Abstract
Malaria and HIV infection are both very common in many developing countries. With the increasing availability of therapy for HIV infection, it was of interest to determine whether antiretroviral drugs exert antimalarial effects. We therefore tested the in vitro activity of 19 antiretroviral drugs against the W2 and 3D7 strains of Plasmodium falciparum at concentrations up to 50 μM. None of 5 tested nucleoside reverse transcriptase inhibitors demonstrated activity. Two nonnucleoside reverse transcriptase inhibitors, efavirenz (mean 50% inhibitory concentration [IC50] of 22 to 30 μM against the two strains) and etravirine (3.1 to 3.4 μM), were active; nevirapine was not active. Also active were the fusion inhibitor enfuvirtide (6.2 to 7.9 μM) and the entry inhibitor maraviroc (15 to 21 μM). Raltegravir was not active. However, for all active drugs mentioned above, the IC50s were considerably greater than the concentrations achieved with standard dosing. The effects most likely to be clinically relevant were with HIV protease inhibitors. Of the tested compounds, activity was seen with lopinavir (2.7 to 2.9 μM), atazanavir (3.3 to 13.0 μM), saquinavir (5.0 to 12.1 μM), nelfinavir (6.5 to 12.1 μM), ritonavir (9.5 to 10.9 μM), tipranavir (15.5 to 22.3 μM), and amprenavir (28.1 to 40.8) but not darunavir. Lopinavir was active at levels well below those achieved with standard dosing of coformulated lopinavir-ritonavir. Lopinavir also demonstrated modest synergy with the antimalarial lumefantrine (mean fractional inhibitory concentration index of 0.66 for W2 and 0.53 for 3D7). Prior data showed that lopinavir-ritonavir also extends the pharmacokinetic exposure of lumefantrine. Thus, when used to treat HIV infection, lopinavir-ritonavir may have clinically relevant antimalarial activity and also enhance the activity of antimalarials.
INTRODUCTION
Every year, 300 to 400 million people become ill and nearly 1 million die from malaria, mostly caused by Plasmodium falciparum and mostly in sub-Saharan Africa (13). HIV currently infects approximately 33 million people worldwide. The pandemic is dynamic, with 2.6 million new infections and nearly 2 million deaths per year (45). With the overlap of malaria and HIV infection in tropical regions, it is important that control measures for one disease consider impacts on the other. Importantly, the treatment of both malaria and HIV infection has changed dramatically in recent years. For malaria, new artemisinin-based combination therapies (ACTs) are now recommended for the treatment of uncomplicated falciparum malaria in nearly all countries where malaria is endemic (52). For HIV infection, antiretroviral therapy is increasingly available in the developing world, and there is a move toward initiating therapy earlier in the course of infection (28). Thus, many patients will be at risk of malaria and also treated for malaria while concomitantly receiving antiretroviral drugs.
The effects of some antiretroviral drugs on malaria have been studied previously. In particular, HIV protease inhibitors have been shown to have antimalarial activity (31, 40). Initially, saquinavir, ritonavir, and indinavir, all older protease inhibitors of relatively little importance for HIV therapy today, were shown to be active against cultured P. falciparum (40). Subsequently, a wider range of protease inhibitors was shown to be active, including lopinavir, atazanavir, and amprenavir, drugs that are more widely used than the agents studied earlier (31). Importantly, lopinavir was active at concentrations well below those that circulate in plasma when the drug is coadministered with a second protease inhibitor, ritonavir, to enhance lopinavir levels (31). Subsequent studies showed activity of multiple protease inhibitors against P. falciparum in vitro and Plasmodium chabaudi in vivo (1), activity against clinical isolates of P. falciparum and Plasmodium vivax (22), and in vitro activity of sera from patients receiving protease inhibitors against P. falciparum (36). Considering other classes of antiretroviral drugs, limited data are available. The nonnucleoside reverse transcriptase inhibitor nevirapine was not active (40). The antimalarial activity of most classes of antiretroviral drugs and of some important new antiretroviral protease inhibitors has not been reported previously (41).
Another important consideration concerning antimalarial and antiretroviral therapy is drug interactions leading to changes in pharmacokinetics or antimalarial efficacy. Considering pharmacokinetics, the HIV protease inhibitor ritonavir is widely used to enhance the circulating concentrations of other protease inhibitors, but it may also affect exposure to many other drugs that are metabolized by cytochrome P450 3A4. Indeed, in normal volunteers, exposure to the antimalarial lumefantrine, a component of the ACT artemether-lumefantrine, was markedly enhanced by coadministration of lopinavir-ritonavir (12), and exposure to a number of other antimalarial drugs may be affected by ritonavir or other agents (41). Considering antimalarial efficacy, HIV protease inhibitors have been shown to potentiate the activity of chloroquine (14, 15, 23) and to either augment (29) or antagonize (16) the activity of artemisinins. To help clarify the impacts of antiretroviral therapy on malaria, we systematically evaluated the activity of representatives of all currently used classes of antiretroviral drugs against two strains of P. falciparum. We also studied the effects of combinations of standard antimalarial drugs with lopinavir, the antiretroviral drug that appears to have the most important antimalarial activity.
MATERIALS AND METHODS
Parasites.
The P. falciparum strains studied, which were obtained from the Malaria Research and Reference Reagent Resource Center, were 3D7, which is sensitive to all standard antimalarial drugs, and W2, which is resistant to many agents.
Drugs.
All antimalarial drugs tested were obtained from Sigma Chemical Co., and all the antiretroviral drugs used in this study were obtained through the NIH AIDS Research and Reference Reagent Program.
In vitro drug susceptibility assay.
P. falciparum (W2 and 3D7 strains) was cultured in human erythrocytes at 2% hematocrit in RPMI medium supplemented with 0.5% Albumax, 2 mM l-glutamine, 100 mM hypoxanthine, and 5 μg/ml gentamicin. The parasites were synchronized by serial treatments with 5% d-sorbitol. Ninety-six-microwell culture plates were prepared with serial dilutions of HIV drugs diluted in dimethyl sulfoxide (DMSO), with concentrations ranging from 50 nM to 50 μM and negative controls containing the same concentrations of DMSO. Parasites were then cultured for 48 h beginning at the ring stage. After 48 h, cultures were fixed with 4% formaldehyde overnight and then stained with 4 nM YOYO-1 dye (Molecular Probes) in 0.1% Triton X-100 in phosphate-buffered saline. Antimalarial activity was assessed by fluorescence-activated cell sorter analysis as previously described (39). Fifty percent inhibitory concentrations (IC50s) were calculated by nonlinear regression with the Prism 5.0 program (GraphPad Software).
Drug combination assay.
Drug interaction studies were performed using the checkerboard technique as previously described (51). Briefly, parasites were cultured in wells containing different combinations of antimalarial drugs (0.05 to 10,000 nM) and lopinavir (5 to 10,000 nM). Combinations based on serial dilutions of each drug, each set up in duplicate, were evaluated. Concentrations that yielded 45 to 55% of control parasitemia were plotted on isobolograms and used to calculate fractional inhibitory concentrations (FICs) as the ratio of the IC50 of the drug in combination and the IC50 of the drug alone (11). The FIC index for two drugs was the FIC of drug A plus the FIC of drug B.
RESULTS
In vitro antimalarial activity.
We surveyed in vitro activity against the P. falciparum W2 and 3D7 strains of representatives of all available classes of drugs to treat HIV infection. Nineteen drugs were assessed at concentrations up to 50 μM, including nucleoside reverse transcriptase inhibitors, nonnucleoside reverse transcriptase inhibitors, protease inhibitors, entry/fusion inhibitors, and an integrase inhibitor (Table 1). None of the nucleoside reverse transcriptase inhibitors displayed antimalarial activity. From the nonnucleoside reverse transcriptase inhibitor class, efavirenz showed midmicromolar and etravirine low micromolar antimalarial activity. However, for both active nonnucleoside reverse transcriptase inhibitors, the IC50s were well above the plasma concentrations achieved with standard dosing. HIV protease inhibitors have previously been shown to have antimalarial activity; we broadened previous observations by including a wide range of protease inhibitors, including newer agents not previously studied. Of these agents, lopinavir was most active, and atazanavir, nelfinavir, ritonavir, and saquinavir also had fairly good activity, with IC50s of <10 μM against at least one of the plasmodial strains tested. However, only for lopinavir was the IC50 for both strains well below achievable plasma concentrations. In the case of lopinavir, the drug circulates at ∼10 to 20 μM with standard dosing of lopinavir/ritonavir, well above the calculated IC50s of 2.6 to 2.9 μM for the two tested strains. Tipranavir showed modest activity, with IC50s of 15 to 22 μM for the two strains. However, those values are below the plasma concentrations achieved when tripranavir is boosted by ritonavir (33 to 165 μM). Among newer agents, the two entry/fusion inhibitors had modest activity, although their IC50s were well above typical plasma concentrations. The integrase inhibitor raltegravir did not show antimalarial activity.
Table 1.
Activity of antiretroviral drugs against cultured P. falciparum
| Classa | Drug | IC50 (μM) for P. falciparum strain: |
Serum concn (μM)b |
||||||
|---|---|---|---|---|---|---|---|---|---|
| W2 | 3D7 | Standard dosing |
Ritonavir coadministration |
||||||
| Cmin | Cmax | Reference | Cmin | Cmax | Reference | ||||
| NRTIs | Abacavir | >50 | >50 | 0.1 | 10.5 | 26 | |||
| Emtricitabine | >50 | >50 | 0.7 | 8.3 | 50 | ||||
| Lamivudine | >50 | >50 | 0.3 | 8.3 | 7 | ||||
| Stavudine | >50 | >50 | 0.1 | 1.7 | 17 | ||||
| Zidovudine | >50 | >50 | 0.4 | 4.1 | 47 | ||||
| NNRTIs | Efavirenz | 21.9 | 29.7 | 0.4 | 33.9 | 21 | |||
| Etravirine | 3.4 | 3.1 | 0.4 | 0.9 | 4 | ||||
| Nevirapine | >50 | >50 | 16.7 | 26.2 | 8 | ||||
| PIs | Amprenavir | 28.1 | 40.8 | 0.7 | 9.5 | 2 | 2.9 | 14.3 | 2 |
| Atazanavir | 3.3 | 13 | 0.2 | 3.6 | 27 | 0.7 | 4.5 | 49 | |
| Darunavir | >50 | >50 | 0.7 | 8.7 | 48 | 4.1 | 12.8 | 4 | |
| Lopinavir | 2.7 | 2.9 | 5.7 | 10.4 | 10 | 9.5 | 19.4 | 20 | |
| Nelfinavir | 6.5 | 12.1 | 1.3 | 8.3 | 43 | 0.9 | 6.4 | 18 | |
| Ritonavir | 9.5 | 10.9 | 3.6 | 14.8 | 38 | ||||
| Saquinavir | 5 | 12.1 | 0.1 | 1.4 | 30 | 0.5 | 3.7 | 6 | |
| Tipranavir | 15.5 | 22.3 | 0.8 | 22.5 | 25 | 32.4 | 131.3 | 24 | |
| Entry/fusion inhibitor | Enfuvirtide | 7.9 | 6.2 | 0.1 | 1.3 | 44 | |||
| Maraviroc | 15.2 | 21.1 | 0 | 0.8 | 19 | ||||
| Integrase inhibitor | Raltegravir | >50 | >50 | 0.27 | 2.71 | 4 | |||
Abbreviations: NRTI, nucleoside reverse transcriptase inhibitors; NNRTIs, nonnucleoside reverse transcriptase inhibitors; PIs, protease inhibitors.
Serum concentrations achieved with standard dosing, including those for protease inhibitors boosted by coadministration with ritonavir, are shown, with the sources of this information cited. Values represent the means of two assays, each performed in duplicate.
Interaction between lopinavir and antimalarials.
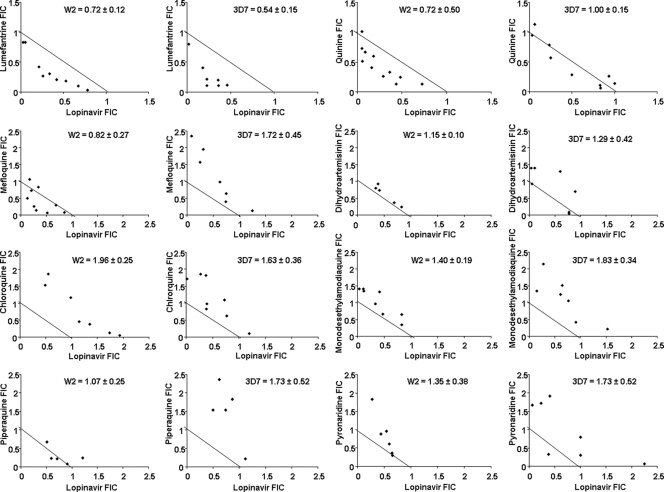
Consistent with previous studies, our broad survey identified lopinavir as the HIV drug most likely to offer clinically relevant activity against P. falciparum. To add insight into studies of the antimalarial activity of lopinavir, we assessed interactions between lopinavir and antimalarial agents. We used standard methods to test activities of combinations of lopinavir and chloroquine, monodesethylamodiaquine (the active metabolite of amodiaquine), quinine, mefloquine, lumefantrine, piperaquine, pyronaridine, and dihydroartemisinin, based on the IC50s determined for each drug against W2 and 3D7 strain parasites (Table 2). One combination, lopinavir and lumefantrine, showed a trend toward synergism, with the mean FIC index ± standard deviation being 0.53 ± 0.23 for the 3D7 strain and 0.66 ± 0.32 for W2 (Fig. 1). All other combinations studied showed additive effects, in some cases with modest trends toward synergism or antagonism.
Table 2.
Activities of antimalarial drugs against the strains of P. falciparum used in the isobologram analyses
| Antimalarial | IC50 (nM) for P. falciparum strain: |
|
|---|---|---|
| W2 | 3D7 | |
| Lumefantrine | 31.86 | 42.27 |
| Quinine | 89.50 | 21.53 |
| Chloroquine | 29.04 | 9.30 |
| Monodesethylamodiaquine | 9.51 | 2.70 |
| Mefloquine | 11.22 | 7.32 |
| Dihydroartemisinin | 1.31 | 1.23 |
| Piperaquine | 9.98 | 12.08 |
| Pyronaridine | 0.99 | 1.24 |
Fig. 1.
Isobolograms describing the interaction between lopinavir and the antimalarials indicated on the graphs for the P. falciparum W2 and 3D7 strains. Mean fractional inhibitory concentration (FIC) index ± standard deviation is shown for each combination and each strain.
DISCUSSION
We assessed the antimalarial activity of different HIV drugs. No activity was seen at concentrations up to 50 μM for all 5 nucleoside reverse transcriptase inhibitors tested, the nonnucleoside reverse transcriptase inhibitor nevirapine, the protease inhibitor darunavir, or the integrase inhibitor raltegravir. However, since nucleoside reverse transcriptase inhibitors function as prodrugs (35), we cannot rule out the possibility that the drugs exert antimalarial activity after intracellular phosphorylation. Among nonnucleoside reverse transcriptase inhibitors, efavirenz and etravirine showed antimalarial activity, although their IC50s were well above the levels of the drugs that circulate with standard therapy. The fusion inhibitor enfuvirtide and entry inhibitor maraviroc were also active although, as with the active nonnucleoside reverse transcriptase inhibitors, concentrations with meaningful antimalarial activity are probably not achieved with standard dosing. Consistent with older reports (22, 31, 36, 40), the most meaningful antimalarial activity was seen with HIV protease inhibitors. All tested protease inhibitors except darunavir showed antimalarial activity. Also consistent with older reports, lopinavir was the most potent of these agents. When provided in standard dosing with ritonavir, lopinavir circulates in the plasma at 10 to 19 μM, well above the IC50s of 2 to 3 μM determined in this study and 1 to 5 μM seen in other studies using a variety of methods (1, 31, 32, 33). Serum from patients treated with lopinavir-ritonavir also exerted in vitro antimalarial activity (36). Thus, when administered in modern regimens to treat HIV infection, lopinavir is likely to exert clinically relevant antimalarial activity.
The antimalarial activities of different HIV protease inhibitors vary greatly. Indeed, among newer agents not previously studied, tipranavir had only modest activity, although due to high circulating concentrations of this drug, the activity may be clinically important. Darunavir was inactive at 50 μM. Thus, lopinavir remains the HIV protease inhibitor with the most promising antimalarial activity. This is a fortuitous result, as lopinavir-ritonavir is increasingly available in countries where malaria is endemic, and it is available in a heat-stable formulation (9).
The antimalarial mechanism of action of HIV protease inhibitors is uncertain. Their action differs from that of the generic aspartic protease inhibitor pepstatin, as unlike pepstatin, they did not display synergy with cysteine protease inhibitors or enhanced activity against a cysteine protease knockout parasite (32). These results suggest that, although lopinavir and ritonavir inhibited the food vacuole hemoglobinase plasmepsin II (32), the HIV protease inhibitors may target other plasmodial proteases. P. falciparum contains 10 aspartic protease genes, which encode 4 food vacuole hemoglobinases (plasmepsins I to IV [3]), a protease that processes proteins for extracellular export (plasmepsin V [5, 37]), and other putative proteases with unknown functions. HIV protease inhibitors probably target plasmepsins, but this has not been confirmed, and it is unclear which plasmepsins are targeted or if all active protease inhibitors target the same enzymes.
We also evaluated the antimalarial activities of combinations of lopinavir and standard antimalarial agents. Interestingly, modest synergy was seen between lopinavir and lumefantrine. Synergy was not seen between lopinavir and any other tested antimalarial. Most combinations appeared to have additive effects. There was a trend toward antagonism between lopinavir and the aminoquinolines chloroquine, amodiaquine (studied as its active metabolite monodesethylamodiaquine), and piperaquine; for piperaquine and mefloquine, a trend toward antagonism was seen only for the chloroquine-resistant strain W2 and not the sensitive strain 3D7. Of interest, prior studies showed potentiation of chloroquine activity by HIV protease inhibitors (14, 15, 23), although the results with lopinavir were modest. It is unclear whether differences in results between our groups are due to the different protease inhibitors studied, differences in methodology, or other factors. Other groups have reported synergy (29) or antagonism (16) between HIV protease inhibitors and artemisinins, although only older protease inhibitors and not lopinavir were studied; we found additive effects between lopinavir and dihydroartemisinin.
Our data and the results of other recent studies suggest that there are three means by which the use of lopinavir-ritonavir may have an impact upon the incidence of malaria. First, the antimalarial activity of lopinavir, with drug levels boosted by ritonavir, may kill erythrocytic parasites before infections progress to clinical illness. Second, after therapy for a prior infection, inhibition of cytochrome P450 3A4 by ritonavir may extend exposure to antimalarial drugs, as has been demonstrated for lumefantrine (12), thereby prolonging the period during which a drug circulates at concentrations adequate to prevent new infections. Third, through synergistic effects, cocirculating antimalarials and antiretrovirals may prevent new infections more effectively than the antimalarials alone. For the last two described mechanisms, the evidence best supports an effect of lopinavir-ritonavir on the posttreatment prophylactic activity of lumefantrine. Therefore, it is of interest to evaluate the effect of lopinavir-ritonavir on those at high risk of malaria and on those treated for malaria in high-transmission areas with artemether-lumefantrine. Indeed, studies of the effects on malaria of treatment of HIV with lopinavir-ritonavir are under way. More broadly, as the use of antiretroviral therapy is increasing in countries where malaria is endemic, our results highlight the importance of evaluating the antimalarial effects of antiretroviral drugs, both in laboratory settings and in clinical trials.
ACKNOWLEDGMENTS
All the antiretroviral drugs used in this study were obtained through the NIH AIDS Research and Reference Reagent Program. Malaria parasites were obtained from the Malaria Research and Reference Reagent Resource Center. We thank Jiri Gut, Jenny Legac, and Danica Helb for expert technical assistance and Sunil Parikh and Fran Aweeka for advice regarding the pharmacology of antiretroviral drugs.
This work was supported by a Prospective Researchers' Fellowship from the Swiss National Science Foundation (PBNEP3-129883) and a grant from the National Institutes of Health (HD059454).
Footnotes
Published ahead of print on 29 August 2011.
REFERENCES
- 1. Andrews K. T., et al. 2006. Potencies of human immunodeficiency virus protease inhibitors in vitro against Plasmodium falciparum and in vivo against murine malaria. Antimicrob. Agents Chemother. 50:639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arvieux C., Tribut O. 2005. Amprenavir or fosamprenavir plus ritonavir in HIV infection: pharmacology, efficacy and tolerability profile. Drugs 65:633–659 [DOI] [PubMed] [Google Scholar]
- 3. Banerjee R., et al. 2002. Four plasmepsins are active in the Plasmodium falciparum food vacuole, including a protease with an active-site histidine. Proc. Natl. Acad. Sci. U. S. A. 99:990–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barrail-Tran A., et al. 2010. Pharmacokinetics of etravirine, raltegravir and darunavir/ritonavir in treatment experienced patients. AIDS 24:2581–2583 [DOI] [PubMed] [Google Scholar]
- 5. Boddey J. A., et al. 2010. An aspartyl protease directs malaria effector proteins to the host cell. Nature 463:627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boffito M., et al. 2005. Boosted saquinavir hard gel formulation exposure in HIV-infected subjects: ritonavir 100 mg once daily versus twice daily. J. Antimicrob. Chemother. 55:542–545 [DOI] [PubMed] [Google Scholar]
- 7. Bouazza N., et al. 2011. Developmental pharmacokinetics of lamivudine in 580 pediatric patients from neonates to adolescents. Antimicrob. Agents Chemother. 55:3498–3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Byakika-Kibwika P., et al. 2008. Steady-state pharmacokinetic comparison of generic and branded formulations of stavudine, lamivudine and nevirapine in HIV-infected Ugandan adults. J. Antimicrob. Chemother. 62:1113–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chandwani A., Shuter J. 2008. Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Ther. Clin. Risk Manag. 4:1023–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fätkenheuer G., Römer K., Kamps R., Salzberger B., Burger D. 2001. Pharmacokinetics of amprenavir and lopinavir in combination with nevirapine in highly pretreated HIV-infected patients. AIDS 15:2334–2335 [DOI] [PubMed] [Google Scholar]
- 11. Fivelman Q. L., Adagu I. S., Warhurst D. C. 2004. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug-resistant strains of Plasmodium falciparum. Antimicrob. Agents Chemother. 48:4097–4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. German P., et al. 2009. Lopinavir/ritonavir affects pharmacokinetic exposure of artemether/lumefantrine in HIV-uninfected healthy volunteers. J. Acquir. Immune Defic. Syndr. 51:424–429 [DOI] [PubMed] [Google Scholar]
- 13. Hay S. I., et al. 2010. Estimating the global clinical burden of Plasmodium falciparum malaria in 2007. PLoS Med. 7:e1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. He Z., et al. 2008. Synergy of human immunodeficiency virus protease inhibitors with chloroquine against Plasmodium falciparum in vitro and Plasmodium chabaudi in vivo. Antimicrob. Agents Chemother. 52:2653–2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He Z., Chen L., You J., Qin L., Chen X. 2009. Antiretroviral protease inhibitors potentiate chloroquine antimalarial activity in malaria parasites by regulating intracellular glutathione metabolism. Exp. Parasitol. 123:122–127 [DOI] [PubMed] [Google Scholar]
- 16. He Z., Chen L., You J., Qin L., Chen X. 2010. In vitro interactions between antiretroviral protease inhibitors and artemisinin endoperoxides against Plasmodium falciparum. Int. J. Antimicrob. Agents. 35:191–193 [DOI] [PubMed] [Google Scholar]
- 17. Hemanth Kumar A. K., et al. 2009. Pharmacokinetics of lamivudine & stavudine in generic fixed-dose combinations in HIV-1 infected adults in India. Indian J. Med. Res. 130:451–457 [PMC free article] [PubMed] [Google Scholar]
- 18. Justesen U. S., et al. 2005. The long-term pharmacokinetics and safety of adding low-dose ritonavir to a nelfinavir 1,250 mg twice-daily regimen in HIV-infected patients. HIV Med. 6:334–340 [DOI] [PubMed] [Google Scholar]
- 19. Kakuda T. N., et al. 2011. Pharmacokinetic interactions of maraviroc with darunavir-ritonavir, etravirine, and etravirine-darunavir-ritonavir in healthy volunteers: results of two drug interaction trials. Antimicrob. Agents Chemother. 55:2290–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kityo C., et al. 2010. Pharmacokinetics of lopinavir-ritonavir with and without nonnucleoside reverse transcriptase inhibitors in Ugandan HIV-infected adults. Antimicrob. Agents Chemother. 54:2965–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Langmann P., Schirmer D., Väth T., Zilly M., Klinker H. 2001. High-performance liquid chromatographic method for the determination of HIV-1 non-nucleoside reverse transcriptase inhibitor efavirenz in plasma of patients during highly active antiretroviral therapy. J. Chromatogr B Biomed. Sci. Appl. 755:151–156 [DOI] [PubMed] [Google Scholar]
- 22. Lek-Uthai U., et al. 2008. Stronger activity of human immunodeficiency virus type 1 protease inhibitors against clinical isolates of Plasmodium vivax than against those of P. falciparum. Antimicrob. Agents Chemother. 52:2435–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li X., et al. 3 May 2011. Synergy of the antiretroviral protease inhibitor indinavir and chloroquine against malaria parasites in vitro and in vivo. Parasitol. Res. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 24. McCallister S., Sabo J. P., Mayers D. L., Galitz L. 2002. An open-label, steady-state investigation of the pharmacokinetics (PK) of tipranavir (TPV) and ritonavir (RTV) and their effects on cytochrome-P450 (3A4) activity in normal, healthy volunteers (BI 1182.5), abstr. 434W. Abstr. 9th Conf. Retrovir. Opportun. Infect. Seattle, WA, 24 to 28 February 2002 [Google Scholar]
- 25. McCallister S., et al. 2004. A 14-day dose-response study of the efficacy, safety, and pharmacokinetics of the nonpeptidic protease inhibitor tipranavir in treatment-naive HIV-1-infected patients. J. Acquir. Immune Defic. Syndr. 35:376–382 [DOI] [PubMed] [Google Scholar]
- 26. McDowell J. A., Lou Y., Symonds W. S., Stein D. S. 2000. Multiple-dose pharmacokinetics and pharmacodynamics of abacavir alone and in combination with zidovudine in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 44:2061–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McRae M., Clay P. G., Anderson P. L., Glaros A. G. 2009. Pharmacokinetics of concurrent administration of fosamprenavir and atazanavir without ritonavir in human immunodeficiency virus-negative subjects. Pharmacotherapy 29:937–942 [DOI] [PubMed] [Google Scholar]
- 28. Menon S. 2010. Early initiation of antiretroviral therapy and universal HIV testing in sub-Saharan Africa: has WHO offered a milestone for HIV prevention? J. Public Health Policy 31:385–400 [DOI] [PubMed] [Google Scholar]
- 29. Mishra L. C., Bhattacharya A., Sharma M., Bhasin V. K. 2010. HIV protease inhibitors, indinavir or nelfinavir, augment antimalarial action of artemisinin in vitro. Am. J. Trop. Med. Hyg. 82:148–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moyle G. J., et al. 2002. Interaction between saquinavir soft-gel and rifabutin in patients infected with HIV. Br. J. Clin. Pharmacol. 54:178–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parikh S., et al. 2005. Antimalarial activity of human immunodeficiency virus type 1 protease inhibitors. Antimicrob. Agents Chemother. 49:2983–2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parikh S., et al. 2006. Antimalarial effects of human immunodeficiency virus type 1 protease inhibitors differ from those of the aspartic protease inhibitor pepstatin. Antimicrob. Agents Chemother. 50:2207–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peatey C. L., et al. 2010. Antimalarial asexual stage-specific and gametocytocidal activities of HIV protease inhibitors. Antimicrob. Agents Chemother. 54:1334–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reference deleted.
- 35. Pretorius E., Klinker H., Rosenkranz B. 2011. The role of therapeutic drug monitoring in the management of patients with human immunodeficiency virus infection. Ther. Drug Monit. 33:265–274 [DOI] [PubMed] [Google Scholar]
- 36. Redmond A. M., et al. 2007. Antimalarial activity of sera from subjects taking HIV protease inhibitors. AIDS 21:763–765 [DOI] [PubMed] [Google Scholar]
- 37. Russo I., et al. 2010. Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte. Nature 463:632–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shelton M. J., et al. 2003. Pharmacokinetics of ritonavir and delavirdine in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 47:1694–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sijwali P. S., Rosenthal P. J. 2004. Gene disruption confirms a critical role for the cysteine protease falcipain-2 in hemoglobin hydrolysis by Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 101:4384–4389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Skinner-Adams T. S., McCarthy J. S., Gardiner D. L., Hilton P. M., Andrews K. T. 2004. Antiretrovirals as antimalarial agents. J. Infect. Dis. 190:1998–2000 [DOI] [PubMed] [Google Scholar]
- 41. Skinner-Adams T. S., McCarthy J. S., Gardiner D. L., Andrews K. T. 2008. HIV and malaria co-infection: interactions and consequences of chemotherapy. Trends Parasitol. 24:264–271 [DOI] [PubMed] [Google Scholar]
- 42. Reference deleted.
- 43. Stocker H., et al. 2007. Saquinavir, nelfinavir and M8 pharmacokinetics following combined saquinavir, ritonavir and nelfinavir administration. J. Antimicrob. Chemother. 59:560–564 [DOI] [PubMed] [Google Scholar]
- 44. Tebas P., et al. 2008. Enfuvirtide does not require dose adjustment in patients with chronic kidney failure: results of a pharmacokinetic study of enfuvirtide in HIV-1-infected patients with impaired kidney function. J. Acquir. Immune Defic. Syndr. 47:342–345 [DOI] [PubMed] [Google Scholar]
- 45. UNAIDS 2010. Global report. UNAIDS report on the global AIDS epidemic 2010. Joint United Nations Programme on HIV/AIDS (UNAIDS), Geneva, Switzerland. http://www.unaids.org/globalreport/documents/20101123_GlobalReport_full_en.pdf
- 46. Reference deleted.
- 47. Venhoff N., et al. 2008. Pharmacokinetics of zidovudine and lamivudine during oral uridine supplementation with NucleomaxX. J. Acquir. Immune Defic. Syndr. 48:114–116 [DOI] [PubMed] [Google Scholar]
- 48. Vermeir M., et al. 2009. Absorption, metabolism, and excretion of darunavir, a new protease inhibitor, administered alone and with low-dose ritonavir in healthy subjects. Drug Metab. Dispos. 37:809–820 [DOI] [PubMed] [Google Scholar]
- 49. von Hentig N., et al. 2008. The steady-state pharmacokinetics of atazanavir/ritonavir in HIV-1-infected adult outpatients is not affected by gender-related co-factors. J. Antimicrob. Chemother. 62:579–582 [DOI] [PubMed] [Google Scholar]
- 50. Wang L. H., et al. 2004. Pharmacokinetic and pharmacodynamic characteristics of emtricitabine support its once daily dosing for the treatment of HIV infection. AIDS Res. Hum. Retroviruses 20:1173–1182 [DOI] [PubMed] [Google Scholar]
- 51. Wei G. X., Bobek L. A. 2004. In vitro synergic antifungal effect of MUC7 12-mer with histatin-5 12-mer or miconazole. J. Antimicrob. Chemother. 53:750–758 [DOI] [PubMed] [Google Scholar]
- 52. WHO 2010. World malaria report 2010. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/world_malaria_report_2010/worldmalariareport2010.pdf [Google Scholar]