2; and (H) TNF-α transcripts.
Absence of TGF-β signaling reduces leukocyte infiltration and cell death in the corneas and TG of infected mice.
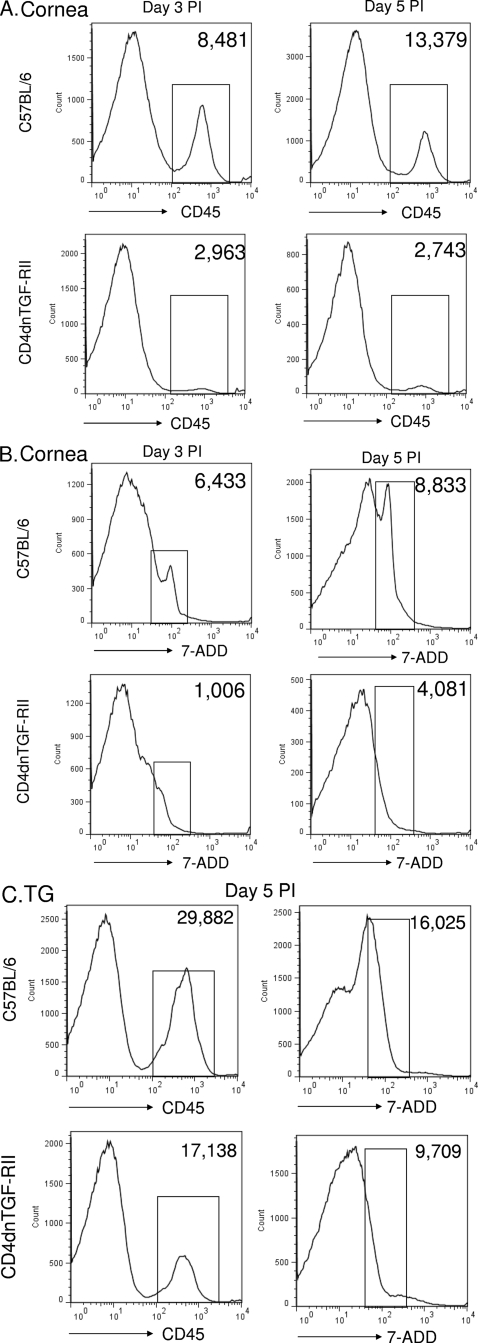
To determine if reduced latency was due to changes in leukocyte infiltration and/or cell death in the corneas and TG of transgenic mice compared with that of WT animals, we performed FACS analyses on days 3 and 5 PI as described in Materials and Methods. We detected an approximate 4-fold decrease in the number of CD45-positive cells in the corneas of CD4dnTGF-RII mice on day 3 PI compared with that in the corneas of WT mice (Fig. 5A; day 3 PI, 2,963 versus 8,481 cells). Similarly, we observed a 5-fold decrease in the number of CD45-positive cells by day 5 PI in the corneas of CD4dnTGF-RII mice compared with that in the corneas of WT mice (Fig. 5A; day 5 PI, 2,743 versus 13,379 cells). Corneal cells were also stained with the cell death marker 7-ADD, and there was a 5-fold decrease in cell death on day 3 PI (Fig. 5B; day 3 PI, 1,006 versus 6,433 cells) and a 2-fold decrease on day 5 PI (Fig. 5B; day 5 PI, 4,081 versus 8,833 cells) when comparing CD4dnTGF-RII with WT mice. Levels of CD45-positive cells were also measured in the TG of infected mice on day 5 PI. Similar to corneal results, there was a 2-fold reduction in cell death in CD4dnTGF-R11 versus WT TG (Fig. 5C; 9,709 versus 16,025 cells). However, no significant differences in annexin V signals (as a surrogate for apoptosis) were detected in either the TG or corneas of infected mice (data not shown). Taken together, our results suggest that the reduced latency and reactivation observed in T cell TGF-β signaling-deficient mice positively associates with less cell death and leukocyte infiltration observed in these animals. Although we were unable to conduct similar experiments in CD11cdnTGF-RII mice due to constraints on mouse availability, we would expect similar results in mice with innate immune compartment restriction of TGF-β signaling inhibition.
Fig. 5.
Absence of T cell TGF-β signaling decreases leukocyte infiltration and cell death in corneas and TG after primary infection. CD4dnTGF-RII and WT mice were infected as described in Materials and Methods. On days 3 and 5 PI, corneas and TGs from 3 individual mice per group were digested with collagenase and stained with anti-CD45 antibody, 7-ADD, or anti-annexin V antibody as described in Materials and Methods. (A) Representative CD45 histograms from corneas of infected mice on days 3 and 5 PI; (B) representative 7-ADD histograms from corneas of infected mice on days 3 and 5 PI; (C) representative CD45 and 7-ADD histograms from the TG of infected mice on day 5 PI. The average numbers of positive cells in the bracket are indicated in the upper right quadrants. Similar results were observed in three independent experiments for corneas and two separate experiments for TG.
DISCUSSION
HSV-1 infections are among the most frequent serious viral eye infections in the United States and are a major cause of virally induced blindness (28, 40, 41, 72). Following HSV ocular infection, the virus establishes a permanent latent infection in the neurons of the trigeminal ganglia (TG) (58, 63, 69, 70). Once acquired, these infections demonstrate a lifelong pattern of episodic recurrence, such that infected individuals serve as permanent carriers who are intermittently infectious (32, 62). In individuals with ocular infection, recurrent rather than primary infections are associated clinically with HSV-induced CS (40). Thus, reducing primary virus replication in the eye and consequently the establishment of latency will at least in principle help to alleviate problems associated with HSV-1 recurrences.
Previously, we have shown that lymphoid dendritic cells are involved in enhancing HSV-1 latency (44, 47). More recently, we demonstrated that HSV-1 subclinical reactivation increases T cell exhaustion in the TG of infected mice (3). We have also shown that T cell exhaustion can be altered by immunization using different HSV-1-expressed glycoproteins (5). TGF-β is one of the most potent immunosuppressive cytokines and acts as a master regulator of immune responses (39). The cytokine is produced by almost all cells, including leukocytes, and its expression controls differentiation and proliferation of innate and adaptive immune cells (14, 39, 67, 68). Surprisingly, very little is known about a putative role for TGF-β during primary and latent stages of HSV-1 infection. Since complete TGF-β deficiency is embryonic lethal for mice or mice with this deficiency do not survive past 3 to 4 weeks of age (30, 35, 55, 59), we instead used transgenic CD11cdnTGF-RII and CD4dnTGF-RII mice in which TGF-β signaling is blocked only in innate cells (i.e., dendritic cells [DCs], macrophages, and natural killer [NK] cells) and T cells, respectively, and is unaffected in other cell types. Therefore, these mice lack most of the problems associated with TGF-β deficiency and give a powerful approach for specifically delineating the role of TGF-β signaling in innate or adaptive immunity to HSV-1 infection. The results presented here demonstrate that blocking TGF-β signaling in innate cells reduced virus replication in the eyes of ocularly infected mice, while blocking TGF-β in the T cell compartment had no effect on primary virus replication in the eyes of infected mice. In this study, we also detected lower virus replication in the TG of CD4dnTGF-RII mice than in the TG of WT mice, while no differences were detected in the level of virus replication in the eyes of infected mice between the two groups. This discrepancy between viral titers in the TG and corneas in CD4dnTGF-RII mice could be due to the significantly higher levels of TNF-α observed in the TG of these animals (Fig. 4H). In contrast to some differences on virus replication after primary infection, TGF-β signal inhibition in either innate or adaptive cells reduced the establishment of HSV-1 latency in ocularly infected mice. Abrogation of TGF-β signaling in innate cells or T cells led to reduced T cell exhaustion, and this most likely resulted in more efficient virus clearance by T cells in the TG of infected mice.
TGF-β plays an important role in the regulation of major histocompatibility complex (MHC) antigens (38, 50). In TGF-β1 knockout mice, both MHC classes I and II are upregulated on multiple tissues (18). Similar to the function of TGF-β, herpesvirus family members have also been reported to affect MHC-I and MHC-II expression and processing, possibly as another mechanism of immune evasion. ICP47 and US11 gene products of HSV-1 are reported to inhibit the presentation of MHC-I on the cell surface and thus suppress the cytotoxic T lymphocyte responses in order to prolong their own survival (56). In addition, HSV-1 has been shown to downregulate MHC-II antigen presentation via virion host shutoff (VHS) and γ34.5 genes (64). Similar results with regard to downregulation of MHC-I and MHC-II were reported for other members of the herpesvirus family (1, 10, 29, 42, 60, 71). Thus, both HSV-1 infection and TGF-β expression have negative effects on MHC expression. Previously, we have shown that both MHC-I and MHC-II immune responses were essential for efficient protection of mice against lethal ocular challenge with HSV-1 (25). Thus, upregulated expression of MHC might, in turn, overcome the suppressive effects of HSV-1 and be responsible for more efficient antigen presentation to T cells and the initiation of a stronger adaptive immune response in these mice. This more efficient adaptive immune response may better clear the virus and therefore cause less T cell exhaustion. Alternatively, since TGF-β has been shown to regulate PD-1 expression (51), the absence of TGF-β in this study may reduce T cell exhaustion and promote more efficient virus clearance. Also, since TGF-β plays a key role in regulatory T cell (Treg) development and hence the suppression of effector T cells (66), a lack of TGF-β may have contributed to reduced latency in the TGF-β dominant negative receptor mice utilized in this study.
Previous studies have suggested that TGF-β may play a role in the severity of HSV-1-associated disease. Specifically, enhanced expression of TGF-β in HSV-infected neonates contributed to impaired HSV-specific CTL responses (15). In addition, it was shown that elevated TGF-β abundance was involved in the increased susceptibility to genital infection with HSV-2 in IL-15 transgenic mice (31). Furthermore, increased pneumonitis in murine allogeneic bone marrow transplant recipients with graft-versus-host disease after pulmonary HSV-1 infection was associated with high concentrations of TGF-β in bronchoalveolar lavage (2). Finally, it was shown that a single administration of TGF-β DNA caused marked suppression of antigen-specific T cell responses and increased susceptibility to HSV-1 infection in mice (34).
TGF-β is essential for the maintenance of T cell homeostasis, and in the absence of TGF-β signaling, most of the T cells differentiate into effectors capable of secreting IL-4 and/or IFN-γ (27). It is likely that exuberant effector T cells clear virus more efficiently from the TG of infected mice than from their wild-type counterparts, leading to reduced levels of latency, contributing to less subclinical reaction and thus less T cell exhaustion. It is interesting in this regard that the blockade of TGF-β in T cells using the CD4 promoter increases T cell effector function (27) that is associated with better protection from latency but leaves primary virus replication unaffected in the eye.
The blockade of TGF-β signaling under the CD11c promoter in innate immune cells did not affect the production of IL-12 in our HSV-1 infection paradigm, while it greatly increased the number of NK cells capable of producing large amounts of IFN-γ responsible for T helper type 1 (TH1) development and protection from Leishmania major infection (36). We previously showed that a strong TH1 response correlated with higher protection against virus replication in the eye and latency in the TG of infected mice (23, 26). We have also shown that the majority of NK cell activity responsible for protecting C57BL/6 mice against ocular HSV-1 challenge was due to an NK function other than IFN-γ production (22). This was supported by the finding that injection of recombinant IFN-γ into C57BL/6 NK cell-depleted mice did not improve survival following HSV-1 challenge.
HSV-1 infection has both pro- and antiapoptotic activities (7, 17, 53, 73) as well as necrosis-inducing elements (6, 54) in vitro and in vivo. In this study, we measured both necrosis by 7-ADD staining and apoptosis by annexin V in the corneas and TG of infected mice. While the absence of T cell TGF-β signaling did not impact apoptosis in the corneas or TG of infected mice, necrosis was significantly reduced in CD4dnTGF-RII transgenic mouse corneas and TG compared to that in WT mouse corneas and TG. This higher cell death may have contributed to less efficient virus clearance in the TG of WT mice. Although less CS occurred in CD4dnTGF-RII transgenic mice than in WT mice, these differences did not reach statistical significance. Following ocular HSV-1 infection, increased immune infiltrates in the eyes are associated with exacerbated immunopathology (9, 21, 25, 33, 65). Interestingly, blocking T cell TGF-β signaling in CD4dnTGF-RII transgenic mice reduced infiltrating leukocytes in the corneas and TG of infected mice. Similar to this study, it was previously reported that the absence of CXCL9 reduced both CD4+ T cell migration and viral load in the corneas of deficient mice (74). Thus, decreased levels of leukocytes detected in the corneas and TG of TGF-β mice cannot be ruled out as potentially causative of the observed reduction in cell death in infected mice. Yet, it is more likely that elevated activation of adaptive immunity in the CD4dnTGF-RII transgenic mice promoted HSV-1 clearance, leading to reduced viral abundance and therefore fewer infiltrating leukocytes that would otherwise respond in the case of copious virus. In this context, it is interesting that blocking T cell TGF-βR signaling reduced leukocyte infiltration into the eye and TG but increased protection against the establishment of latency and reactivation compared to that of the WT mice.
In summary, our results demonstrate that both innate and adaptive TGF-β immune signaling led to increased HSV-1 latency and reactivation. The coordinated activity of TGF-β signaling in these two immune compartments seems to be of essential importance in regulating the latent phase of viral infection. If these results extrapolate to clinical HSV-1 infection, then blocking TGF-β signaling in immune cells may represent an important new therapeutic approach to virus-associated disease, particularly HSV-1-associated eye disease.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants EY15557 and EY13615 from the National Eye Institute to H.G. S.L.W. was supported by Public Health Service grant EY013191 and a Research to Prevent Blindness Challenge grant. S.J.A. was supported by NIH training grant 1T32AI089553.
Footnotes
Published ahead of print on 31 August 2011.
REFERENCES
- 1. Abendroth A., et al. 2000. Modulation of major histocompatibility class II protein expression by varicella-zoster virus. J. Virol. 74:1900–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adler H., et al. 1998. A role for transforming growth factor-beta1 in the increased pneumonitis in murine allogeneic bone marrow transplant recipients with graft-versus-host disease after pulmonary herpes simplex virus type 1 infection. Blood 92:2581–2589 [PubMed] [Google Scholar]
- 3. Allen S. J., et al. 2011. The role of LAT in increased CD8+ T cell exhaustion in trigeminal ganglia of mice latently infected with herpes simplex virus type 1. J. Virol. 85:4184–4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allen S. J., Mott K. R., Ljubimov A. V., Ghiasi H. 2010. Exacerbation of corneal scarring in HSV-1 gK-immunized mice correlates with elevation of CD8+CD25+ T cells in corneas of ocularly infected mice. Virology 399:11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allen S. J., Mott K. R., Zandian M., Ghiasi H. 2010. Immunization with different viral antigens alters the pattern of T cell exhaustion and latency in HSV-1 infected mice. J. Virol. 84:12315–12324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aubert M., Krantz E. M., Jerome K. R. 2006. Herpes simplex virus genes Us3, Us5, and Us12 differentially regulate cytotoxic T lymphocyte-induced cytotoxicity. Viral Immunol. 19:391–408 [DOI] [PubMed] [Google Scholar]
- 7. Aurelian L. 2005. HSV-induced apoptosis in herpes encephalitis. Curr. Top. Microbiol. Immunol. 289:79–111 [DOI] [PubMed] [Google Scholar]
- 8. Barber D. L., et al. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687 [DOI] [PubMed] [Google Scholar]
- 9. Brandt C. R., Salkowski C. A. 1992. Activation of NK cells in mice following corneal infection with herpes simplex virus type-1. Invest. Ophthalmol. Vis. Sci. 33:113–120 [PubMed] [Google Scholar]
- 10. Cebulla C. M., et al. 2002. Human cytomegalovirus disrupts constitutive MHC class II expression. J. Immunol. 169:167–176 [DOI] [PubMed] [Google Scholar]
- 11. Chemnitz J. M., et al. 2007. RNA fingerprints provide direct evidence for the inhibitory role of TGFbeta and PD-1 on CD4+ T cells in Hodgkin lymphoma. Blood 110:3226–3233 [DOI] [PubMed] [Google Scholar]
- 12. Day C. L., et al. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350–354 [DOI] [PubMed] [Google Scholar]
- 13. de Caestecker M. 2004. The transforming growth factor-beta superfamily of receptors. Cytokine Growth Factor Rev. 15:1–11 [DOI] [PubMed] [Google Scholar]
- 14. Dunker N., Krieglstein K. 2000. Targeted mutations of transforming growth factor-beta genes reveal important roles in mouse development and adult homeostasis. Eur. J. Biochem. 267:6982–6988 [DOI] [PubMed] [Google Scholar]
- 15. Fernandez M. A., et al. 2008. T regulatory cells contribute to the attenuated primary CD8+ and CD4+ T cell responses to herpes simplex virus type 2 in neonatal mice. J. Immunol. 180:1556–1564 [DOI] [PubMed] [Google Scholar]
- 16. Fraser N. W., Block T. M., Spivack J. G. 1992. The latency-associated transcripts of herpes simplex virus: RNA in search of function. Virology 191:1–8 [DOI] [PubMed] [Google Scholar]
- 17. Galvan V., Roizman B. 1998. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc. Natl. Acad. Sci. U. S. A. 95:3931–3936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Geiser A. G., et al. 1993. Transforming growth factor beta 1 (TGF-beta 1) controls expression of major histocompatibility genes in the postnatal mouse: aberrant histocompatibility antigen expression in the pathogenesis of the TGF-beta 1 null mouse phenotype. Proc. Natl. Acad. Sci. U. S. A. 90:9944–9948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ghiasi H., Bahri S., Nesburn A. B., Wechsler S. L. 1995. Protection against herpes simplex virus-induced eye disease after vaccination with seven individually expressed herpes simplex virus 1 glycoproteins. Invest. Ophthalmol. Vis. Sci. 36:1352–1360 [PubMed] [Google Scholar]
- 20. Ghiasi H., Cai S., Nesburn A. B., Wechsler S. L. 1996. Vaccination with herpes simplex virus type 1 glycoprotein K impairs clearance of virus from the trigeminal ganglia resulting in chronic infection. Virology 224:330–333 [DOI] [PubMed] [Google Scholar]
- 21. Ghiasi H., Cai S., Perng G., Nesburn A. B., Wechsler S. L. 1999. Perforin pathway is essential for protection of mice against lethal ocular HSV-1 challenge but not corneal scarring. Virus Res. 65:97–101 [DOI] [PubMed] [Google Scholar]
- 22. Ghiasi H., Cai S., Perng G. C., Nesburn A. B., Wechsler S. L. 2000. The role of natural killer cells in protection of mice against death and corneal scarring following ocular HSV-1 infection. Antiviral Res. 45:33–45 [DOI] [PubMed] [Google Scholar]
- 23. Ghiasi H., et al. 1999. The role of interleukin (IL)-2 and IL-4 in herpes simplex virus type 1 ocular replication and eye disease. J. Infect. Dis. 179:1086–1093 [DOI] [PubMed] [Google Scholar]
- 24. Ghiasi H., Perng G., Nesburn A. B., Wechsler S. L. 1999. Either a CD4(+)or CD8(+)T cell function is sufficient for clearance of infectious virus from trigeminal ganglia and establishment of herpes simplex virus type 1 latency in mice. Microb. Pathog. 27:387–394 [DOI] [PubMed] [Google Scholar]
- 25. Ghiasi H., et al. 1997. The importance of MHC-I and MHC-II responses in vaccine efficacy against lethal herpes simplex virus type 1 challenge. Immunology 91:430–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ghiasi H., Wechsler S. L., Cai S., Nesburn A. B., Hofman F. M. 1998. The role of neutralizing antibody and T-helper subtypes in protection and pathogenesis of vaccinated mice following ocular HSV-1 challenge. Immunology 95:352–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gorelik L., Flavell R. A. 2000. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 12:171–181 [DOI] [PubMed] [Google Scholar]
- 28. Hill T. J. 1987. Ocular pathogenicity of herpes simplex virus. Curr. Eye Res. 6:1–7 [DOI] [PubMed] [Google Scholar]
- 29. Jones T. R., et al. 1996. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc. Natl. Acad. Sci. U. S. A. 93:11327–11333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaartinen V., et al. 1995. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat. Genet. 11:415–421 [DOI] [PubMed] [Google Scholar]
- 31. Kagimoto Y., et al. 2008. A regulatory role of interleukin 15 in wound healing and mucosal infection in mice. J. Leukoc. Biol. 83:165–172 [DOI] [PubMed] [Google Scholar]
- 32. Kaufman H. E., et al. 2005. HSV-1 DNA in tears and saliva of normal adults. Invest. Ophthalmol. Vis. Sci. 46:241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koelle D. M., Ghiasi H. 2005. Prospects for developing an effective vaccine against ocular herpes simplex virus infection. Curr. Eye Res. 30:929–942 [DOI] [PubMed] [Google Scholar]
- 34. Kuklin N. A., Daheshia M., Chun S., Rouse B. T. 1998. Immunomodulation by mucosal gene transfer using TGF-beta DNA. J. Clin. Invest. 102:438–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kulkarni A. B., et al. 1993. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl. Acad. Sci. U. S. A. 90:770–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Laouar Y., Sutterwala F. S., Gorelik L., Flavell R. A. 2005. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat. Immunol. 6:600–607 [DOI] [PubMed] [Google Scholar]
- 37. Laouar Y., et al. 2008. TGF-beta signaling in dendritic cells is a prerequisite for the control of autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U. S. A. 105:10865–10870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee Y. J., et al. 1997. TGF-beta suppresses IFN-gamma induction of class II MHC gene expression by inhibiting class II transactivator messenger RNA expression. J. Immunol. 158:2065–2075 [PubMed] [Google Scholar]
- 39. Li M. O., Wan Y. Y., Sanjabi S., Robertson A. K., Flavell R. A. 2006. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 24:99–146 [DOI] [PubMed] [Google Scholar]
- 40. Liesegang T. J. 1999. Classification of herpes simplex virus keratitis and anterior uveitis. Cornea 18:127–143 [DOI] [PubMed] [Google Scholar]
- 41. Liesegang T. J. 2001. Herpes simplex virus epidemiology and ocular importance. Cornea 20:1–13 [DOI] [PubMed] [Google Scholar]
- 42. Lin A., Xu H., Yan W. 2007. Modulation of HLA expression in human cytomegalovirus immune evasion. Cell. Mol. Immunol. 4:91–98 [PubMed] [Google Scholar]
- 43. Massague J. 1990. The transforming growth factor-beta family. Annu. Rev. Cell Biol. 6:597–641 [DOI] [PubMed] [Google Scholar]
- 44. Mott K. R., Ghiasi H. 2008. Role of dendritic cells in enhancement of herpes simplex virus type 1 latency and reactivation in vaccinated mice. Clin. Vaccine Immunol. 15:1859–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mott K. R., et al. 2007. The corneas of naive mice contain both CD4+ and CD8+ T cells. Mol. Vis. 13:1802–1812 [PubMed] [Google Scholar]
- 46. Mott K. R., Perng G. C., Osorio Y., Kousoulas K. G., Ghiasi H. 2007. A recombinant herpes simplex virus type 1 expressing two additional copies of gK is more pathogenic than wild-type virus in two different strains of mice. J. Virol. 81:12962–12972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mott K. R., UnderHill D., Wechsler S. L., Ghiasi H. 2008. Lymphoid-related CD11c+CD8a+ dendritic cells are involved in enhancing HSV-1 latency. J. Virol. 82:9870–9879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mueller S. N., Ahmed R. 2009. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. U. S. A. 106:8623–8628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Osorio Y., Ghiasi H. 2003. Comparison of adjuvant efficacy of herpes simplex virus type 1 recombinant viruses expressing TH1 and TH2 cytokine genes. J. Virol. 77:5774–5783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Panek R. B., Lee Y. J., Benveniste E. N. 1995. TGF-beta suppression of IFN-gamma-induced class II MHC gene expression does not involve inhibition of phosphorylation of JAK1, JAK2, or signal transducers and activators of transcription, or modification of IFN-gamma enhanced factor X expression. J. Immunol. 154:610–619 [PubMed] [Google Scholar]
- 51. Park H. B., Paik D. J., Jang E., Hong S., Youn J. 2004. Acquisition of anergic and suppressive activities in transforming growth factor-beta-costimulated CD4+CD25− T cells. Int. Immunol. 16:1203–1213 [DOI] [PubMed] [Google Scholar]
- 52. Perng G. C., et al. 1994. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J. Virol. 68:8045–8055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Perng G. C., et al. 2000. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science 287:1500–1503 [DOI] [PubMed] [Google Scholar]
- 54. Posavad C. M., Rosenthal K. L. 1992. Herpes simplex virus-infected human fibroblasts are resistant to and inhibit cytotoxic T-lymphocyte activity. J. Virol. 66:6264–6272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Proetzel G., et al. 1995. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat. Genet. 11:409–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Radosevich T. J., Seregina T., Link C. J. 2003. Effective suppression of class I major histocompatibility complex expression by the US11 or ICP47 genes can be limited by cell type or interferon-gamma exposure. Hum. Gene Ther. 14:1765–1775 [DOI] [PubMed] [Google Scholar]
- 57. Rock D. L., et al. 1987. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J. Virol. 61:3820–3826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Roizman B., Sears A. E. 1987. An inquiry into the mechanisms of herpes simplex virus latency. Annu. Rev. Microbiol. 41:543–571 [DOI] [PubMed] [Google Scholar]
- 59. Sanford L. P., et al. 1997. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development 124:2659–2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schwyzer M., et al. 2002. Transduction of Vero cells and bovine monocytes with a herpes simplex virus-1 based amplicon carrying the gene for the bovine herpesvirus-1 Circ protein. Vet. Microbiol. 86:165–174 [DOI] [PubMed] [Google Scholar]
- 61. Shvets A., et al. 2009. Impaired negative regulation of homeostatically proliferating T cells. Blood 113:622–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Steiner I. 1996. Human herpes viruses latent infection in the nervous system. Immunol. Rev. 152:157–173 [DOI] [PubMed] [Google Scholar]
- 63. Stevens J. G. 1989. Human herpesviruses: a consideration of the latent state. Microbiol. Rev. 53:318–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Trgovcich J., Johnson D., Roizman B. 2002. Cell surface major histocompatibility complex class II proteins are regulated by the products of the gamma(1)34.5 and U(L)41 genes of herpes simplex virus 1. J. Virol. 76:6974–6986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tumpey T. M., et al. 1998. Absence of macrophage inflammatory protein-1alpha prevents the development of blinding herpes stromal keratitis. J. Virol. 72:3705–3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. von Boehmer H. 2005. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 6:338–344 [DOI] [PubMed] [Google Scholar]
- 67. Wahl S. M. 2007. Transforming growth factor-beta: innately bipolar. Curr. Opin. Immunol. 19:55–62 [DOI] [PubMed] [Google Scholar]
- 68. Wahl S. M., Wen J., Moutsopoulos N. 2006. TGF-beta: a mobile purveyor of immune privilege. Immunol. Rev. 213:213–227 [DOI] [PubMed] [Google Scholar]
- 69. Wechsler S. L., Nesburn A. B., Watson R., Slanina S., Ghiasi H. 1988. Fine mapping of the major latency-related RNA of herpes simplex virus type 1 in humans. J. Gen. Virol. 69:3101–3106 [DOI] [PubMed] [Google Scholar]
- 70. Wechsler S. L., Nesburn A. B., Watson R., Slanina S. M., Ghiasi H. 1988. Fine mapping of the latency-related gene of herpes simplex virus type 1: alternative splicing produces distinct latency-related RNAs containing open reading frames. J. Virol. 62:4051–4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wiertz E. J., et al. 1996. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell 84:769–779 [DOI] [PubMed] [Google Scholar]
- 72. Wilhelmus K. R., et al. 1996. Risk factors for herpes simplex virus epithelial keratitis recurring during treatment of stromal keratitis or iridocyclitis. Herpetic Eye Disease Study Group. Br. J. Ophthalmol. 80:969–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wilson S. E., Pedroza L., Beuerman R., Hill J. M. 1997. Herpes simplex virus type-1 infection of corneal epithelial cells induces apoptosis of the underlying keratocytes. Exp. Eye Res. 64:775–779 [DOI] [PubMed] [Google Scholar]
- 74. Wuest T., Farber J., Luster A., Carr D. J. 2006. CD4+ T cell migration into the cornea is reduced in CXCL9 deficient but not CXCL10 deficient mice following herpes simplex virus type 1 infection. Cell Immunol. 243:83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zuniga J. E., et al. 2005. Assembly of TbetaRI:TbetaRII:TGFbeta ternary complex in vitro with receptor extracellular domains is cooperative and isoform-dependent. J. Mol. Biol. 354:1052–1068 [DOI] [PubMed] [Google Scholar]