Abstract
Infections due to caspofungin-resistant Candida isolates in patients exposed to caspofungin therapy are increasing. We report here a nested case-control study which aimed at identifying factors associated with bloodstream infections caused by Candida spp. having reduced susceptibility to caspofungin (CRSC) in adults suffering from hematological malignancies. In univariate and multivariate analyses, infections with CRSC were associated with caspofungin exposure in the previous 30 days (odds ratio [OR] = 5.25; 95% confidence interval [95% CI], 1.68–16.35) and with an age of ≤65 years (OR = 3.27; 95% CI, 1.26–8.50).
INTRODUCTION
Caspofungin is the first echinocandin approved for clinical use in France and has emerged as a major choice for primary treatment of invasive candidiasis and for empirical therapy in patients with persistent fever and neutropenia (4, 16). A growing number of breakthrough infections with Candida species that have low susceptibility to echinocandins have been reported for patients receiving echinocandin therapy (1, 3, 13, 17, 18, 20–22, 25). The Candida parapsilosis complex (i.e., C. parapsilosis, C. metapsilosis, and C. orthopsilosis) and Candida guilliermondii are intrinsically less susceptible than other Candida species to echinocandin due to naturally occurring point mutations in the FKS gene (6, 10, 19, 26). These point mutations were identified in clinical isolates having low susceptibilities to caspofungin, are clustered on two hot-spot regions (HS1 and HS2) (6, 10, 17, 19), and confer resistance to all three echinocandins (7, 18, 24). Recent epidemiological studies have reported concomitant increases in global caspofungin usage and in the incidence of C. parapsilosis fungemia (9, 23). We reported previously that the spectrum of species causing bloodstream infections (BSI) in patients who had been exposed to caspofungin differed significantly from that in BSI patients who had not been treated with this drug (15). Here, we explored whether recent prior exposure to caspofungin was associated with an increased risk of BSI with Candida spp. having reduced susceptibility to caspofungin (CRSC) in adults with hematological malignancies.
A matched case-control study was nested within the YEASTS program, an active surveillance program on yeast BSI implemented in the Paris area in France (5). The study population included patients >17 years of age suffering from hematological diseases and Candida BSI between 1 October 2002 and 1 February 2010. If several Candida BSI occurred in the same patient during the study period, only the episode due to CRSC—or the first episode if the caspofungin MICs were low—was considered in the analysis. Cases corresponding to mixed infection (with ≥2 species) were analyzed as a single infection due to the species for which the MIC was highest. Isolates were identified at the species level by phenotypic and/or molecular methods as described previously (15). In vitro caspofungin susceptibility was determined according to the EUCAST procedure (24) and tested in AM3 medium (6) as described previously (15). Sequencing of the hot-spot regions of FKS genes was performed for C. albicans and C. glabrata isolates for which caspofungin MICs were ≥0.5 mg/liter (17). Cases were patients with BSI due to (i) species known for their intrinsically low susceptibilities to caspofungin, i.e., the C. parapsilosis complex and C. guilliermondii (regardless of the caspofungin MICs) or to (ii) other Candida spp. for which MICs were ≥0.5 mg/liter (7). Controls were patients with BSI due to non-C. parapsilosis or -C. guilliermondii species for which caspofungin MICs were <0.5 mg/liter. Controls were randomly selected in a ratio of 2 per case and matched by center and a time period within 1 year. Patient information (sociodemographic data, the presence of underlying conditions, and histories of antifungal treatment with caspofungin or with an azole antifungal in the 30 days prior to BSI) was also gathered. These data were systematically recorded within the framework of the YEASTS program at each center by either the microbiologist or the clinician, using a standardized anonymous questionnaire. Conditional logistic regression was used to estimate odds ratios (OR) and the associated 95% confidence intervals (95% CI) for univariate analysis (unadjusted OR) and multivariate analysis (adjusted OR). The final multivariate model was built through back stepwise elimination of variables of interest with the highest P value. P values of less than 0.05 for associations were considered to be statistically significant. Stata version 10.0 (StataCorp, College Station, TX) was used.
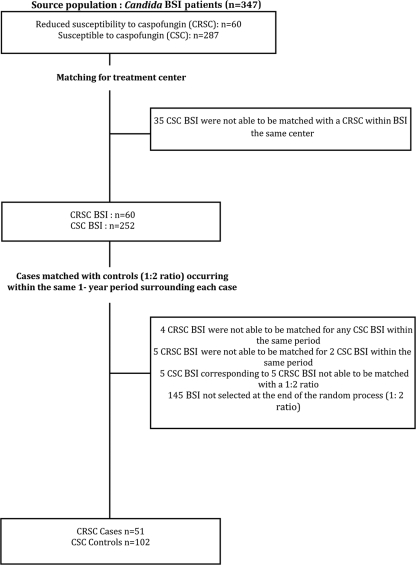
A total of 347 patients were eligible. The matching of two controls for each case by using treatment center and 1-year time period criteria led to a total of 153 patients (51 in the case group and 102 in the control group) being included in the study (Fig. 1). Of the 153 patients included in the study, 88 (57.5%) were male, and the median patient age was 48.0 years for the case group and 58.4 for the control group (Table 1). No patient had been exposed to any echinocandin other than caspofungin. The median MICs of caspofungin were 0.25 mg/liter for the case group (interquartile range [IQR] = 0.356) and 0.06 mg/liter for the control group (IQR = 0). Candida isolates identified in patients preexposed to caspofungin were C. parapsilosis and C. guilliermondii (accounting for 86.3% of the cases), as well as C. albicans, C. glabrata, C. krusei, and C. lipolytica. Point mutations in the FKS gene were identified in all C. albicans isolates (n = 2) and in one C. glabrata isolate.
Fig. 1.
Flow chart of the process used to match cases (infections with CRSC) with controls (infections with CSC) at a 1:2 ratio for cases and controls occurring within the same 1-year period.
Table 1.
Univariate and multivariate analyses of factors associated with BSI caused by Candida spp. with decreased susceptibility to caspofungin in 153 adults suffering from hematological diseasesa
| Characteristic or parameter | No. (%) among case group (n = 51) or values for group | No. (%) among control group (n = 102) or values for group | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |||
| Sex | 1.71 | 0.87–3.36 | 0.12 | |||||
| Male | 34 (66.7) | 54 (52.9) | ||||||
| Female | 17 (33.3) | 48 (47.1) | ||||||
| Age at fungemia | 0.005 | 0.015 | ||||||
| ≤65 years | 45 (88.2) | 66 (64.7) | 3.81 | 1.51–9.57 | 3.27 | 1.26–8.50 | ||
| >65 years | 6 (11.8) | 36 (35.3) | 1 | 1 | ||||
| Median length (days) of stayb (IQR) in treatment center | 15 (28.0) | 14 (25.1) | 1.01 | 0.98–1.03 | 0.51 | |||
| Prior exposure to caspofungin (within 30 days) | 14 (27.5) | 6 (5.9) | 6.31 | 2.06–19.33 | 0.001 | 5.25 | 1.68–16.35 | 0.004 |
| Prior exposure to azole antifungal agent (within 30 days) | 5 (9.8) | 12 (7.9) | 0.81 | 0.27–2.46 | 0.71 | |||
| Presence of hematological disease(s): | 0.09 | |||||||
| Acute leukemia | 24 (47.1) | 35 (34.3) | 2.62 | 1.06–6.46 | ||||
| Lymphoma | 17 (33.3) | 31 (30.4) | 2.10 | 0.79–5.59 | ||||
| Other(s) | 10 (19.6) | 36 (35.3) | 1 | |||||
| Presence of cancer | 1 (2.0) | 5 (4.9) | 0.36 | 0.04–3.36 | 0.36 | |||
| History of: | ||||||||
| Previous surgery (<30 days) | 2 (3.9) | 11 (10.8) | 0.36 | 0.08–1.64 | 0.19 | |||
| Allogeneic HSCT | 10 (19.6) | 12 (11.8) | 1.87 | 0.74–4.75 | 0.19 | |||
| Autologous HSCT | 4 (7.8) | 4 (3.9) | 2.00 | 0.50–8.00 | 0.33 | |||
| GVHD | 6 (11.8) | 6 (5.9) | 2.50 | 0.67–9.31 | 0.17 | |||
| Use of or exposure to: | ||||||||
| Immunosuppressive agents (including corticosteroids) | 14 (27.5) | 28 (27.5) | 0.86 | 0.40–1.85 | 0.69 | |||
| Broad-spectrum antimicrobial agents | 25 (49.0) | 56 (54.9) | 0.72 | 0.32–1.60 | 0.42 | |||
| Central venous catheter | 46 (90.2) | 87 (85.3) | 1.60 | 0.54–4.72 | 0.39 | |||
| Indwelling venous catheter | 11 (21.6) | 28 (27.5) | 0.70 | 0.30–1.63 | 0.41 | |||
| Arterial catheter | 3 (5.9) | 11 (10.8) | 0.55 | 0.15–1.96 | 0.35 | |||
| Urinary probe | 5 (9.8) | 12 (11.8) | 0.81 | 0.27–2.46 | 0.71 | |||
| Other foreign material | 3 (5.9) | 7 (6.9) | 0.86 | 0.22–3.32 | 0.82 | |||
| Death before day 30 | 15 (29.4) | 40 (39.2) | ||||||
HSCT, hematopoietic stem cell transplantation; GVHD, graft-versus-host disease. Values in boldface indicate statistical significance.
Values are available for 18 patients in the case group and 41 patients in the control group admitted for inpatient treatment.
In the univariate analysis, patients with CRSC isolates were more likely than those without CRSC isolates to be younger than 65 years (P = 0.005) and to have been exposed to caspofungin in the 30 days prior to BSI (P = 0.001) (Table 1). In the multivariate analysis, the risk of CRSC BSI was independently higher in patients less than 65 years old than it was in older patients (OR = 3.27; P = 0.015) and in patients with prior exposure to caspofungin than in those without (OR = 5.25; P = 0.004) (Table 1). Thirty days after the onset of BSI, 15 patients in the case group (29.4%) and 40 in the control group (39.2%) had died.
We report a significant association between prior exposure to caspofungin and an elevated risk of CRSC BSI in adults with hematological malignancies. An association between prior exposure to an echinocandin, especially caspofungin, and CRSC BSI had been suspected previously, but earlier studies were hampered by limited numbers of patients and by the lack of a specific, case-control design (3, 11, 14, 22, 23). The incidence of C. parapsilosis BSI has been increasing over the past 6 years (8, 12), and this has been reported to correlate significantly with more prevalent caspofungin usage in some centers (9). Here, 44 cases of CRSC BSI (86.3%) were due to species whose susceptibility to caspofungin is intrinsically low. Similar to what has been strongly suspected in previous studies (15, 23), our main hypothesis is that the prevalent use of echinocandins is having a progressive ecologic impact, resulting in the selection in the gut or on the skin of Candida species with decreased (intrinsic or acquired) susceptibility to these drugs (2). However, fungal colonization prior to BSI could not be demonstrated here due to lack of recorded details. In conclusion, similar to previous exposure to azoles, recent exposure to caspofungin should be considered in prescribing initial treatment of Candida BSI in patients with hematological malignancies.
Acknowledgments
The YEASTS program was supported in part by the Institut de Veille Sanitaire and the Institut Pasteur. It was approved by the Institut Pasteur Institutional Review Board (IRB 00006966). The founders had no role in study design, data collection, analysis, or interpretation.
The following investigators from the French Mycosis Study Group participated in the YEASTS program: for collection of data in each participating center, C. Bouges-Michel (Hôpital Avicenne, Bobigny), I. Poilane (Hôpital Jean Verdier, Bondy), J. Dunan (Hôpital Ambroise Paré, Boulogne), G. Galeazzi (Hôpital Louis Mourier, Colombes), F. Botterel (Hôpital Henri Mondor, Créteil), N. Fauchet (Centre Intercommunal, Créteil), E. Forget (Hôpital Beaujon, Clichy), C. Lawrence (Hôpital Raymond Poincaré, Garches), C. Bonnal, F. Botterel, and P. Bourée (Hôpital du Kremlin Bicêtre, Kremlin Bicêtre), O. Eloy (Centre Hospitalier, Le Chesnay), M.-F. David, N. Khassis, and L. Milhaila (Hôpital Paul Brousse, Villejuif), E. Chachaty (Institut Gustave Roussy, Villejuif), C. Chochillon (Hôpital Bichat, Paris), A. Paugam and M.-T. Baixench (Hôpital Cochin, Paris), M. Cornet (Hôtel Dieu, Paris), M.-C. Escande (Institut Curie, Paris), M.-E. Bougnoux, Y. Sterckers, and S. Challier (Hôpital Necker, Paris), E. Dannaoui and V. Lavarde (Hôpital Européen Georges Pompidou, Paris), A. Datry, B. Lmimouni, S. Brun, and A. Fekkar (Hôpital de la Pitié-Salpétrière, Paris), J.-L. Poirot (Hôpital Saint-Antoine, Paris), C. Lacroix (Hôpital Saint Louis, Paris), D. Moissenet (Hôpital Trousseau, Paris), M. Develoux (Hôpital Tenon, Paris), and S. Bonacorsi (Hôpital Robert Debré, Paris), and for technical analysis of the isolates at the National Reference Center for Mycoses and Antifungals, Dorothée Raoux and Damien Hoinard.
D.G. and O.L. are consultants to Merck, and O.L. discloses lecture fees from Merck. F.D., K.B.-S., M.D.-O., and E.B. do not report receiving any fees, honoraria, grants, or consultancies that would constitute a conflict of interest with the present study.
Footnotes
Published ahead of print on 22 August 2011.
REFERENCES
- 1. Baixench M.-T., et al. 2007. Acquired resistance to echinocandins in Candida albicans: case report and review. J. Antimicrob. Chemother. 59:1076–1083 [DOI] [PubMed] [Google Scholar]
- 2. Charles P. E., et al. 2005. Candida spp. colonization significance in critically ill medical patients: a prospective study. Intensive Care Med. 31:393–400 [DOI] [PubMed] [Google Scholar]
- 3. Dannaoui E., et al. 2010. Infections due to Candida spp. with reduced susceptibility to caspofungin in France, communication O–346. In Proceedings of the 20th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID). European Society of Clinical Microbiology and Infectious Diseases, Basel, Switzerland [Google Scholar]
- 4. Denning D. W. 2003. Echinocandin antifungal drugs. Lancet 362:1142–1151 [DOI] [PubMed] [Google Scholar]
- 5. Desnos-Ollivier M., et al. 2008. Clonal population of flucytosine-resistant Candida tropicalis from blood cultures, Paris, France. Emerg. Infect. Dis. 14:557–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Desnos-Ollivier M., et al. 2008. Mutations in the fks1 gene in Candida albicans, C. tropicalis, and C. krusei correlate with elevated caspofungin MICs uncovered in AM3 medium using the method of the European Committee on Antibiotic Susceptibility Testing. Antimicrob. Agents Chemother. 52:3092–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Desnos-Ollivier M., Dromer F., Dannaoui E. 2008. Detection of caspofungin resistance in Candida spp. by Etest. J. Clin. Microbiol. 46:2389–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dizbay M., et al. 2010. High incidence of Candida parapsilosis candidaemia in non-neutropenic critically ill patients: epidemiology and antifungal susceptibility. Scand. J. Infect. Dis. 42:114–120 [DOI] [PubMed] [Google Scholar]
- 9. Forrest G. N., Weekes E., Johnson J. K. 2008. Increasing incidence of Candida parapsilosis candidemia with caspofungin usage. J. Infect. 56:126–129 [DOI] [PubMed] [Google Scholar]
- 10. Garcia-Effron G., Lee S., Park S., Cleary J. D., Perlin D. S. 2009. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-beta-d-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob. Agents Chemother. 53:3690–3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hachem R., Hanna H., Kontoyiannis D., Jiang Y., Raad I. 2008. The changing epidemiology of invasive candidiasis: Candida glabrata and Candida krusei as the leading causes of candidemia in hematologic malignancy. Cancer 112:2493–2499 [DOI] [PubMed] [Google Scholar]
- 12. Horasan E. S., et al. 2010. Increase in Candida parapsilosis fungemia in critical care units: a 6-years study. Mycopathologia 170:263–268 [DOI] [PubMed] [Google Scholar]
- 13. Kabbara N., et al. 2008. Breakthrough C. parapsilosis and C. guilliermondii blood stream infections in allogeneic hematopoietic stem cell transplant recipients receiving long-term caspofungin therapy. Haematologica 93:639–640 [DOI] [PubMed] [Google Scholar]
- 14. Kofteridis D. P., Lewis R. E., Kontoyiannis D. P. 2010. Caspofungin-non-susceptible Candida isolates in cancer patients. J. Antimicrob. Chemother. 65:293–295 [DOI] [PubMed] [Google Scholar]
- 15. Lortholary O., et al. 2011. Recent exposure to caspofungin or fluconazole influences the epidemiology of candidemia: a prospective multicenter study involving 2,441 patients. Antimicrob. Agents Chemother. 55:532–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pappas P. G., et al. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park S., et al. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 49:3264–3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perlin D. S. 2011. Current perspectives on echinocandin class drugs. Future Microbiol. 6:441–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perlin D. S. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 10:121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pfaller M., et al. 2011. Use of epidemiological cutoff values to examine 9-year trends in susceptibility of Candida species to anidulafungin, caspofungin, and micafungin. J. Clin. Microbiol. 49:624–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pfaller M. A., Castanheira M., Messer S. A., Moet G. J., Jones R. N. 2011. Echinocandin and triazole antifungal susceptibility profiles for Candida spp., Cryptococcus neoformans, and Aspergillus fumigatus: application of new CLSI clinical breakpoints and epidemiologic cutoff values to characterize resistance in the SENTRY Antimicrobial Surveillance Program (2009). Diagn. Microbiol. Infect. Dis. 69:45–50 [DOI] [PubMed] [Google Scholar]
- 22. Pfeiffer C. D., et al. 2010. Breakthrough invasive candidiasis in patients on micafungin. J. Clin. Microbiol. 48:2373–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sipsas N. V., et al. 2009. Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001–2007): stable incidence but changing epidemiology of a still frequently lethal infection. Cancer 115:4745–4752 [DOI] [PubMed] [Google Scholar]
- 24. Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing 2008. EUCAST definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 14:398–405 [DOI] [PubMed] [Google Scholar]
- 25. Sun H.-Y., Singh N. 2010. Characterisation of breakthrough invasive mycoses in echinocandin recipients: an evidence-based review. Int. J. Antimicrob. Agents 35:211–218 [DOI] [PubMed] [Google Scholar]
- 26. Walker L. A., Gow N. A. R., Munro C. A. 2010. Fungal echinocandin resistance. Fungal Genet. Biol. 47:117–126 [DOI] [PMC free article] [PubMed] [Google Scholar]