Abstract
Resistance to the nonnucleoside reverse transcriptase inhibitors etravirine and rilpivirine (RPV) is conferred by the E138K mutation in human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT). Clinical trials of RPV administered with lamivudine or emtricitabine showed the emergence of E138K together with M184I, which confers lamivudine and emtricitabine resistance in most patients with virologic failure. To understand why M184I was favored over M184V, we determined the drug susceptibility, infectivity, relative fitness, and reverse transcriptase activity of HIV-1 carrying E138K/M184I or E138K/M184V mutations. Whereas the replication capacity (RC) of the single mutants was reduced compared to that of the wild type (WT), the RC of the two double mutants was comparable to that of the WT in the absence of drug. The RC of the E138K/M184I mutant in the presence of etravirine was significantly greater than that of the E138K and E138K/M184V mutants; the RC of the double mutants was greater than that of the M184I or M184V mutant. Fitness profiles and growth competition experiments showed that the E138K/M184I mutant had a significant replicative advantage over the E138K/M184V mutant in the presence of etravirine and lamivudine. The virion-associated RT activity of the E138K, M184I, or M184V virus was significantly reduced compared to that of the WT, whereas the RT activity of the E138K/M184I virus was significantly greater than that of the WT or E138K/M184V virus. These results suggest that the E138K and M184I/V mutations are mutually compensatory and may explain the frequent occurrence of E138K/M184I after the virologic failure of rilpivirine-, lamivudine-, and emtricitabine-containing regimens.
INTRODUCTION
Nonnucleoside reverse transcriptase inhibitors (NNRTIs) are key components of combination antiretroviral therapy. Etravirine (ETV; formerly TMC125) and rilpivirine (RPV; formerly TMC278) are potent next-generation NNRTIs that retain activity against efavirenz (EFV)-resistant viruses carrying the K103N mutation in human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT) (1, 3); both are diarylpyrimidine (DAPY) compounds. The E138K mutation, which emerges both in vitro and in vivo, confers resistance to ETV and RPV (2, 3, 22, 23). Although in vitro passage experiments suggested that resistance to ETV and RPV emerges more slowly than resistance to first-generation NNRTIs (e.g., nevirapine [NVP] or EFV) (3, 25), this finding has not been confirmed in clinical trials, which show the emergence of ETV and RPV resistance at the time of virologic failure in most patients treated with these drugs (17, 18).
Most antiretroviral regimens include the cytosine analogs lamivudine (3TC) or emtricitabine (FTC) administered as fixed-dose combinations together with zidovudine (ZDV) or abacavir (ABC) in the case of ZDV/3TC and ABC/3TC or with tenofovir (TDF) in the case of TDF/FTC. Resistance to 3TC and FTC is conferred by a valine or isoleucine substitution for the methionine normally found at position 184, which lies in the conserved YMDD motif at the polymerase active site (5, 19, 24). Viruses carrying the M184I or M184V mutation show reduced replication capacity, and in the absence of drug they are less fit than wild-type (WT) virus, presumably because these mutations reduce the overall activity and processivity of HIV-1 RT (4, 26). The M184I mutation emerges first but is rapidly replaced by the M184V mutation (9, 20). Viruses carrying M184V are fitter than the M184I mutants (12).
Clinical trials of RPV in treatment-naïve patients show that HIV-1 carrying the E138K mutation emerged in approximately 60% of patients with virologic failure, usually together with the M184I mutation (6, 17). To understand why M184I was favored over M184V in those studies and to explore potential interactions between E138K and the M184I or M184V mutations, we determined the susceptibility, relative infectivity, replication capacity, relative fitness, and virion-associated RT activity of recombinant HIV-1 carrying the E138K/M184I or E138K/M184V mutations.
(These data were presented in part at the following meetings: 18th Conference on Retroviruses and Opportunistic Infections, 27 February to 2 March 2011, Boston, MA [abstract 594], and 20th International HIV-1 Drug Resistance Workshop, 7 to 11 June 2011, Los Cabos, Mexico [abstract 12].)
MATERIALS AND METHODS
Reagents and cells.
Etravirine and 3TC were kindly provided by the AIDS Research and Reference Reagent Program. The 293T cell line (ATCC, Manassas, VA), TZM-bl cells, and U87-X4 cells were propagated in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 U/ml). MT-2 cells were grown in R-10 medium (RPMI 1640 [Cellgro, Herndon, VA] supplemented with 10% FBS, 2 mM l-glutamine, penicillin [100 U/ml], and streptomycin [100 μg/ml]).
Site-directed mutagenesis.
Protease- and RT-coding segments of the pol gene of HIV-1NL4-3 were amplified by PCR and cloned into pGEM-T-easy vector. The E138K, M184I, M184V, E138K/M184I, and E138K/M184V mutations were introduced into the cloned pol gene using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). The presence of mutant sequences was confirmed by the automated sequencing of the final plasmid clone on an ABI 377 automated sequencer.
Generation of virus stocks.
Infectious recombinant marker viruses expressing WT or mutant RTs were generated by cotransfecting 293T cells with a pol-deleted proviral clone of NL4-3 together with the PCR-amplified pol gene of interest (7, 14). Virus-containing supernatants were harvested 72 h after transfection, the recombinant virus stocks were expanded, and titers were determined as described previously (8). The RT coding region of pol was amplified from proviral DNA of infected cells at the end of virus culture and analyzed by automated DNA sequencing to verify the presence of the correct alleles at RT codons 138 and 184.
Drug susceptibility testing.
The susceptibility of HIV-1 recombinants to ETV and 3TC was determined by a standard drug susceptibility assay as described previously (8).
Replication capacity assay.
Viral replication capacity was determined by a single-cycle assay on TZM-bl cells by luciferase assay. The TZM-bl cells were plated in 24-well plates at 4 × 104 cells/well the day before infection. After removing the medium, virus stocks at a multiplicity of infection (MOI) of 0.05 were adsorbed onto cells in triplicate wells for 2 h. Subsequently, DMEM was added to a final volume of 1 ml/well, and cultures were incubated at 37°C in the absence and presence of 100 μM 3TC or 25 nM ETV. After 3 days, the medium was removed; cells were washed with phosphate-buffered saline (PBS) and lysed in 500 μl of lysis buffer (5 mM MgCl2 and 0.1% NP-40 in PBS). Viral replication was quantified by measuring luciferase activity in cell lysates. Experiments were performed twice and the results averaged.
Infectivity and fitness profile assays.
To determine viral infectivity, virus stocks were normalized according to β-galactosidase activity on TZM-bl cells, and an amount of virus sufficient to produce β-galactosidase activity equivalent to 1 × 105 chemiluminescence units per well in 96-well plates was used to infect 1 × 104 TZM-bl cells. Two-fold serial dilutions of RT inhibitors (ranges, 100 to 1.5 nM for ETV and 100 to 1.5 μM for 3TC) were added to triplicate wells of 96-well microtiter plates in a total volume of 200 μl DMEM. After incubation at 37°C for 48 h, β-galactosidase activity was quantified using Galacto-Light Plus (Applied Biosystems, Foster City, CA) and expressed as chemiluminescence units. Infectivity was determined from the mean chemiluminescence units in the triplicate wells.
To determine the replicative advantage of mutant viruses as a function of RT inhibitor concentration, the infectivity ratio of the mutants to WT (measured as relative β-galactosidase activity) was determined at each concentration of ETV or 3TC. The ratios then were interpolated as a continuous profile across the range of different drug concentrations tested using GraphPad Prism 5 (GraphPad Software, Ann Arbor, MI). Assays were performed in triplicate, and independent experiments were repeated twice.
Virus growth competition assays.
Pairwise growth competition assays were performed as described previously (7, 14). Briefly, recombinant marker viruses of interest carrying the hisD or GFP sequence tags were mixed together at ratios of 50:50 and inoculated into triplicate wells of a 12-well plate containing 8 × 104 MT-2 cells per well in 300 μl of R-10 medium to yield an MOI of 0.001. After incubation at 37°C for 2 h, cells were washed twice with PBS, resuspended in 1.5 ml of R-10 medium, seeded into 12-well plates, and reincubated. On days 1, 4, 7, 11, and 14 postinfection, 200 μl of culture supernatant was removed and replaced with 250 μl of fresh medium. The proportion of the two competing viral variants was estimated by quantifying GFP and hisD sequences present in culture supernatants using real-time reverse transcriptase-coupled PCR (RT-PCR) with an ABI PRISM 7300 sequence detection system (Applied Biosystems, Inc.). Viral RNA was extracted from culture supernatants using the Qiagen kit and treated with RNase-free DNase (Qiagen, Valencia, CA). Parameters for the real-time PCR were as described above, except that the initial reverse transcription reaction was performed at 50°C for 30 min. Quantitative real-time RT-PCR was performed in triplicate for each sample. Experiments were performed twice and the results averaged.
Virion-associated reverse transcriptase assay.
Virion-associated RT activity of the wild-type and mutant virus stocks was assayed using a colorimetric reverse transcriptase assay (Roche Applied Science, Indianapolis, IN). Viral input was standardized by p24 antigen. The viruses were pelleted by centrifugation at 17,000 rpm for 1 h at 4°C. Virus pellets containing 100 ng of p24 capsid protein were resuspended and lysed by adding 40 μl lysis buffer. Reactions were performed in triplicate, and experiments were performed twice and the results averaged.
Statistical analysis.
Results are presented as means ± standard deviations (SD). Samples were compared by analysis of variance. Posttest comparisons (performed only if P was <0.05) were made with the two-sample comparison test. A P value of <0.05 was considered significant.
RESULTS
Drug susceptibility.
The 50% inhibitory concentration (IC50) of ETV for WT virus was 6.4 ± 0.5 nM. The E138K mutation conferred 2-fold resistance to ETV; M184I and M184V had no effect on ETV susceptibility (Table 1). The combination of E138K plus M184I or M184V conferred 3.3- and 2.7-fold resistance to ETV, respectively. The IC50 of 3TC for WT virus was 1.6 ± 0.13 μM, and for the E138K virus, 1.5 ± 0.19 μM. The IC50 of 3TC for viruses carrying the M184I or M184V mutation with or without E138K was greater than 100 μM (Table 1).
Table 1.
ETV and 3TC susceptibility of HIV-1 recombinants carrying resistance mutations in reverse transcriptase
| Virus | Susceptibility tod: |
|||
|---|---|---|---|---|
| ETV |
3TC |
|||
| IC50a (nM) | Fold changeb | IC50 (μM) | Fold change | |
| Wild type | 6.4 ± 0.45 | 1.6 ± 0.13 | ||
| E138K | 13.2 ± 1.30 | 2.0 | 1.5 ± 0.19 | 0.9 |
| M184I | 6.0 ± 0.66 | 0.9 | >100 | NCc |
| M184V | 6.1 ± 0.54 | 0.9 | >100 | NC |
| E138K/M184I | 21.3 ± 1.63 | 3.3 | >100 | NC |
| E138K/M184V | 17.5 ± 1.90 | 2.7 | >100 | NC |
IC50, 50% inhibitory concentration.
Fold change in IC50 compared to the IC50 of the wild type.
NC, not calculated.
Data shown are the means from three independent determinations.
Replication capacity of etravirine- and lamivudine-resistant viruses.
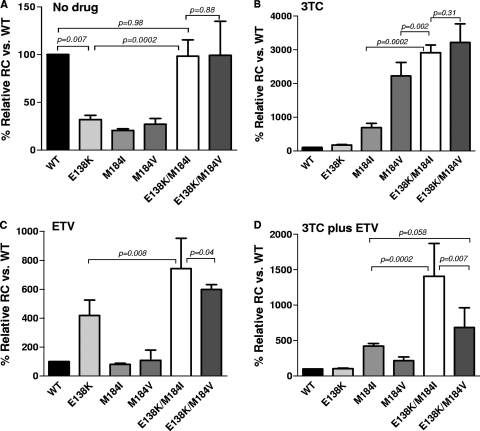
The single-cycle RC assay showed that in the absence of drug, the RC of the E138K, M184I, and M184V mutants decreased by 3- to 5-fold compared to that of the WT, whereas the E138K/M184I and E138K/M184V double mutants had RC values similar to those of WT virus (Fig. 1A). In the presence of 100 μM 3TC, the RC of the M184I and M184V mutants increased 6.9- and 22.2-fold, respectively, compared to that of the WT (Fig. 1B). The RC of the E138K/M184I mutant increased 29.1-fold compared to that of the wild type. This increase was significantly greater than that of the M184I (P = 0.0002) and M184V (P = 0.002) mutants. The RC of the E138K/M184V mutant increased 32.2-fold, which was not significantly different from the RC of the E138K/M184I mutant (P = 0.31). In the presence of 25 nM ETV, the RC of the E138K mutant increased 4.2-fold above that of the WT, whereas that of the E138K/M184I and E138K/M184V mutants increased 7.4- and 5.9-fold, respectively (Fig. 1C). The RC increase of the E138K/M184I mutant was significantly greater than that of the E138K/M184V mutant (P = 0.04). The difference in RC between the two double mutants was accentuated in the presence of ETV plus 3TC (Fig. 1D).
Fig. 1.
Replication capacity of HIV recombinants carrying resistance mutations at codon 138 or 184 in RT, respectively. The bar graphs show that the results of RC assays in the absence of drug (A) or in the presence of 100 μM 3TC (B), 25 nM ETV (C), or 100 μM 3TC plus 25 nM ETV (D). The y axis shows the relative RC for each mutant as a percentage of the wild-type value, which was set at 100%. The data shown are the means ± SD of two experiments.
Fitness profiles of etravirine- and lamivudine-resistant viruses.
The infectivity of ETV- and 3TC-resistant viruses was compared to that of WT virus over a range of drug concentrations (Fig. 2). As expected, in the presence of ETV the E138K, E138K/M184V, and E138K/M184I mutants all showed a replicative advantage over viruses without the E138K mutation (Fig. 2A). Of note, both double mutants showed a significantly greater replication advantage over the WT compared to that of the E138K mutant at most concentrations of ETV. At ETV concentrations greater than 12.5 nM, the E138K/M184I mutant appeared to have a greater advantage than the E138K/M184V mutant, but this difference was not statistically significant. In the presence of lamivudine, the M184V and M184I mutants had a replication advantage over the WT and the E138K mutant (Fig. 2B); as expected, M184V conferred a substantially greater advantage compared to that provided by M184I. The double mutants had a replicative advantage that was similar to that of the M184V mutant at most 3TC concentrations but had a significantly greater advantage compared to that of M184V at the highest 3TC concentration tested (100 μM). When tested in the combined presence of ETV and 3TC, the E138K/M184I mutant had a significantly greater advantage over the WT compared to that of the E138K/M184V mutant (Fig. 2C).
Fig. 2.
Fitness profiles of HIV-1 recombinants carrying the E138K and/or M184I or M184V mutation in RT. The infectivity ratio of the mutants to wild type (measured as relative β-galactosidase activity) was determined in the presence of 1.5 to 100 nM ETV (A), 1.5 to 100 μM 3TC (B), or 0.78 to 100 nM ETV plus 0.78 to 100 μM 3TC (C).
Relative fitness of E138K/M184I and E138K/M184V mutants.
The relative fitness of recombinant viruses carrying the E138K/M184I and E138K/M184V mutations was determined by growth competition assays performed in the absence and presence of 3TC or ETV (Fig. 3). Under each condition the E138K/M184I mutant outcompeted the E138K/M184V mutation, demonstrating the greater fitness of the M184I-containing mutant. The difference between the two viruses was most pronounced in the presence of 25 nM ETV (Fig. 3C).
Fig. 3.
Growth competition assays comparing the fitness of HIV recombinants carrying the E138K/M184I or E138K/M184V mutation in RT. Shown are the results of competition assays between E138K/M184I and E138K/M184V inoculated at a ratio of 50:50 in the absence of drug (A) or in the presence of 100 μM 3TC (B) or 25 nM ETV (C). The data shown are the means ± SD from two independent experiments.
Virion-associated RT polymerase activity of wild-type and mutant viruses.
Figure 4 shows the relative virion-associated RT activity of the wild-type and mutant viruses. Viruses carrying the single E138K, M184I, or M184V mutation each had reduced RT activity relative to that of the WT. Whereas the introduction of the M184V mutation into the E138K mutant resulted in comparable RT activity, the introduction of the M184I mutation into the E138K/M184I mutant resulted in significantly greater RT activity compared to that of the E138K or E138K/M184V mutant (P = 0.03 and 0.04, respectively).
Fig. 4.
Virion-associated RT activity. The y axis shows the relative RT activity for each mutant as a percentage of wild-type activity, which was set at 100%. The data shown are the means ± SD from two independent experiments.
DISCUSSION
We determined the drug susceptibility, replication capacity, fitness profiles, relative fitness, and virion-associated RT activity of recombinant HIV-1 carrying mutations that confer resistance to the NNRTIs ETV and RPV and the nucleoside reverse transcriptase inhibitors (NRTIs) 3TC and FTC. We found that when present alone, the E138K, M184I, and M184V mutations each reduced the RC of HIV-1 3- to 4-fold compared to that of the WT in the absence of drug. This observation confirms and extends a previous report that found a 2-fold decrease in the RC in E138K mutants (2). In contrast, the combined presence of E138K and either M184I or M184V resulted in an RC equal to that of the WT. In the presence of ETV, the E138K/M184I mutant had the greatest RC and relative infectivity. The relative advantage of the E138K/M184I mutant over the E138K/M184V mutant was accentuated in the presence of ETV plus 3TC. The E138K/M184I mutant also had greater relative fitness and greater virion-associated RT activity than the E138K/M183V mutant. In the presence of 3TC, both double mutants had similar RC and relative infectivity that was greater than that of either the M184I or M184V single mutant. When tested in growth competition assays, the E138K/M184I mutant was fitter in the presence of 3TC than the E138K/M184V mutant. Taken together, these results suggest that the M138K and M184I or M184V mutations are mutually compensatory, and that the combination of E138K plus M184I confers a significant advantage over E138K plus M184V. This observation provides a plausible explanation for the frequent combined occurrence of E138K/M184I rather than E183K/M184V after the virologic failure of RPV-, 3TC-, or FTC-containing regimens.
Although initial studies of in vitro selection with ETV reported a variety of resistance mutations (25), a more recent report using clinical isolates from several different HIV-1 subtypes found that the E138K mutation was selected in all isolates and was usually the first mutation to emerge (2). A number of amino acid substitutions at RT position 138, including E138K, were identified in HIV-1 after the failure of ETV in the phase 3 trials (22). In the phase 3 trials of RPV, E138K was detected in plasma HIV-1 RNA from 28 of the 39 subjects (71%) who had virologic failure and in whom genotyping was successfully performed (17). Thus, it appears that E138K is a major resistance mutation for ETV and RPV.
The improved RC and fitness of the E138K/M184I double mutant compared to those of the respective single mutants suggests that these mutations are mutually compensatory. Compensatory mutations have been described in association with a number of so-called primary drug resistance mutations in HIV-1. For example, secondary mutations in HIV-1 protease and at gag cleavage sites improve the fitness of protease inhibitor-resistant virus (15, 30), the E92Q or G140S mutation in integrase improves the fitness of raltegravir-resistant virus carrying the N155H or Q148H mutation, respectively (8, 16), and mutations in the second helical coil domain (HR-2) of gp41 improve the fitness of enfuvirtide-resistant virus (29).
An unresolved question is the order of appearance of the E138K and M184I mutations. Does the E138K mutation emerge in viruses carrying the M184I mutation or vice versa? In patients receiving 3TC, M184I emerges prior to M184V, presumably because HIV-1 RT favors the G-to-A change encoding that substitution (ATG→ATA) over the A-to-G change required to produce an M184V substitution (ATG→GTG) (9). The superior fitness of E138K/M184I over E138K/M184V demonstrated in the current study suggests the possibility that the emergence of E138K in a virus carrying M184I locks in the M184I substitution, as there is no further advantage to the virus for the emergence of M184V. Resolving this question requires the single-genome sequencing of closely timed samples collected around the time of virologic failure on a RPV or ETV regimen with 3TC or FTC.
A possible explanation for our findings is that the presence of the M184I or M184V mutation results in a higher level of ETV and RPV resistance than is conferred by E138K alone. Although the M184I and M184V mutations themselves did not shift the IC50 for ETV, we did observe a slightly greater fold increase in IC50s for the E138K/M184I and E138K/M184V mutants (3.3- and 2.7-fold, respectively) compared to those of the E138K mutant (2.0-fold). Whether relatively modest differences in ETV and RPV susceptibility are clinically significant and can account for the selection of E138K/M184I over E138K/M184V in patients is unresolved. The difference in ETV resistance would not explain the superior RC and fitness of the E138K/M184I mutant we observed in the absence of drug. Molecular modeling studies suggest that the E138K mutation disrupts a salt bridge between Lys101 and Glu138, expanding the NNRTI binding pocket and reducing affinity for the DAPY compounds (11). It has been proposed that the M184I mutation contributes to reduced RPV (and ETV) susceptibility by further distorting the NNRTI binding pocket (11). Detailed enzyme kinetic studies are needed to determine the effect of the M184I and M184V mutations on the Ki for etravirine and rilpivirine in RT carrying E138K.
An alternative explanation for the fitness differences we observed is the effect on virion-associated RT activity of the different mutants. We found that the E138K, M184I, and M184V mutations each resulted in reduced virion-associated RT activity, and that the E138K/M184I combination restored RT activity to WT levels. Our findings are consistent with results of a more detailed biochemical assessment of HIV-1 RT using purified enzyme preparations that noted the improved processivity of the E138K-M184I/V mutant enzyme compared to that of the WT or M184I/V RT (28). These results provide another example of cross-talk between mutations affecting the nucleoside and nonnucleoside RT inhibitor binding pockets (10, 21). Other NNRTI resistance mutations result in a reduction in the amount of virion-associated RT, an effect correlated with observed fitness differences of the mutant viruses (27). Whether the E138K mutation also reduces the amount of virion-associated RT and whether that effect is counteracted by the M184V mutation are important points for future study.
Our study has a number of limitations. Because we were unable to obtain RPV, we used ETV as a surrogate. As the E138K mutation confers similar levels of resistance to both drugs (3, 22), it is likely that similar results would be obtained if the experiments reported here were conducted with RPV. The salutary effect of E138K on the fitness of HIV-1 carrying M184V raises the question of why E138K does not appear to arise as a compensatory mutation under the selective pressure of 3TC or FTC in the absence of ETV or RPV. We examined the interactions among the E138K and M184I/V mutations in a single viral genetic backbone (NL4-3), and different results might be obtained in different backbones. However, the E138K mutation appears to have similar effects in a variety of viral isolates and subtypes (2). The fitness profiles and growth competition experiments were performed in the presence of one or two drugs, whereas most antiretroviral regimens involve three-drug combinations. It is possible that the presence of a third drug (most often tenofovir, abacavir, or zidovudine in the RPV studies [6]) modifies the relative fitness of mutant viruses. Lastly, adaptive and innate immune responses as well as variation in the HIV-1 genome outside the RT-coding region contribute to overall viral fitness in infected persons, but the contribution of these factors was not assessed in our in vitro system.
In conclusion, the greater relative fitness and virion-associated RT activity of the E138K/M184I mutant compared to those of the E138K/M184V mutant likely explains the frequent association of E138K with M184I in HIV-1 from patients with virologic failure in clinical trials of RPV. It would be interesting to determine the rate at which these mutations disappear in patients who happen to interrupt treatment with RPV or ETV after virologic failure, and to know whether preexisting minority virus populations carrying E138K or M184I predispose patients to the failure of these drugs (13).
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service grants from the National Center for Research Resources (K24 RR16482) and the National Institute of Allergy and Infectious Diseases (through a Virology Support Laboratory contract from the AIDS Clinical Trials Group [U01 AI068636]).
We thank Sébastien Gallien and Jonathan Li for assistance with some experiments, Françoise Giguel for technical support, and Janet Steele and Jaclyn Coté for administrative support.
Footnotes
Published ahead of print on 17 August 2011.
REFERENCES
- 1. Andries K., et al. 2004. TMC125, a novel next-generation nonnucleoside reverse transcriptase inhibitor active against nonnucleoside reverse transcriptase inhibitor-resistant human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 48:4680–4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asahchop E. L., et al. 2011. Characterization of the E138K resistance mutation in HIV-1 reverse transcriptase conferring susceptibility to etravirine in B and non-B HIV-1 subtypes. Antimicrob. Agents Chemother. 55:600–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Azijn H., et al. 2010. TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob. Agents Chemother. 54:718–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Back N. K. T., et al. 1996. Reduced replication of 3TC-resistant HIV-1 variants in primary cells due to a processivity defect of the reverse transcriptase enzyme. EMBO J. 15:4040–4049 [PMC free article] [PubMed] [Google Scholar]
- 5. Boucher C. A. B., et al. 1993. High-level resistance to (-) enantiomeric 2′-deoxy-3′-thiacytidine in vitro is due to one amino acid substitution in the catalytic site of human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 37:2231–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cohen C., et al. 2011. Pooled week 48 efficacy and safety results from ECHO and THRIVE, two double-blind, randomised, phase III trials comparing TMC278 versus efavirenz in treatment-naive, HIV-1-infected patients. Abstr. XVIII Int. AIDS Conf., abstr. THLBB206. [Google Scholar]
- 7. Hu Z., et al. 2006. Fitness comparison of thymidine analog resistance pathways in human immunodeficiency virus type 1. J. Virol. 80:7020–7027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hu Z., Kuritzkes D. R. 2010. Effect of raltegravir resistance mutations in HIV-1 integrase on viral fitness. J. Acquir. Immune Defic. Syndr. 55:148–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keulen W., Back N. T., van Wijk A., Boucher C. A. B., Berkhout B. 1997. Initial appearance of the 184Ile variant in lamivudine-treated patients is caused by the mutational bias of human immunodeficiency virus type 1 reverse transcriptase. J. Virol. 71:3346–3350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koval C. E., Dykes C., Wang J., Demeter L. M. 2006. Relative replication fitness of efavirenz-resistant mutants of HIV-1: correlation with frequency during clinical therapy and evidence of compensation for the reduced fitness of K103N + L100I by the nucleoside resistance mutation L74V. Virology 353:184–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kulkarni R., et al. 2011. Cross-talk between the HIV reverse transcriptase NRTI and NNRTI binding pockets: interactions between E138K and M184I and drug resistance. Antivir. Ther. 16(Suppl. 1):A21 [Google Scholar]
- 12. Larder B. A., Kemp S. D., Harrigan P. R. 1995. Potential mechanism for sustained antiretroviral efficacy of AZT-3TC combination therapy. Science 269:696–699 [DOI] [PubMed] [Google Scholar]
- 13. Li J. Z., et al. 2011. Low-frequency HIV-1 drug resistance mutations and risk of NNRTI-based antiretroviral treatment failure: a systematic review and pooled analysis. JAMA 305:1327–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu J., Kuritzkes D. R. 2001. A novel recombinant virus assay for comparing the relative fitness of HIV-1 reverse transcriptase variants. J. Acquir. Immune Defic. Syndr. 27:7–13 [DOI] [PubMed] [Google Scholar]
- 15. Nijhuis M., et al. 1999. Increased fitness of drug resistant HIV-1 protease as a result of acquisition of compensatory mutations during suboptimal therapy. AIDS 13:2349–2360 [DOI] [PubMed] [Google Scholar]
- 16. Quercia R., Dam E., Perez-Bercoff D., Clavel F. 2009. Selective-advantage profile of human immunodeficiency virus type 1 integrase mutants explains in vivo evolution of raltegravir resistance genotypes. J. Virol. 83:10245–10249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rimsky L., Eron J., Clotet B. 2011. Characterization of the resistance profile of TMC278: 48-week analysis of the phase 3 studies ECHO and THRIVE. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., abstr. H-1810. [Google Scholar]
- 18. Ruxrungtham K., et al. 2008. Impact of reverse transcriptase resistance on the efficacy of TMC125 (etravirine) with two nucleoside reverse transcriptase inhibitors in protease inhibitor-naive, nonnucleoside reverse transcriptase inhibitor-experienced patients: study TMC125-C227. HIV Med. 9:883–896 [DOI] [PubMed] [Google Scholar]
- 19. Schinazi R. F., et al. 1993. Characterization of human immunodeficiency viruses resistant to oxathiolane-cytosine nucleosides. Antimicrob. Agents Chemother. 37:875–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schuurman R., et al. 1995. Rapid changes in human immunodeficiency virus type 1 RNA load and appearance of drug-resistant virus populations in persons treated with lamivudine (3TC). J. Infect. Dis. 171:1411–1419 [DOI] [PubMed] [Google Scholar]
- 21. Selmi B., et al. 2003. The Y181C substitution in 3′-azido-3′-deoxythymidine-resistant human immunodeficiency virus, type 1, reverse transcriptase suppresses the ATP-mediated repair of the 3′-azido-3′-deoxythymidine 5′-monophosphate-terminated primer. J. Biol. Chem. 278:40464–40472 [DOI] [PubMed] [Google Scholar]
- 22. Tambuyzer L., Nijs S., Daems B., Picchio G., Vingerhoets J. 2011. Effect of mutations at position E138 in HIV-1 reverse transcriptase on phenotypic susceptibility and virologic response to etravirine. J. Acquir. Immune Defic. Syndr. 58:18–22 [DOI] [PubMed] [Google Scholar]
- 23. Tambuyzer L., et al. 2010. Characterization of genotypic and phenotypic changes in HIV-1-infected patients with virologic failure on an etravirine-containing regimen in the DUET-1 and DUET-2 clinical studies. AIDS Res. Hum. Retrovir. 26:1197–1205 [DOI] [PubMed] [Google Scholar]
- 24. Tisdale M., Kemp S. D., Parry N. R., Larder B. A. 1993. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc. Natl. Acad. Sci. U. S. A. 90:5653–5656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vingerhoets J., et al. 2005. TMC125 displays a high genetic barrier to the development of resistance: evidence from in vitro selection experiments. J. Virol. 79:12773–12782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wakefield J. K., Jablonski S. A., Morrow C. D. 1992. In vitro enzymatic activity of human immunodeficiency virus type 1 reverse transcriptase mutants in the highly conserved YMDD amino acid motif correlates with the infectious potential of the proviral genome. J. Virol. 66:6806–6812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang J., Bambara R. A., Demeter L. M., Dykes C. 2010. Reduced fitness in cell culture of HIV-1 with nonnucleoside reverse transcriptase inhibitor-resistant mutations correlates with relative levels of reverse transcriptase content and RNase H activity in virions. J. Virol. 84:9377–9389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu H.-T., et al. 2011. Compensation by the E138K mutation in HIV-1 reverse transcriptase for deficits in viral replication capacity and enzyme processivity associated with the M184I/V mutation. 85:11300–11308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu L., et al. 2005. Emergence and evolution of enfuvirtide resistance following long-term therapy involves heptad repeat 2 mutations within gp41. Antimicrob. Agents Chemother. 49:1113–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Y. M., et al. 1997. Drug resistance during indinavir therapy is caused by mutations in the protease gene and in its gag substrate cleavage sites. J. Virol. 71:6662–6670 [DOI] [PMC free article] [PubMed] [Google Scholar]